ABSTRACT
The field of immunotherapeutics is living an exceptional time as new antibodies that take brakes off T-cells and unleash them on tumours are being approved by the US-Food and Drug Administration (FDA). For the design and development of an HIV-1 therapeutic-vaccine, one would need preferably to shift the balance T-effectors/T-regulatory cells (Teff/Tregs) towards effectors to improve vaccine-specific immune-responses. Given the success with the new immune-checkpoint-blockers (ICB), it is an appropriate time for HIV-1 field to seize this opportunity and develop new therapeutic vaccine-strategies that take into consideration ICB and other immunomodulators such as cytokines. While the vaccine is important to stimulate HIV-1-specific T-cell responses, cytokines will support the expansion of the stimulated virus-specific T-cells and ICB will reverse exhaustion and unchain cytotoxic T-cells. In this commentary, we will spotlight Tregs as another major brake for T-cell immunity and address the main stumbling-blocks that often blurs HIV-1-specific-Tregs status with regards to their role (beneficial or detrimental) and we will recall some proof-of-concept studies where therapeutic immunization skewed the HIV-1-specific response from Tregs to Teffs which impacts on the magnitude of viral replication. We will also suggest some strategies to shift the balance towards Teffs and potentiate HIV-1-specific immune-responses.
Introduction
The main goal of therapeutic vaccines is to strengthen and help the immune system to fight an existing disease. Different strategies of immunotherapy have been described ranging from peptides therapeutic vaccine to targeted-cell therapies.
In HIV-1 infection, different therapeutic vaccine strategies have been developed and trialled, but none of these succeeded in eradicating the virus despite induction of strong specific immune responses (reviewed inCitation1).
CD4+ T-cell subsets including Teffs and Tregs play key roles during infections, and monitoring the development of such immune responses after vaccination is crucial. The role of Tregs has been extensively studied in cancer and HIV-1 vaccine trials.Citation2-4 In HIV-1 infection, these cells have been shown to be both beneficial and deleterious, creating thus some confusion in the field. This is due in part to the variable settings used when Tregs have been looked at and focused on at the time of the study. These include the stage of infection (acute versus chronic), the sampling (blood, lymph node or gut tissue) and/or the phenotypic markers used to identify and quantify Tregs (reviewed inCitation5). Moreover, an additional important factor that has not been substantially discussed so far and may add a layer of confusion in data interpretation, is related to the nature of Tregs, i.e. “bulk” or antigen-specific subset. In this context, recent advances have been made in HIV-1 infected patients, where both subsets (bulk and antigen-specific) have been simultaneously measured and compared in the same patients.Citation6 These aspects will be highlighted below.
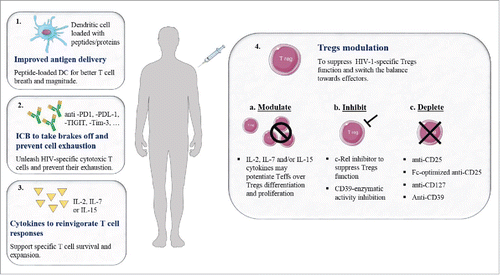
For the design and development of an HIV-1 therapeutic vaccine, one would need preferably to shift the balance of Teff/Tregs toward effector cells in order to improve vaccine-specific immune responses. Given that we are living an exciting time where new therapeutic strategies based on ICB that target negative molecules such as protein-death 1 (PD-1, CD279) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4, CD152),Citation7 it is time to acknowledge that Tregs are a full-fledged additional immune checkpoint that needs to be considered for depletion and/or modulation in combination with an anti-PD-1 antibody for example, in order to strengthen specific immune responses (particularly CD8 T cell response). In this commentary, we will highlight the main stumbling-blocks that often blurs HIV-1-specific-Tregs status in the field with regards to their role during anti-HIV responses and we will recall some proof-of concept studies where therapeutic immunization skewed the HIV-1-specific immune response from Tregs to Teffs which impacts on the magnitude of viral replication following treatment interruption. We will also suggest some strategies to shift the balance towards Teffs and potentiate HIV-1-specific immune responses ().
