ABSTRACT
Vaccination is the most effective method of preventing the spread of the influenza virus. However, the traditional intramuscular (IM) immunization causes fear, pain, and cross infection. In contrast, needle-free (NF) immunization is quick and easy for medical personnel and painless and safe for patients. In this study, we assessed the safety and protective efficacy of NF intradermal (ID) immunization with the influenza H7N9 split vaccine (Anhui H7N9/PR8). A preliminary safety evaluation showed that ID immunization with 15 μg of the H7N9 influenza vaccine was not toxic in rats. Moreover, the antigen was metabolized more rapidly after ID than after IM immunization, as determined by in vivo imaging, and ID immunization accelerated the generation of a specific immune response. Additionally, ID immunization with a 20% dose of the H7N9 split vaccine Anhui H7N9/PR8 offered complete protection against lethal challenge by the live H7N9 virus. Taken together, our findings suggest that NF ID immunization with the H7N9 influenza vaccine induces effective protection, has a good safety profile, requires little antigen, and elicits an immune response more rapidly than does IM immunization. This approach may be used to improve the control of influenza H7N9 outbreaks.
Introduction
China has experienced five influenza A(H7N9) virus epidemics since the first report in March 2013. As of May 16, 2017, 1,486 infections in 25 provinces of China have been reported by the World Health Organization, and 586 of these led to the death of the patient (mortality rate, ∼ 40%).Citation1 Therefore, Chinese health organizations have focused on fighting this novel influenza virus. By means of reverse genetics, we prepared a novel H7N9/PR8 virus cleavage vaccine that protects mice from lethal challenge with the wild-type virus.Citation2 We evaluated alternative immunization methods, as the traditional method is hampered by fear of needles and can cause needle-stick injuries.
Needle-free (NF) intradermal (ID) immunization is safe, simple, and requires a small quantity of antigen.Citation3 For example, ID immunization is effective against influenza, rabies, and hepatitis B virus infection.Citation4 The skin is a major immunocompetent organ and is easily accessible, making it ideal for vaccination.Citation5 Compared to the traditional intramuscular (IM) influenza vaccine, NF ID immunization with even a low dose of antigen elicits a rapid and protective immune response.Citation6 However, the safety and efficacy of NF ID administration of the H7N9 influenza split vaccine are unclear. More importantly, ID immunization may reduce the quantity of antigen required and accelerate the immune response, thus reducing the time required to halt an influenza H7N9 epidemic.Citation5,Citation7,Citation8 In this study, we evaluated the safety and protective efficacy of the ID delivery of a fractional dose of an influenza H7N9 split vaccine in mice and rats. Our results revealed that ID immunization requires only 20% of the antigen dose administered IM.
Results
Safety and accuracy of the MP-0.1 NF injection system
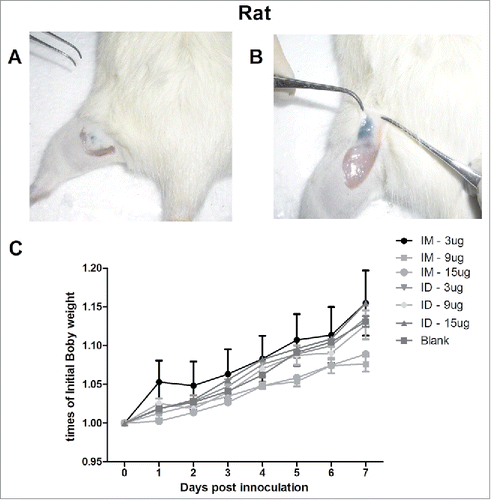
We evaluated the safety and accuracy of ID immunization with the monovalent H7N9 split vaccine using the MP-0.1 system in rats, using 10% ink (HERO232) as an indicator. The MP-0.1 system with a suitable spring was used to immunize rats ID; no residue was observed super- or sub-cutaneously (). The daily increase in body weight was similar after IM and ID injection of 100 µL of the H7N9 split vaccine (3, 9, or 15 µg hemagglutinin [HA]) or of saline as a control (), suggesting the safety of the NF injection system and the split H7N9 vaccine. Next, we evaluated micro- and macro-pathological damage. Skin specimens were collected, and pathological changes were analyzed histologically. From days 1 to 3, both MP-0.1 (ID) and syringe (IM) immunization with 3, 9, or 15 µg of HA caused slight hyperkeratosis of the epidermis and a slight inflammatory exudation and hyperemia in the dermis, sebaceous glands, and subcutaneous muscle. On day 7, epidermis excoriation, fewer infiltrating inflammatory cells (IICs), superficial fascia capillary proliferation, and muscle fiber hyperplasia were observed, which led to complete recovery of the injection injuries (Fig. S1). Consistent with the pathological results, the Draize test scores showed that skin irritation had recovered at day 7. The initially high Draize test scores in the ID groups decreased from days 1 to 3, suggesting gradual absorption of the antigen (Fig. S1). Thus, the MP-0.1 system facilitates safe and accurate NF injection of 100 µL of the H7N9 vaccine.
