ABSTRACT
Immunogenicity of hepatitis B vaccine between 20 μg with 3-dose schedule and 60 μg with 2-dose regimens was compared 2 years after primary immunization. A total of 353 healthy adults aged 18–25 years were enrolled in the study and randomly assigned (1: 1: 1) into 3 vaccine groups: A (20 μg, 0–1–6 month), B (60 μg, 0–1 month) and C (60 μg, 0–2 month). Serum samples were collected at 1 month after a series vaccination and 12 months, 24 months after the first-dose. The GMC level of anti-HBs antibody was measured using Chemiluminescent Microparticle ImmunoAssay (CMIA). There were 59, 45 and 55 vaccinees available to follow-up with 2 year later in vaccine groups A, B and C, respectively. No significant differences existed in sex ratio, age and body mass index (BMI) among vaccinees at month 24 and the corresponding participants at baseline in each group (P > 0.05). The seroprotection rates in group A, B and C were 98.31%, 88.37% and 85.19%, respectively (P = 0.014), reflecting the fact that the rate of group A was significantly higher than that in group C (P = 0.026). Also, the GMC level of anti-HBs antibody in group A was significantly higher than those of other two groups (427.46 mIU/ml vs. 89.74 mIU/ml, 89.80 mIU/ml, respectively; all P < 0.01). This data suggested that the standard 20 μg (0–1–6 month) regimen of hepatitis B vaccine should be recommended as a priority on the premise of complete compliance in adults.
Introduction
Hepatitis B virus (HBV) infection is a major public health concern worldwide due to the high morbidity and mortality associated with liver cirrhosis and hepatocellular carcinoma (HCC). The World Health Organization (WHO) estimates that 2 billion people worldwide have been infected with HBV, and 257 million have chronic HBV infections. Around 885,000 die each year from the complications of chronic hepatitis B.Citation1
Universal HBV immunization program has significantly reduced the prevalence of HBsAg carriers. Since 1992, the Chinese government has implemented infant vaccination with hepatitis B vaccine as a basic state policy.Citation2 Given the huge population in China, a reduction of the HBsAg prevalence rate in the general population from 9.75% (1992) to 7.18% (2006) and 2.64% (1–29 years old, 2014)Citation3 implies that millions of persons have been protected against HBV infection and proves that vaccination is a simple and cost-effective measure to prevent HBV infection. At present, HBV carrier rate in the general population increases with advancing age (0.32%, 1–4 years old; 0.94%, 5–14 years old; 4.38%, 15–29 years old),Citation3 demonstrating the fact that adult HBV infection is not being effectively controlled and suggesting the need for further improvements in current immunization strategies.
A complete standard vaccination schedule for adults exists in 3 doses that are most commonly administered on a 0, 1, and 6-month schedule. The conventional vaccine contains 20 μg of HBsAg antigen per dose.Citation4 The 3rd dose of immunization is often missed or forgotten because of the too-long interval between the previous two shots and the third dose in the immunization protocol. This results in an explicit relationship of poorer immunological response and the shorter duration of the seroprotection of anti-HBs.Citation5 On the other hand, the increased HBsAg content in a vaccine formulation has been investigated as a strategy to improve immunogenicity.Citation6-7 In order to increase the vaccinees compliance and vaccination coverage, the present clinical trial administered the 60 μg vaccine on an accelerated schedule given at 0–1 or 0–2 month as the primary vaccination to young adults (college students) in order to evaluate the immunogenicity compared to the standard HB vaccine containing 20 μg of HBsAg given in 3 doses at 0–1–6 month.Citation8 The participants of the study have been followed for 2 years and the purpose of research document is to report the 2-year follow-up results.