Figure 1. To potentiate antigen-specific immune responses in the context of HIV-1-therapeutic vaccine. T cell breath and magnitude will be improved by using peptides-loaded-DC (1). Immune response is regulated by inhibitory and co-stimulatory signals mediated by immune-checkpoint. These signals can be modulated using IC blockers (ICB) in order to amplify HIV-1-specific responses and to prevent cell exhaustion (2). T cell proliferation can be boosted using cytokines (IL-2, IL-7 or IL-15) in combination with therapeutic vaccines in order to promote antigen-specific T cell survival and expansion (3). It is important to block Tregs expansion and shift the balance toward effectors (4). To this end, cytokines, i.e IL-7 will modulate Tregs phenotype and function towards and potentiates Teffs differentiation (4a). Vaccines could be combined with other molecules that will suppress Treg-activity (c-Rel inhibitor, anti-CD39) (4b). Antibodies, i.e anti-CD25 (Daclizumab), Fc-optimized anti-CD25, anti-CD127 or anti-CD39 may be used to efficiently deplete Tregs (4c).
Monitoring vaccine impact: How to detect the full range of Th responses
Monitoring vaccine responses is crucial for predicting its impact on the immune system and improving therapeutic strategies. These responses are usually evaluated by assessing T-cell phenotype and proliferation as well as T-cell cytotoxicity and cytokine release following stimulation with peptides/proteins.Citation8 However, these commonly used assays do not include Tregs responses. Indeed, the lack of tools that one can easily use in clinical trials setting has prevented the standartized measurement of Tregs specific responses. Therefore, Tregs frequency and/or function have often been studied on bulk CD4+CD25hi CD127lowFoxP3+ Tregs (reviewed inCitation5,Citation9 and less often on antigen-specific Tregs. Angin et al., reported the presence of gag-specific Tregs in infected individuals by using MHC Class II tetramer loaded with gag peptides.Citation9 This approach is interesting but very challenging when used in clinical trials due to the genetic variability of MHC Class II as well as the limited availability of Class II tetramers. Tregs could also have different affinity with MHC comparing to Teffs, which could lead to differential staining and probable under- or over- estimation of their frequencies. Therefore, more practical tools have been developped, and one of them is the “OX40 assay”Citation10 that simultaneously detects a full range of Th responses including antigen-specific TregsCitation11 and T follicular helper (Tfh) responses (Seddiki et al., unpublished). Moreover, it could be combined with other methods i.e, intracellular staining (ICS) or genomic and proteomic analyses. Importantly, this assay has served as a platform for the development of other assays such as the activation-induced marker (AIM) which is efficient at detecting antigen-specific CD4 T cells.Citation12,Citation13 A newer version of the AIM assay based on the use of OX40/PD-L1 and OX40/4-1BB in human and rhesus macaque samples respectively, has been reported.Citation14 However, these assays, contrary to the « OX40 assay», do not allow antigen-specific Tregs response detection, which are part of the immune response and should not be neglected.
Taking advantage of the reliability of the « OX40 assay », we demonstrated that HIV-1-infected individuals harbor high levels of HIV-1-specific Tregs at baseline.Citation15 After vaccination with autologous dendritic cells (DC) loaded with HIV-1-derived peptides, patients who exhibited lower viral load after treatment interruption, displayed lower levels of HIV-1-specific CD4+CD134+CD25+CD39+FoxP3+ Tregs by shifting the HIV-1-specific Tregs:Teffs ratio towards polyfunctional effector responses.Citation15 Of note, there was no difference in bulk CD4+CD25+CD127lowFoxp3+Tregs levels before and after vaccination.
In a more recent work, we showed that therapeutic vaccination, of HIV-1-infected patients under therapy (cART), using ALVAC-HIV and Lipo-6T followed by 3 cycles of subcutaneous interleukin-2 (IL-2), decreased CD4+CD25+OX40+CD39+Foxp3+ HIV-1-specific Tregs thus shifting the balance towards effectors, while peripheral bulk CD4+CD25hiCD127lowFoxP3+ increased significantly.Citation6 Moreover, we have been able to demonstrate that increased bulk Tregs frequency impacts on T-cell exhaustion while decreased HIV-specific Tregs impact on the balance towards effectors. This demonstrates the need for assessing different subsets of Tregs as each population is provided with its specific phenotype and/or function, playing thus different roles in HIV-1 infection.
How could we potentiate HIV-1-specific responses with therapeutic vaccines?