Figure 1. The ventral sites of rats were wiped and injected ID with 100 µL of 10% ink-PBS using the MP-0.1 system. The animals were euthanized, and their skin was dissected and lifted to confirm the accuracy of the injection (A, B). On day 0, the rats were immunized IM (syringe) or ID (MP-0.1 system) with 3, 9, or 15 µg of HA, or PBS, in a total volume of 100 µL, and body weight changes were recorded daily (C).
Optimum vaccine dose
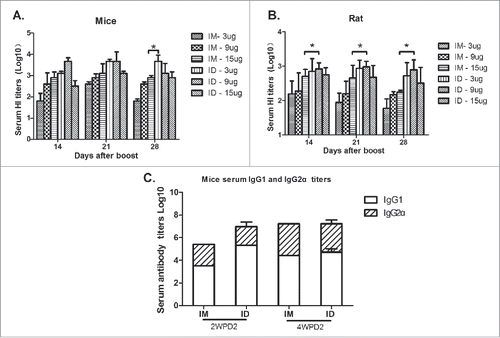
ID immunization with an antigen dose as low as 20% of that typically administered IM can be effective,Citation5, Citation7, Citation9, Citation10 possibly due to the specific immunogenic structure of the skin.Citation11, Citation12 To determine the optimal HA dose for ID immunization, mice and rats were immunized ID or IM with 3, 9, or 15 µg HA, and serum was collected on days 14, 21, and 28 after boosting for measurement of hemagglutination-inhibition (HI) titers. The HI titers of mice immunized IM with 3, 9, and 15 µg of HA were 101.80 ± 0.10, 102.60 ± 0.10, and 102.90 ± 0.10, respectively, at 28 days after the second immunization (). The serum HI titers of mice that received 3 µg HA ID were 103.10±0.10, 103.67 ± 0.10, and 103.67 ± 0.30 at 14, 21, and 28 days after boosting, respectively. However, serum HI titers were lower following the ID immunization of mice and rats with 15 µg HA (), indicating that a higher HA dose does not elicit a stronger immune response. Thus, the optimal HA dose for ID immunization in mice was 3 µg, which was lower than that of rats, possibly because of reduced intradermal antigen absorption in the former. ID or IM immunization with the H7N9 influenza vaccine preferentially induced a Th2-type immune response in BALB/c mice (). Therefore, NF ID immunization induced a Th2-type immune response in mice and rats, and a 20% dose in mice yielded serum HI titers similar to those following IM immunization, which were maintained for a longer time.
Figure 2. Six-to-eight-week-old BALB/c mice (n = 6) and SD rats (n = 6) were twice immunized ID or IM with 3, 9, or 15 µg of HA of the split H7N9 influenza vaccine at 2-week intervals, and serum samples were collected at 2, 3, and 4 weeks post-boosting for HI antibody measurements (A, B). Mice and rats were immunized with the H7N9 influenza vaccine at 0 and 14 days, and serum HI antibody titers and IgG subtypes were assayed at 2 and 4 weeks post-boosting (C).