Results
Recruitment and follow-up of subjects
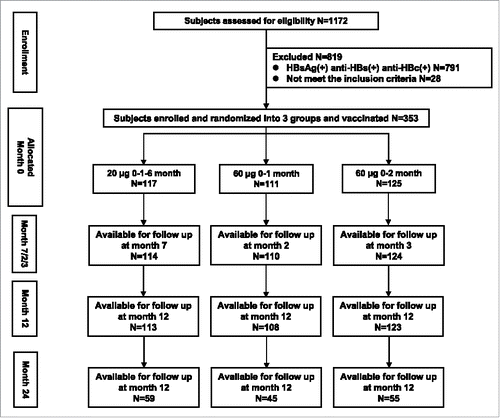
Of 1,172 subjects assessed for eligibility, 791 were excluded because of pre-existing serologic markers (HBsAg, anti-HBc and anti-HBs) for HBV infection or immunization and 28 failed to meet one or more of the criteria for inclusion described in the previous paper.Citation8 As a result, 353 qualified participants were enrolled into the current study and randomized into three groups: Group A included 117 subjects were vaccinated with HB vaccine of 20 μg (0–1–6 month), and Group B with 111 and Group C with 125 subjects were immunized with HB vaccine of 60 μg (0–1 or 0–2 month), respectively. Blood samples were collected from 98.6% participants (348/353, 5 participants quit the study after the inoculation of only one dose) at 1 month post the full series vaccination, 97.5% (344/353, 4 were eliminated from the immunogenicity analysis because of non-compliance) at 12 months after the first dose vaccination, and 45.0% (159/353, an additional 185 were unable to follow) at 24 months after the first injection. After 2 years of follow-up, 156 participants had a serial serum sample and were included in immunogenicity analysis. The flow diagram of the study was shown in .
Demography of participants
Comparison of the demographic characteristics involving the follow-ups of three groups revealed no significant differences in age (P = 0.481), sex ratio (P = 0.934), Body Mass Index (P = 0.550), but demonstrated a significant difference in race ratio (P = 0.013), as is shown in . Also, the comparison between the present and missing vaccinees of three groups showed no significant differences in age, BMI and race ratio (P > 0.05), except the sex ratio of the 60 μg groups (P < 0.05) because fewer male vaccinees were part of follow up blood sampling. Moreover, the demographic characteristics were comparable between the participants at 2 years post the first injection and the correspondent vaccinees at baseline among the three vaccine groups (P > 0.05, data not shown). Furthermore, there were no significant differences in the GMC levels between the follow-up and drop-out vaccinees at the prior two time-points (P > 0.05, data not shown).
Table 1. Demographic characteristics of participants after 2 years of follow up.
Immunogenicity
The dynamic changes of seroprotection rates and GMCs of anti-HBs at different time points in subjects immunized with different regimens of hepatitis B vaccines are shown in . The seroprotection rates of the group A (20 μg, 0–1–6 month), B (60 μg, 0–1 month) and C (60 μg, 0–2 month) were 100%, 93.64%, 99.19% and 100%, 96.04%, 95.90% at 1 month post series vaccination and 12 months post first injection, respectively. No significant differences in seroprotection rate were revealed between three groups at both time points (P > 0.05). Whereas, the seroprotection rates of the vaccine recipients dropped to 98.31% (58/59, 95%CI: 94.91% – 100%), 88.37% (38/43, 95%CI: 78.39% – 98.35%) and 85.19% (46/54, 95%CI: 75.40% – 94.97%) after 2 years of follow up, respectively, and did differ significantly by vaccine groups (P = 0.014), reflecting that, the rate of 20 μg (0–1–6 month) was significantly higher than that of 60 μg (0–2 month) (P = 0.026), but comparable with that of 60 μg (0–1 month) (P = 0.093).
Figure 2. Seroprotection rates (A) and GMCs of anti-HBs antibody (B) at different time points in subjects immunized with different regimens of hepatitis B vaccines.