An immune response is initiated following recognition of antigen by T cell receptor (TCR) and is regulated by inhibitory and co-stimulatory signals mediated by immune checkpoint (IC) molecules. In physiological conditions, IC protect from autoimmunity and immune system over-reaction following pathogenic infection. PD-1 and CTLA-4 constitute the most targeted molecules in immunotherapy as they have proven to be good candidates in cancer (reviewed inCitation7,Citation16). In cART HIV-1 individuals, the first clinical trial with anti-PDL1 antibody has been completed and only two patients showed increased percentage of Gag-specific CD8+ T cells at day 28 after infusion.Citation17 In SIV (simian immunodeficiency virus) infection, anti-PD-1 infusion resulted in significant enhancement of specific-immune responses and decrease in plasma RNA level.Citation18 However, none of these studies has examined the impact of IC inhibitors on Tregs subsets dynamics. A recent study analyzed the effect of pembrolizumab (anti-PD1) on different populations of Tregs. Authors showed that PD-1 was mainly expressed on CD4+CD25+ cells of healthy donors and patients with primary breast cancer. However, pembrolizumab did not affect the frequency of FoxP3+ Tregs subsets but rather blocked signalling cascades via the PD-1/PD-L1 axis in activated T cells, suggesting that pembrolizumab may reverse immune escape through blockage of PD-1/PD-L1 interaction and not by altering Tregs phenotype or function.Citation19
Given that therapeutic vaccines and cytokines have been commonly used to enhance HIV-1-specific cell-mediated immune responses and to suppress virus replication, combined immunotherapy including ICB, cytokines and vaccines represents potent combinatorial interventions in HIV-1-infected patients. While the vaccine is important to stimulate HIV-specific T-cell responses, cytokines and ICB may support the expansion of the stimulated virus-specific T cells.
The common γ-chain cytokines IL-2, IL-7 and IL-15 are crucial for T-lymphocyte development and survival, therefore their use in immunotherapy was proposed to improve quantitative and qualitative CD4+ T cell restoration.
Studies using a mouse model for LCMV indicated that combined IL-2 and PD-1 blockade therapy represents a promising strategy for increasing CD8 T cell function and reducing viral load during chronic infection despite an increase in Tregs.Citation20 In cancer, AIPV combination, i.e. tumor-antigen specific antibody (A), IL-2 (I), anti-PD1 (P) and amphiphile-vaccine (V, specific tumor peptides or CpG), induced tumor regression, tumor infiltration of CD8+ T cells and cross-presentation promotion in mice treated with subcutaneous syngeneic tumor models (melanoma, mouse breast cancer and tumors expressing the human papilloma virus (HPV) onco-antigens E6 and E7).Citation21 In HIV-1 infection, recombinant IL-2 administration to patients on cART, showed sustained increase in CD4 T cell countCitation22 but no clinical benefits were noted.Citation23 These results were explained by the expansion of CD4+CD25+FoxP3+ Tregs.Citation24 However, unlike IL-2 therapy alone, the use of IL-2 as an adjuvant to vaccines highlighted more positive effects by decreasing HIV-1-specific CD4+ CD39+FOXP3+CD25+CD134+ Tregs as mentioned above,Citation6 emphasizing thus the promising character of this cytokine in combinatorial therapeutic strategies for HIV-1+ patients.
Il-7 therapy was seen as an alternative strategy to IL-2 therapy. Clinical trials performed in HIV-1 infected patients under cART revealed that IL-7 administration induced immunological improvement, particularly on T cellsCitation25-27 with a relative decrease of Tregs.Citation28,Citation29 Indeed, IL-7 treatment on Tregs led to reduced expression of CD39 and reduced suppressive activity with a shift toward Th17 phenotype.Citation30 However, IL-7 therapy failed to reduce viral reservoirCitation31,Citation32 probably due to central memory CD4 T cell expansion.Citation33
IL-15 has emerged as another highly-promising candidates in immunotherapy. This cytokine shares many of the biological properties of IL-2.Citation34 Interestingly, IL-15Rα/IL-15 complex emerged as a potent adjuvant for dendritic cell immunotherapy or combinatorial therapy for the developpement of sustained memory-CD8 T cell responses.Citation35,Citation36 In HIV-1-infected humanized mice, IL-15 superagonist (ALT-803) treatment inhibited HIV-1 infection through NK cell activation which highlights the pluricellular effect of IL-15.Citation37,Citation38 ALT-803 molecule is moving into a clinical trial in cART HIV-1 infected subjects (ClinicalTrials.gov identifier: NCT02191098). It is noteworthy to mention that combined therapy with anti-PD-1, anti-PD-L1 or anti-CTLA-4, greatly enhanced the efficacy of ALT-803, in remission of primary and advanced tumors in different preclinical studies.Citation39,Citation40
Treg depletion or modulation: An additional therapeutic strategy to potentiate vaccines
Meta-analysis work reviewing various cancer research studies, found a negative prognosis associated with tumor-infiltrating FoxP3+ Tregs.Citation41
In HIV-1 and SIV infections, Tregs were associated with lower specific immune responsesCitation42-44 and contributed to HIV-1 reservoir persistence.Citation45 Moreover, cART-patients who received a DC-based vaccine showed a better anti-HIV response that was correlated with lower frequency of HIV-specific CD4+CD134+CD25+CD39+FoxP3+ Tregs.Citation15 Indeed, Tregs negatively impact anti-HIV-1 specific responses, it is thus necessary to pay attention to them and they should not be neglected.