ID immunization elicits a rapid serum antibody response
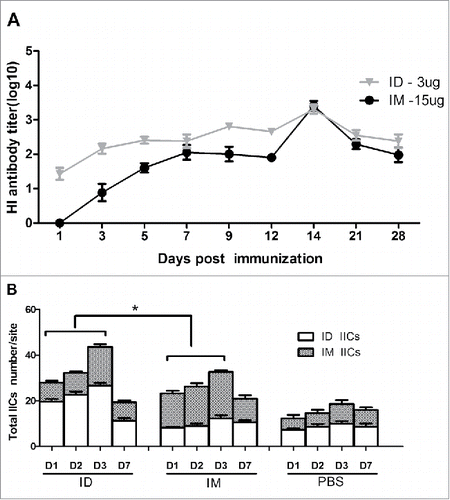
Next, we analyzed IICs in the dermis and muscle tissue in rats and measured HI antibody titers after immunization in mice (, ). Pathologic analysis of the skin of rats immunized ID at days 1, 2, 3, and 7 showed that intradermal IIC began on day 1, peaked at day 3, and returned to a normal level at day 7. In muscle tissue, IIC began at day 3 after IM immunization. Interestingly, the serum HI antibody titers of mice immunized ID began to increase at 3 days after immunization, which was 2 days earlier than in mice immunized IM. These data indicate that ID immunization elicited a more rapid immune response than IM immunization.
Figure 3. BALB/c mice (n = 6) were immunized IM or ID with 3 or 15 µg HA of the H7N9 influenza vaccine, and serum HI antibody titers were measured beginning at day 0 (A). Thirty rats were injected with 3 µg of HA of the H7N9 influenza vaccine, or saline as a control, using a syringe or the MP-0.1 system, and skin biopsies were obtained at 0, 1, 2, 3, and 7 days for assessment of inflammatory cell infiltration into the dermis and muscular layer (B).
Intradermal immunization prolongs antigen deposition
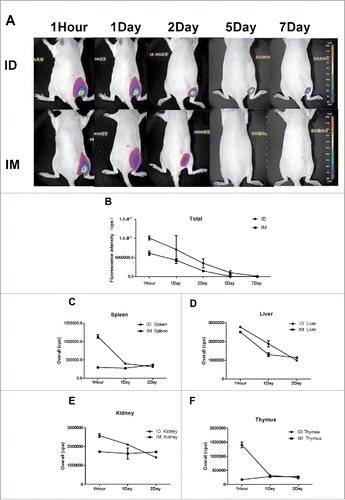
To explore antigen distribution and metabolism following IM or ID immunization, in vivo imaging was performed. Rats were immunized IM or ID with 3 µg of Cy5-labeled-HA, and in vivo fluorescence imaging was performed at 1 hour, 1 day, 2 days, 5 days, and 7 days ( and ). NF ID immunization resulted in a diffuse distribution of HA, whereas injection using a syringe resulted in a small concentrated pool of HA. The diffuse HA distribution likely facilitated uptake of HA by antigen-presenting cells (APCs). This could explain the earlier immune response after ID immunization with the H7N9 influenza virus vaccine. Moreover, fluorescence could be detected for up to 7 days in the rats immunized ID but had disappeared by day 5 in those immunized IM. Maintenance of the antigen in situ for a longer time likely resulted in a stronger antigen-specific immune response. Next, antigen metabolism in the kidney, liver, spleen, thymus, and brain of rats was evaluated by in vivo imaging (, , , ). HA was metabolized more rapidly after ID immunization than after IM immunization, as the fluorescence intensity in the organs of mice immunized ID was markedly higher up to 24 h. After 24 h, there was no difference between IM and ID immunization. Notably, no fluorescence was observed in the brain tissue of mice immunized ID or IM. These in vivo imaging results indicated earlier HA metabolism and prolonged deposition after ID immunization, possibly due to diffuse spread of the antigen.