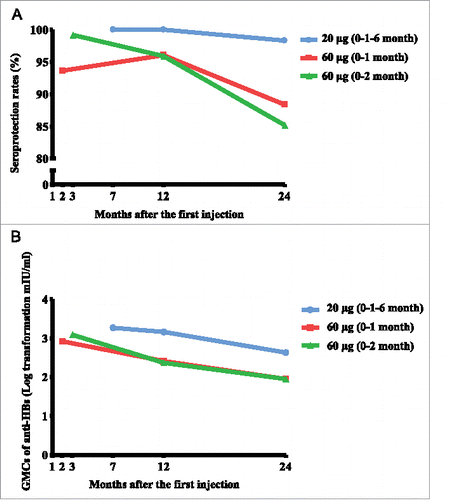
The GMC of anti-HBs was significantly higher in the standard regimen of 20 μg (0–1–6 month) than that in the 60 μg (0–1 month) group at 1 month post the series inoculation (1847.99 vs. 839.27 mIU/ml, P = 0.004), but was similar to the 60 μg (0–2 month) group (1847.99 vs. 1244.80 mIU/ml, P = 0.138). After 12 months of the first-dose injection, the GMC of the 20 μg (0–1–6 month) group was significantly higher than both 60 μg groups (1456.63 vs. 256.30, 235.15 mIU/ml, respectively, P < 0.001). While the two-year follow-up results indicated that all three groups experienced a dramatic decline in the GMC levels to 427.46 (95%CI: 274.92 – 664.51 mIU/ml), 89.74 (95%CI: 50.43 – 159.70 mIU/ml), 89.80 (95%CI: 56.96 – 141.58 mIU/ml) mIU/ml, respectively, however, the GMC of 20 μg (0–1–6 month) group was significantly higher than the two 60 μg groups (P < 0.001).
Further immunogenicity analysis performed on the distribution of subjects according to anti-HBs category response 24 months after application of the first vaccine dose is shown in , and implied the anti-HBs response level distribution was statistically different among the three vaccine groups (P = 0.002). The proportions of moderate-responders (100 ≤ anti-HBs < 1000 mIU/ml) among the three vaccine groups did not show significant differences (P = 0.192). Whereas, with respect to low-responders (10 ≤ anti-HBs < 100 mIU/ml) and hyper-responders (anti-HBs ≥ 1000 mIU/ml), the proportions presented statistically significant differences among the three vaccine groups (P = 0.03, P = 0.013, respectively). The highest proportion of hyper-responders and smallest proportion of low-responders to HB vaccination were detected among 20 μg (0–1–6 month) vaccine recipients. Additionally the 3-dose regimen also showed significantly higher good-responder rates (anti-HBs ≥ 100 mIU/ml) to vaccination at 24 months after the first injection compared with the 2-dose schedule groups (P < 0.001). For the subjects with anti-HBs antibody < 10 mIU/ml at 2 years, only one (1.69%) was found in 20 μg 3-dose group, but 5 (11.6%) and 8 (14.8%) were observed in both 60 μg 2-dose groups, respectively.
Table 2. Distribution of low-responders, moderate-responders and hyper-responders at 24 months after the first dose of the inoculation in subjects immunized with different regimens of hepatitis B vaccines.