Depletion of Tregs using anti-CD25 has been used therapeutically in combination with vaccines in cancer studies.Citation46 In a promising study, patients with breast cancer receiving Daclizumab (anti-CD25 mAb) followed by peptide-vaccination, displayed a prolonged depletion of CD25highCD45RA− Tregs with an increase in Teffs responses .Citation47 Moreover, in a recent study, Treg depletion was shown to synergize with PD-1 blockade to eradicate tumor-infiltrating Tregs.Citation48 Indeed, the use of an anti-CD25 antibody with enhanced binding to activating Fcγ-Receptors led to effective depletion of tumor-infiltrating Tregs and improved control of established tumors. More importantly, combination of anti-CD25 with anti-PD-1 promoted complete tumor rejection, demonstrating the relevance of CD25 as a therapeutic target and promising substrate for future combination approaches for immunotherapy.Citation48 In another recent study it has been shown that melanoma cell growth is drastically reduced in mice lacking NF-κB c-Rel subunit, but not p65, in Tregs. Interestingly, chemical inhibition of c-Rel function delayed melanoma growth by impairing Tregs-mediated immunosuppression and potentiated the effects of anti-PD-1 immunotherapy. These results therefore establish inhibition of NF-κB c-Rel as a viable therapeutic approach for enhancing checkpoint-targeting immunotherapy protocols.Citation49 Altogether, these studies open the field to novel molecules and possibilities of combinatorial strategies, i.e simultaneous Tregs inhibition and ICB use. Other strategies of Treg-depletion were developed via the targeting of CTLA-4,Citation50,Citation51 CD27,Citation52 ICOSCitation53 or OX40 (CD134).Citation54 These major advances are very attractive and provide the scientific basis for developing potent anti-HIV-1 combinatorial therapeutics to enhance immune responses and gain more efficient control of virus replication.
Conclusion
Despite major advances in the field of HIV-1 therapeutic vaccination, it became apparent that HIV cure will need to combine cytokines, i.e IL-2, IL-7 or IL-15, which are effective at increasing expansion, differenciation and maintenance of virus-specific memory T cells, in addition to ICB that have proven to be effective in cancer immunotherapy. Furthermore, the recent concepts of Tregs depletion based on NF-kB c-Rel inhibitionCitation49 or the use of anti-CD25 antibody with enhanced binding to activating Fcγ-Receptors leading to effective Tregs depletion,Citation48 offer excellent opportunities to block Tregs which represent a major immune checkpoint that should not be neglected in future clinical studies.
Disclosure of potential conflict of interest
There are no conflicts of interests.
Additional information
Funding
References
- Pantaleo G, Levy Y. Therapeutic vaccines and immunological intervention in HIV infection: a paradigm change. Curr Opin HIV AIDS. 2016;11:576-84. doi:10.1097/COH.0000000000000324. PMID:27607591.
- Seddiki N, Brezar V, Draenert R. Cell exhaustion in HIV-1 infection: role of suppressor cells. Curr Opin HIV AIDS. 2014;9:452-8. doi:10.1097/COH.0000000000000087. PMID:25010895.
- Welters MJ, Kenter GG, de Vos van Steenwijk PJ, Lowik MJ, Berends-van der Meer DM, Essahsah F, Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci U S A. 2010;107:11895-9. doi:10.1073/pnas.1006500107. PMID:20547850.
- Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110:17945-50. doi:10.1073/pnas.1316796110. PMID:24127572.
- Simonetta F, Bourgeois C. CD4+FOXP3+ Regulatory T-Cell Subsets in human immunodeficiency virus infection. Front Immunol. 2013;4:215. doi:10.3389/fimmu.2013.00215. PMID:23908654.
- Brezar V, Hani L, Surenaud M, Hubert A, Lacabaratz C, Lelievre JD, Levy Y, Seddiki N. Negative modulation of suppressive HIV-specific regulatory T cells by IL-2 adjuvanted therapeutic vaccine. PLoS Pathog. 2017;13:e1006489. doi:10.1371/journal.ppat.1006489. PMID:28708863.
- Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2017;18:91–104. doi:10.1038/nri.2017.112. PMID:28990586.
- Apostolopoulos V, Marincola FM. Methods to measure vaccine immunity. Expert Rev Vaccines. 2010;9:545-6. doi:10.1586/erv.10.61. PMID:20518709.
- Brezar V, Godot V, Cheng L, Su L, Levy Y, Seddiki N. T-regulatory cells and vaccination “pay attention and do not neglect them”: lessons from hiv and cancer vaccine trials. Vaccines (Basel). 2016;4:1–13. doi:10.3390/vaccines4030030. PMID:27608046.
- Zaunders JJ, Munier ML, Seddiki N, Pett S, Ip S, Bailey M, Xu Y, Brown K, Dyer WB, Kim M. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J Immunol. 2009;183:2827-36. doi:10.4049/jimmunol.0803548. PMID:19635903.
- Seddiki N, Cook L, Hsu DC, Phetsouphanh C, Brown K, Xu Y, Xu Y, Kerr SJ, Cooper DA, Munier CM, Pett S. Human antigen-specific CD4(+) CD25(+) CD134(+) CD39(+) T cells are enriched for regulatory T cells and comprise a substantial proportion of recall responses. Eur J Immunol. 2014;44:1644-61. doi:10.1002/eji.201344102. PMID:24752698.
- Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Pena A, Kasturi SP, Dan JM, Bothwell M, Sanders RW. Cytokine-independent detection of antigen-specific germinal center t follicular helper cells in immunized nonhuman primates using a live cell activation-induced marker technique. J Immunol. 2016;197:994-1002. doi:10.4049/jimmunol.1600320. PMID:27335502.
- Dan JM, Lindestam Arlehamn CS, Weiskopf D, da Silva Antunes R, Havenar-Daughton C, Reiss SM, et al. A cytokine-independent approach to identify antigen-specific human germinal center t follicular helper cells and rare antigen-specific CD4+ T cells in blood. J Immunol. 2016;197:983-93. doi:10.4049/jimmunol.1600318. PMID:27342848.
- Reiss S, Baxter AE, Cirelli KM, Dan JM, Morou A, Daigneault A, Brassard N, Silvestri G, Routy JP, Havenar-Daughton C. Comparative analysis of activation induced marker (AIM) assays for sensitive identification of antigen-specific CD4 T cells. PLoS One. 2017;12:e0186998. doi:10.1371/journal.pone.0186998. PMID:29065175.
- Brezar V, Ruffin N, Richert L, Surenaud M, Lacabaratz C, Palucka K, Thiébaut R, Banchereau J, Levy Y, Seddiki N. Decreased HIV-specific T-regulatory responses are associated with effective DC-vaccine induced immunity. PLoS Pathog. 2015;11:e1004752. doi:10.1371/journal.ppat.1004752. PMID:25816350.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-64. doi:10.1038/nrc3239. PMID:22437870.
- Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N. Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J Infect Dis. 2017;215:1725-33. doi:10.1093/infdis/jix191. PMID:28431010.
- Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206-10. doi:10.1038/nature07662. PMID:19078956.
- Toor SM, Syed Khaja AS, Alkurd I, Elkord E. In-vitro effect of pembrolizumab on different T regulatory cell subsets. Clin Exp Immunol. 2018;191:189-97. doi:10.1111/cei.13060. PMID:28963773.
- West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604-15. doi:10.1172/JCI67008. PMID:23676462.