Figure 4. The H7N9 split vaccine was labeled with Cy5 for visualization of antigen deposition and metabolism in vivo. Rats were immunized ID using MP-0.1 or IM using a syringe and anesthetized with 4% isoflurane. Fluorescence signals were visualized at 1 hour, 1 day, 2 days, 5 days, and 7 days, and background auto-fluorescence was subtracted (A). Fluorescence intensity over time (B). At 1, 24, and 48 h, three rats per group were euthanized, and the kidney, liver, spleen, and thymus were removed and subjected to fluorescence imaging (C, D, E, F).
A lower HA dose protected against lethal H7N9 virus challenge
To evaluate the protective efficacy of ID immunization with a reduced dose of HA against live H7N9 influenza virus, mice immunized with 3 or 15 µg HA were, at 2 weeks after boosting, challenged intranasally (IN) with 5.0 × 103 of the 50% tissue culture infective dose (TCID50) of live A/AnHui/1/2013 H7N9 virus. The mice that received ID immunization with 3 µg HA survived and showed the least reduction in body weight; their body weight recovered from day 7 post-challenge (). Similarly, all mice immunized IM with 15 µg HA survived; however, 20% of the mice immunized IM with 3 µg HA died. All mice in the control group died within 10 days. At day 7 post-challenge, mice were euthanized, and the lungs were subjected to histopathological examination. Severe histopathological damage, including fragmentation of alveolar walls and infiltration of lymphocytes, was observed in the control group, whereas only mild inflammatory changes and few IICs were observed in mice immunized ID with 3 µg HA. Therefore, ID immunization with a 3-µg HA dose of the H7N9 split vaccine protected mice against challenge with live H7N9 influenza virus.
Figure 5. At 0 and 14 days, BALB/c mice were immunized IM with PBS or 15 or 3 µg HA, or they were immunized ID with 3 µg HA of the influenza H7N9 split vaccine. Two weeks later, they were challenged IN with a lethal dose of wild-type A/AnHui/1/2013 H7N9 virus (5.0 × 103 TCID50 pfu). Body weight was monitored daily (n = 6), and pathological changes (n = 3) in the lungs were analyzed at 7 days after challenge (A, B, C, D, E).
Discussion
Because NF ID immunization requires penetration of the epidermis, there is a risk of skin damage, such as unexpected penetration.Citation13, Citation14 Our results confirm the safety and accuracy of the NF injection system in rats. Although mouse skin is so thin that slight adjustment of the spring pressure can result in a penetration accident, vaccination of mini pigs, which have thicker skin than mice, resulted in no damage to the skin (data not shown).