Discussion
There was evidence that a fundamental strategy to improve the efficacy of HB immunization is to ensure the vaccinees receive the complete series of vaccination. However, the coverage rate of a complete 3-dose immunization with HB vaccine in Chinese college students was merely around 30%.Citation9 In spite of current standard 20 μg HBV vaccine regimen being well tolerated and highly effective, it has obvious limitations including reduced immunologic responsiveness resulting from poor compliance to a prescribed 3-dose schedule within 6 months.Citation9 Numerous studies have been performed to find new strategies to improve response rates after HB vaccination and long-term antibodies persistence such as using higher HBsAg dosages of HB vaccine,Citation10 increasing the vaccination doses,Citation11 or adding new adjuvants,Citation12 etc. Then, the practicable approach to achieve adequate protective anti-HBs antibody level and to ensure vaccinees receiving the complete series of vaccination at the same time needs to be discussed and evaluated. The 60 μg/1.0 ml recombinant yeast derived hepatitis B vaccine (Saccharomyces cerevisiae) has been commercially available since 2010 and is mainly applied to immunize the subjects who fail to respond to the standard primary vaccination in China.Citation13 In a recent Chinese study, a positive seroconversion rate of 85.7% (36/42) was observed among healthy adults > 16 years who received one dose of 60 μg HB vaccine as the primary vaccination, indicating that the 60 μg vaccine could exert positive immunological effects.Citation14 In the present study, researchers applied the 60 μg HB vaccine on an accelerated schedule given at 0–1 or 0–2 month as the primary vaccination to young adults. The one-year follow-up results represented good reactogenicity and safety profiles of 60 μg HB vaccine.Citation8
In order to evaluate the long-term persistence of anti-HBs antibody induced by 60 μg HB vaccine with 2 doses, blood samples were collected at two years post the first injection and tested for anti-HBs antibody to compare the protective rates and the GMC levels with the 20 μg (0–1–6 month) group. The 2-year data indicated that all three vaccine groups kept a relatively high protective rate (> 85%). However, the seroprotecion rate in 60 μg (0–2 month) group was significantly lower than that in 20 μg (0–1–6 month) group (85.19% vs. 98.31%, P = 0.026), and rate in 60 μg (0–1 month) group also showed a similar decline as noted in 60 μg (0–2 month) group (88.37% vs. 98.31%, P = 0.093).There were 98.31% (58/59) of subjects in the 20 μg (0–1–6 month) group possessing protective levels of anti-HBs antibody with a GMC of 427.46 mIU/ml, which was the highest among the three groups. Similar studies applying the 20 μg (0–1–6 month) regimen showed that the percentage of vaccinated teenagers and young adults with protective antibody levels was 77%-89% 10 years after primary vaccination.Citation15-16 Thus, the standard conventional 20 μg (0–1-6 month) regimen should be recommended as a priority in the adults who follow appropriate compliance.
An anti-HBs level ≥ 10 mIU/ml is conventionally considered to be protective. While immunity after HB vaccination is known to persist for at least more than 10 years in adults, the actual protective rate is independent of the initial post vaccination antibody titer.Citation4 Vaccinees with a higher titer of anti-HBs antibody retain the antibody for a longer period than those with lower anti-HBs titers which indicates that the anti-HBs levels induced by HB vaccine are associated with the longevity of protection in healthy adults.Citation17 Compared with the data of the first year, all three groups experienced a notable decline in GMC levels 24 months following the first inoculation of HB vaccine. The previous studies validated that the peak anti-HBs concentrations following immunization appear 28 days/one month after completion of the vaccination series. In addition, the typical vaccine-induced anti-HBs antibody declines rapidly within the first year and then more slowly during the following years.Citation18-19 A similar phenomenon was also observed in the 60 μg vaccine groups. The decline of anti-HBs antibody titer during the first year was steeper than the second year (582.97 vs. 166.56 mIU/ml, 1009.65 vs. 145.35 mIU/ml, respectively). While in the 20 μg vaccine group, a smaller decline range was detected during the initial year following first injection rather than the second year, namely 391.36 vs 1029.17 mIU/ml, which could be due to the different timing of the immune response measurement. That is, the intervals between the last vaccine dose and the second blood sampling for anti-HBs measurement differed between the three groups. It was 6 months for the 20 μg vaccine group and up to 11 or 10 months for the 60 μg vaccine groups already. Thus, the relatively faster decline of anti-HBs titer occurred at 12 months after the first dose in the 60 μg vaccine groups, whereas the decline occurred at 24 months following the first injection in the 20 μg vaccine group.