- Moynihan KD, Opel CF, Szeto GL, Tzeng A, Zhu EF, Engreitz JM, Williams RT, Rakhra K, Zhang MH, Rothschilds AM. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22:1402-10. doi:10.1038/nm.4200. PMID:27775706.
- Levy Y, Capitant C, Houhou S, Carriere I, Viard JP, Goujard C, et al. Comparison of subcutaneous and intravenous interleukin-2 in asymptomatic HIV-1 infection: A randomised controlled trial. ANRS 048 study group. Lancet. 1999;353:1923-9. doi:10.1016/S0140-6736(98)07345-0. PMID:10371571.
- Group I-ES, Committee SS, Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361:1548-59. doi:10.1056/NEJMoa0903175. PMID:19828532.
- Weiss L, Letimier FA, Carriere M, Maiella S, Donkova-Petrini V, Targat B, Benecke A, Rogge L, Levy Y. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc Natl Acad Sci U S A. 2010;107:10632-7. doi:10.1073/pnas.1000027107. PMID:20498045.
- Beq S, Nugeyre MT, Ho Tsong Fang R, Gautier D, Legrand R, Schmitt N, et al. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol. 2006;176:914-22. doi:10.4049/jimmunol.176.2.914. PMID:16393976.
- Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304-14. doi:10.1182/blood-2008-10-186601. PMID:19380868.
- Sereti I, Estes JD, Thompson WL, Morcock DR, Fischl MA, Croughs T, Beq S, Lafaye de Micheaux S, Yao MD, Ober A. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014;10:e1003890. doi:10.1371/journal.ppat.1003890. PMID:24497828.
- Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boué F, Molina JM, Rouzioux C, Avettand-Fénoêl V. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997-1007. PMID:19287090.
- Thiebaut R, Jarne A, Routy JP, Sereti I, Fischl M, Ive P, Speck RF, D'Offizi G, Casari S, Commenges D. Repeated Cycles of Recombinant Human Interleukin 7 in HIV-Infected Patients With Low CD4 T-Cell Reconstitution on Antiretroviral Therapy: Results of 2 Phase II Multicenter Studies. Clin Infect Dis. 2016;62:1178-85. doi:10.1093/cid/ciw065. PMID:26908786.
- Younas M, Hue S, Lacabaratz C, Guguin A, Wiedemann A, Surenaud M, et al. IL-7 modulates in vitro and in vivo human memory T regulatory cell functions through the CD39/ATP axis. J Immunol. 2013;191:3161-8. doi:10.4049/jimmunol.1203547. PMID:23966629.
- Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, Molina JM, Fischl M, Goujard C, Rodriguez B. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55:291-300. doi:10.1093/cid/cis383. PMID:22550117.
- Katlama C, Lambert-Niclot S, Assoumou L, Papagno L, Lecardonnel F, Zoorob R, Tambussi G, Clotet B, Youle M, Achenbach CJ. Treatment intensification followed by interleukin-7 reactivates HIV without reducing total HIV DNA: a randomized trial. AIDS. 2016;30:221-30. doi:10.1097/QAD.0000000000000894. PMID:26684819.
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893-900. doi:10.1038/nm.1972. PMID:19543283.
- Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965-8. doi:10.1126/science.8178155. PMID:8178155.
- Hasan AN, Selvakumar A, Shabrova E, Liu XR, Afridi F, Heller G, Riviere I, Sadelain M, Dupont B, O'Reilly RJ. Soluble and membrane-bound interleukin (IL)-15 Ralpha/IL-15 complexes mediate proliferation of high-avidity central memory CD8(+) T cells for adoptive immunotherapy of cancer and infections. Clin Exp Immunol. 2016;186:249-65. doi:10.1111/cei.12816. PMID:27227483.
- Van den Bergh JMJ, Smits E, Berneman ZN, Hutten TJA, De Reu H, Van Tendeloo VFI, Dolstra H, Lion E, Hobo W. Monocyte-derived dendritic cells with silenced PD-1 ligands and transpresenting interleukin-15 stimulate strong tumor-reactive t-cell expansion. Cancer Immunol Res. 2017;5:710-5. doi:10.1158/2326-6066.CIR-16-0336. PMID:28637876.
- Seay K, Church C, Zheng JH, Deneroff K, Ochsenbauer C, Kappes JC, Liu B, Jeng EK, Wong HC, Goldstein H. In Vivo Activation of Human NK Cells by Treatment with an Interleukin-15 Superagonist Potently Inhibits Acute In Vivo HIV-1 Infection in Humanized Mice. J Virol. 2015;89:6264-74. doi:10.1128/JVI.00563-15. PMID:25833053.