IN administration of antigen elicits a specific sIgA response at the mucosal surface, which prevents virus invasion.Citation15 Therefore, we hypothesized that intradermal structures also induce the secretion of mucosal antibodies. However, we did not detect sIgA in the mucosa after ID immunization. ID immunization with 20% of the typical antigen dose reportedly elicits a stronger immune response than IM immunization.Citation6,Citation16,Citation17 However, the underlying mechanism is unclear. Considering the advantages of a low antigen dose (e.g., safety for human use, lower cost, faster production, and reduced side effects), ID immunization, and particularly NF ID immunization, will likely be used increasingly frequently.Citation18,Citation20,Citation21
Following ID immunization, LCs [infiltrated inflammatory cells] and dendritic cells (DCs) present antigens to naïve T cells. Thus, ID immunization may result in an earlier immune response, which would reduce the rate of spread of the influenza virus. In this study, NF ID immunization elicited a rapid immune response and HA metabolism in vivo. This may be because NF ID immunization resulted in a diffuse HA distribution, enhancing the uptake of the antigen by APCs. Alternatively, the antigen may have been more rapidly metabolized into the lung, liver, spleen and kidney, as indicated by in vivo imaging. The absence of antigen metabolism in brain tissue is likely due to its inability to cross the blood–brain barrier. Because NF ID immunization removes the fear of needles and is pain-free, it is suitable for vulnerable populations, including the elderly and children.Citation22
NF ID immunization resulted in prolonged deposition of antigen at the site, enhancing its uptake by APCs. Interestingly, NF ID immunization resulted in higher serum antibody titers did than immunization using a syringe, possibly because of the diffuse distribution of the antigen by the NF system. LCs and DCs take up antigens, migrate to peripheral draining lymph nodes, and process and present the antigen to naïve T cells to initiate an immune response.Citation10, Citation15, Citation23–25 These processes differ between IM and ID immunization.Citation26, Citation27 In future research, we plan to examine why ID immunization accelerated the immune response and investigate whether both the cellular and humoral immune responses are accelerated. Moreover, whether the earlier initiation of antigen metabolism in vivo contributed to the more rapid immune response remains unclear. Additionally, further investigations of cross-protective efficacy and the long-term immune response following ID NF immunization are required. To facilitate clinical application, the safety and immunogenicity of NF ID immunization with fractional doses of antigen should be evaluated in ferrets or primates. In summary, NF ID immunization with a lower dose of influenza vaccine stimulates a rapid and protective immune response at a reduced cost and will enable optimum utilization of influenza vaccine production capacity.
Materials and methods
Animals
BALB/c mice and Sprague–Dawley (SD) rats were purchased from the Laboratory Animal Center of the Academy of Military and Medical Sciences and were housed at an appropriate temperature under a standard light/dark cycle. All animal experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee of the Academy of Military and Medical Sciences (AMMS) (Approval no.: SYXK 2015-008), and all facilities were accredited by the AMMS Animal Care and Ethics Committee. All experiments using infectious influenza viruses were conducted under biosafety level 3 containment.
Vaccine
The monovalent influenza A (H7N9) split virus vaccine (Anhui H7N9/PR8) was developed by the HengYe Biological Company, and the seed virus was prepared from the reassortant vaccine H7N9/PR8 virus as described previously.Citation2 Briefly, the reassortant H7N9/PR8 virus was harvested from egg cultures and inactivated with formaldehyde. The inactivated virus was concentrated, purified, and further sterilized by chromatography in a buffer containing Triton X-100. The vaccine contains all viral proteins, and the major component is HA (∼ 30% of total protein). Split virus vaccines containing 3, 9, and 15 μg HA were prepared using WHO-approved protocols.Citation28 The vaccines were stored at 4°C prior to use.
Device accuracy and safety evaluation
SD rats (200 g) were used to assess the accuracy of the MP-0.1 low-pressure NF injection system (Jiangsu Chengyu Mite Medical Technologies Co., Jiangsu, China). The rats were anesthetized, their hair trimmed using clippers, and ventral sites were wiped with alcohol prior to injection. Blue and black ink (10%) in phosphate-buffered saline (PBS) (100 μL) was injected, the animals were euthanized immediately thereafter. The skin of the rats was dissected and lifted to evaluate the accuracy of injection. For safety evaluation, rats were immunized IM (syringe) or ID (MP-0.1 system) with 3, 9, or 15 μg of HA of the H7N9 split vaccine or with PBS as a control in a total volume of 100 μL on day 0, and their body weight was monitored daily. Pathological sections obtained at 1, 2, 3, and 7 days after IM immunization with 15 μg HA or ID immunization with 3 μg HA were used to evaluate damage to the skin.
Draize test and pathology evaluation
Skin irritation was evaluated at 1–7 days after IM or ID immunization. Skin erythema and edema were graded separately, each on a 0–4 scale: 0, no erythema; 1, very slight erythema, barely perceptible erythema; 2, well-defined erythema; 3, moderate-to-severe erythema; and 4, severe erythema (beet redness) to slight eschar formation (deep injury); 0, no edema; 1, very slight edema, barely perceptible; 2, slight edema (edges of the area raised and well defined); 3, moderate edema (raised approximately 1 mm); and 4, severe edema (raised more than 1 mm and extending beyond the area of exposure). Epidermis, fat, and muscle samples from rats at days 1, 2, 3, and 7 were embedded in paraffin wax, sectioned, and subjected to pathologic analysis of IICs in the epidermis and subcutaneous muscle.
Efficacy of ID immunization
To determine the optimum dose for NF ID immunization with the H7N9 split vaccine, 6-week-old BALB/c mice and 6-week-old SD rats were immunized IM or ID with 3, 9, or 15 μg of HA in a total volume of 100 μL on two occasions separated by 14-day intervals. Sera were collected before immunization and at 14, 21, and 28 days after boosting, and HI titers were measured as described previously.Citation7 Briefly, 4 HA units were mixed with serum for 30 min at room temperature in 96-well plates, 0.5% (v/v) turkey erythrocytes were added, and the samples were incubated for 40 min. The highest serum dilution level was defined as the HI titer of the sample. Serum IgG, IgG1, and IgG2a levels were measured as described previously.Citation29 Plates were coated with 5 μg/mL inactivated influenza virions, and samples were serially diluted 10-fold in PBS and added to the coated plates in triplicate. Bound antibodies were detected using 1:20,000 dilutions of goat anti-mouse IgG, IgG1, and IgG2a (Sigma, USA) and goat-anti-rat IgG (Sigma), conjugated to horseradish peroxidase. Plates were stained with 3,3'5,5' tetramethyl benzidine substrate (Sigma), the reaction was stopped by adding 2 M H2SO4, and the optical density (OD) at 450 nm was read. Mice that received PBS alone were used as negative controls. At 14 days after boosting, mice were challenged IN with wild-type A/Anhui/1/2013 (H7N9) virus (5.0 × 103 TCID50), as described previously.Citation2 Mice were observed for signs of illness and weighed daily for 14 days. Mice identified as moribund or who had lost > 25% of their body weight were humanely euthanized. At 7 days post-challenge, mice were euthanized, and lung tissue was collected for histopathological evaluation.
In vivo imaging
Mice immunized IM or ID with Cy5-labeled (1:500) (GE Healthcare USA) H7N9 vaccine were imaged using an FX PRO In Vivo Imaging System (Kodak BioMax, Rochester, NY, USA).Citation30 Briefly, mice were anesthetized in a chamber containing 4% isoflurane in a gas mixture of oxygen, nitrogen, and medical air. Following each imaging session, mice were allowed to recover until they could breathe and walk unassisted. To track antigen metabolism after ID and IM immunization, liver, kidney, thymus, spleen, and brain samples were collected at the indicated time points. The fluorescence intensity (counts per second [cps]) of the target site after the subtraction of the background autofluorescence from control mice was used as a surrogate for antigen concentration.
Statistical analysis
Data were analyzed using GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). Measurements were subjected to analysis of variance (ANOVA), and significant differences were analyzed by two-tailed t-test. Differences between groups were evaluated by ANOVA; p < 0.05 was considered to indicate statistical significance. All experiments were performed in triplicate.
Abbreviations
| DC | = | dendritic cell |
| ELISA | = | enzyme-linked immunosorbent assay |
| GMT | = | geometric mean titer |
| HA | = | hemagglutination |
| HI | = | hemagglutination-inhibition |
| IM | = | intramuscular |
| ID | = | intradermal |
| IN | = | intranasally |
| IIC | = | infiltrated inflammatory cells |
| PBS | = | phosphate buffered saline |
| sIgA | = | secretory immunoglobulin A |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KHVI_A_1423156_Supplemental.zip
Download Zip (7.6 MB)Funding
This work was supported in part by the funding from the National High Technology Research and Development Program of China (2015AA020924) and the National Natural Science Foundation of China (81771700).