- Guo Y, Luan L, Patil NK, Sherwood ER. Immunobiology of the IL-15/IL-15Ralpha complex as an antitumor and antiviral agent. Cytokine Growth Factor Rev. 2017;38:10-21. doi:10.1016/j.cytogfr.2017.08.002. PMID:28888485.
- Mathios D, Park CK, Marcus WD, Alter S, Rhode PR, Jeng EK, Wong HC, Pardoll DM, Lim M. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int J Cancer. 2016;138:187-94. doi:10.1002/ijc.29686. PMID:26174883.
- Kim PS, Kwilas AR, Xu W, Alter S, Jeng EK, Wong HC, Schlom J, Hodge JW. IL-15 superagonist/IL-15RalphaSushi-Fc fusion complex (IL-15SA/IL-15RalphaSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget. 2016;7:16130-45. PMID:26910920.
- Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi:10.1038/srep15179. PMID:26462617.
- Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104:3249-56. doi:10.1182/blood-2004-01-0365. PMID:15271794.
- Estes JD, Li Q, Reynolds MR, Wietgrefe S, Duan L, Schacker T, Picker LJ, Watkins DI, Lifson JD, Reilly C. Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection. J Infect Dis. 2006;193:703-12. doi:10.1086/500368. PMID:16453267.
- Tenorio AR, Martinson J, Pollard D, Baum L, Landay A. The relationship of T-regulatory cell subsets to disease stage, immune activation, and pathogen-specific immunity in HIV infection. J Acquir Immune Defic Syndr. 2008;48:577-80. doi:10.1097/QAI.0b013e31817bbea5. PMID:18645514.
- Li G, Nunoya JI, Cheng L, Reszka-Blanco N, Tsao LC, Jeffrey J, Su L. Regulatory T Cells Contribute to HIV-1 Reservoir Persistence in CD4+ T Cells through cyclic adenosine monophosphate-dependent mechanisms in humanized mice in vivo. J Infect Dis. 2017;216:1579-91. doi:10.1093/infdis/jix547. PMID:29045701.
- Rech AJ, Vonderheide RH. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann N Y Acad Sci. 2009;1174:99-106. doi:10.1111/j.1749-6632.2009.04939.x. PMID:19769742.
- Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ Jr., Colligon TA, Trosko JA, Leinbach LI, Pletcher CH. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4:134ra62. doi:10.1126/scitranslmed.3003330. PMID:22593175.
- Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Miranda Rota E, Dahan R, Georgiou A, Sledzinska A. Fc-Optimized Anti-CD25 depletes tumor-infiltrating regulatory t cells and synergizes with PD-1 blockade to eradicate established tumors. Immunity. 2017;46:577-86. doi:10.1016/j.immuni.2017.03.013. PMID:28410988.
- Grinberg-Bleyer Y, Oh H, Desrichard A, Bhatt DM, Caron R, Chan TA, Schmid RM, Klein U, Hayden MS, Ghosh S. NF-kappaB c-Rel is crucial for the regulatory t cell immune checkpoint in cancer. Cell. 2017;170:1096-108 e13. doi:10.1016/j.cell.2017.08.004.
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32-42. doi:10.1158/2326-6066.CIR-13-0013. PMID:24777248.
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695-710. doi:10.1084/jem.20130579. PMID:23897981.
- Wasiuk A, Testa J, Weidlick J, Sisson C, Vitale L, Widger J, Crocker A, Thomas LJ, Goldstein J, Marsh HC. CD27-mediated regulatory t cell depletion and effector t cell costimulation both contribute to antitumor efficacy. J Immunol. 2017;199:4110-23. doi:10.4049/jimmunol.1700606. PMID:29109120.
- Mo L, Chen Q, Zhang X, Shi X, Wei L, Zheng D, Li H, Gao J, Li J, Hu Z. Depletion of regulatory T cells by anti-ICOS antibody enhances anti-tumor immunity of tumor cell vaccine in prostate cancer. Vaccine. 2017;35:5932-8. doi:10.1016/j.vaccine.2017.08.093. PMID:28923424.
- Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92:475-80. doi:10.1038/icb.2014.26. PMID:24732076.