References
- Iuliano AD JY, Jones J, Davis CT, Wentworth DE, Uyeki TM, Roguski K, Thompson MG, Gubareva L, Fry AM, et al. Increase in human infections with avian influenza A(H7N9) virus during the fifth epidemic—China, October 2016–February 2017. MMWR Morb Mortal Wkly Rep. 2017;66:254–5. doi:10.15585/mmwr.mm6609e2.
- Duan Y GH, Chen R, Zhao Z, Zhang L, Xing L, Lai C, Zhang P, Li Z, Zhang K, et al. Response of Mice and Ferrets to a Monovalent Influenza A (H7N9) Split Vaccine. PLOS one. 2014;9:e99322. doi:10.1371/journal.pone.0099322.
- Levine MM. Can needle-free administration of vaccines become the norm in global immunization? Na Med. 2003;9:99–103. doi:10.1038/nm0103-99.
- Saitoh A, Aizawa Y. Intradermal vaccination for infants and children. Hum Vaccines Immunother. 2016;12:2447–55. doi:10.1080/21645515.2016.1176652.
- Kenney RT FS, Muenz LR, Villar CP, Glenn GM. Dose Sparing with Intradermal Injection of Influenza Vaccine. N Engl J Med. 2004;351:2295–301. doi:10.1056/NEJMoa043540.
- Elsa E, Kis GW JM. Devices for intradermal vaccination. Vaccine. 2012;30:523–38. doi:10.1016/j.vaccine.2011.11.020.
- Darius Soonawalaa PVC, Alienke J, Wijmenga-Monsuurb Claire J, Boogb C, Patrick Koedamd Leo G, Vissera Nynke Y. Rotsb. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine. 2013;6.
- Anthony ES, Nelsona HSL KCC, Wendy CS, Hoc LW, Eva Funga, Frankie WT, Chenga Rita YT, Sunga Michael Royalsd Paul KS. Chanc. A pilot randomized study to assess immunogenicity, reactogenicity,safety and tolerability of two human papillomavirus vaccinesadministered intramuscularly and intradermally to females aged18–26 years. Vaccine. 2013;31:3452. doi:10.1016/j.vaccine.2013.06.034.
- Mohammed AJ AS, Bawikar S, Kurup PJ, Elamir E, Shaban MMA, Sharif SM, van der Avoort HG, Pallansch MA, Malankar P, et al. Fractional Doses of Inactivated Poliovirus Vaccine in Oman. N Engl J Med. 2010;362:2351–9. doi:10.1056/NEJMoa0909383.
- Resik S TA, Sutter RW, Diaz M, Sarmiento L, Alemañi N, Garcia G, Fonseca M, Hung LH, Kahn AL, et al. Priming after a Fractional Dose of Inactivated Poliovirus Vaccine. N Engl J Med. 2013;368:416–24. doi:10.1056/NEJMoa1202541.
- Li N PL-H, Chen X, Nakagawa S, Gao J-Q. Transcutaneous vaccines: Novel advances in technology and delivery for overcoming the barriers. Vaccine. 2011;29:6179–90. doi:10.1016/j.vaccine.2011.06.086.
- Bal SM DZ, van Riet E, Jiskoot W, Bouwstra JA. Advances in transcutaneous vaccine delivery: Do all ways lead to Rome? J Controll Release. 2010;148:266–82. doi:10.1016/j.jconrel.2010.09.018.
- James J, Norman JG, Samirkumar R, Patel Sara Park, Courtney Jarrahian, Darin Zehrung, et al. Reliability and accuracy of intradermal injection by Mantoux technique, hypodermic needle adapter, and hollow microneedle in pigs. Drug Deliv and Transl Res. 2014;4:4.
- Brandtzaeg P ea. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–84. doi:10.1016/j.vaccine.2006.12.001.
- Zhang X CN, Lemoine S, Mozeleski B, Azria E, Ray CL, Schwartz O, Launay O, Leclerc C, Lo-Man R. Plasmacytoid DC engagement by Flu vaccine as a surrogate strategy for driving Th1 responses in human neonatal settings. J Infect Dis. 2014;210:424–34. doi:10.1093/infdis/jiu103.
- Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–94. doi:10.1056/NEJMoa043555.
- Gartlan. KH WJ, Demaria1 MC, Nastovska R, Chang T, L.Jones E, Apostolopoulos V, Pietersz GA, Hickey MJ, van Spriel AB, et al. Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur J Immunol. 2013;43:1208–19. doi:10.1002/eji.201242730.
- Leroux-Roels I WF. Intanza® 9 µg intradermal seasonal influenza vaccine for adults 18 to 59 years of age. Hum Vaccines Immunother. 2013;9:115–21. doi:10.4161/hv.22342.
- Atmar RL, Patel SM, Keitel WA. Intanza((R)): a new intradermal vaccine for seasonal influenza. Expert Rev Vaccines. 2010;9:1399–409. doi:10.1586/erv.10.134.
- Elnekave M, Furmanov K, Hovav AH. Intradermal naked plasmid DNA immunization: mechanisms of action. Expert Rev Vaccines. 2011;10:1169–82. doi:10.1586/erv.11.66.
- Andrianov AK MG. Intradermal immunization using coated microneedles containing an immunoadjuvant. Vaccine. 2012;30:4355–60. doi:10.1016/j.vaccine.2011.09.062.
- Skountzou I, Compans RW. Skin immunization with influenza vaccines. Curr Top Microbiol Immunol. 2015;386:343–69.
- Tuan-Mahmood TM, McCrudden MT, Torrisi BM, McAlister E, Garland MJ, Singh TR, et al. Microneedles for intradermal and transdermal drug delivery. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2013;50:623–37. doi:10.1016/j.ejps.2013.05.005.
- Obeid M FJ, Lorthiois A, Gego A, Grüner AC, Tefit M, Boucheix C, Snounou G, Mazier D. Skin draining lymph node priming is sufficient to induce sterile immunity against pre erythrocytic malaria. EMBO Mol Med. 2013;5:250–63. doi:10.1002/emmm.201201677.
- Weissmueller NT SH, Pollard AJ. Intradermal powder immunization with protein-containing vaccines. Expert Rev Vaccines. 2013;12:687–702. doi:10.1586/erv.13.48.
- Yu S TC, Shi X, Yang P, Xing L, Wang X. Novel Th1-biased adjuvant, SPO1, enhances mucosal and systemic immunogenicity of vaccines administered intranasally in mice. Vaccine. 2012;30:5425–36. doi:10.1016/j.vaccine.2012.05.085.
- Kalluri H KC, Banga AK. Characterization of Microchannels Created by Metal Microneedles: Formation and Closure. AAPS Journal. 2011;13:473–81. doi:10.1208/s12248-011-9288-3.
- Harvey R WJ, Wallis CL, Robertson JS, Engelhardt OG. Quantitation of haemagglutinin in H5N1 influenza viruses reveals low haemagglutinin content of vaccine virus NIBRG-14 (H5N1). Vaccine. 2008 26:6550–4. doi:10.1016/j.vaccine.2008.09.050.
- H. DJ GW, O. CH. Methods for the study of irritation and toxicity of substances applied topically to the skin and the mucous membranes. J Pharmacol Exp Ther. 1944;82(3): 377–390.
- Cao. RG SN, Obermoser G, Lopez SMC, Flano E, Mertz SE, Albrecht RA, García-Sastre A, Mejias A, Xu H, et al. Differences in Antibody Responses between TIV and LAIV Influenza Vaccines Correlate with the Kinetics and Magnitude of Interferon Signaling in Children. J Infect Dis. 2014;210:224–33. doi:10.1093/infdis/jiu079.