When comparing the low-response (10 ≤ anti-HBs < 100 mIU/ml) rates among the three groups after 2 years of follow up, statistically significant differences were detected (P = 0.030), and in comparison with the 60 μg (0–2 month) group, the rate was significant lower in 20 μg vaccine group (16.95% vs. 38.89%, P = 0.009). But at the same time, the 3-dose regimen of 20 μg HB vaccine showed significantly higher moderate and hyper-responder (anti-HBs ≥ 100 mIU/ml) rates (81.36% vs. 55.81%, 46.30%, respectively, P < 0.05) compared with the two 60 μg groups. It suggested that in terms of immunity durability, 20 μg (0–1–6 month) regimen had an advantage over 60 μg regimens of two doses. Several long-term follow-up studies revealed that in spite of a gradual decline in titer of anti-HBs antibody year by year, vaccine-induced immunity may persist for up to 15–20 years or even longer after a complete series.Citation20-22 Another recent meta-analysis confirmed that the duration of protection offered by hepatitis B vaccines was negatively influenced by the schedule of vaccine administration (gap time between last and preceding dose of the primary vaccine series < 6 months), vaccine dosage (lower vaccine dosage than presently recommended) and the characteristics of the population (maternal carrier status),Citation23 which could explain the inferior immunogenicity of the two-dose schedules to some extent.
It is essential to evaluate the immunogenicity of 2-dose 20 μg HBV vaccine (0–1 month) in order to assess 2-dose 60 μg vaccine (0–1/0–2 month) objectively. In a study carried out in India, 100 healthy adolescents aged 11–19 years were immunized with 3-dose (0–1–6 month) of 20 μg HB vaccine, and seroprotection rates and GMCs for anti-HBs antibody were measured at one month after each dose. The results showed one dose induced mild immune response with a seroprotection rate of 19% and GMC of anti-HBs antibody 14 mIU/ml, while moderate immune response was elicited for seroprotection rate increasing to 86% after 2 doses and GMCs of anti-HBs to 94 mIU/ml, respectively.Citation24 In another study, 20.4% and 77.0% of the total 147 healthy subjects aged between 15 and 40 years achieved seroprotection anti-HBs antibody titers ≥ 10 mIU/ml at one month after first and second doses of 3-dose regimen with 20 μg HB vaccine, and GMCs were 9 mIU/ml and 33 mIU/ml respectively.Citation25 As compared with the 20 μg HB vaccine, the seroprotection rate and GMC of anti-HBs antibody induced by 60 μg HB vaccine were higher at the measurement point (two month post the first dose), which were 51.7% (vs. 19%) and 17.16 mIU/ml (vs. 14 mIU/ml), respectively.Citation26 Moreover, in our study, the seroprotective rate and GMC in the 60 μg (0–1 month) vaccine group at month 2 were 93.61% and 839.27 mIU/ml, respectively. Both of which presented higher immunogenicity than that of the 20 μg formulation accompanying the first 2 doses.
The present two-year follow-up study on hepatitis B vaccine applying standard 20 μg (0–1–6 month) and 60 μg (0–1 month) or 60 μg (0–2 month) regimens in healthy young adults suggested that though the high dosage and accelerated schedule groups experienced a more dramatic drop in GMC levels compared with the conventional three-dose regimen, these groups still demonstrated a good immunogenicity with a high seroprotection rate (> 85%). In conclusion, on the assumption of complete compliance by vaccine recipients, the standard three-dose protocol provides the benefits of a high seroconversion of anti-HBs and high GMC levels which are associated with the longevity of protection. Whether the higher dosage and accelerated regimen (60 μg vaccine 0–1 or 0–2 month) can be a useful reference as an initial vaccination regimen providing earlier protection, higher compliance and vaccination coverage needs more additional clinical studies.
There are some limitations to our study: (1) The effects of the natural exposure to HBV during the follow-up of vaccinees were not evaluated. (2) The anamnestic responses in individuals with anti-HBs <10 mIU/ml were not measured. (3) Blood samples should be collected after each dose to compare immunogenicity in more detail. (4) Loss of subjects during follow up could have resulted in selection bias.
Materials and methods
Study design
The single-blind, randomized, parallel-group clinical trial presented in this paper enrolled healthy freshmen aged 18–25 years from one college in Liuzhou city of Guangxi Zhuang Autonomous Region in March 2014, and who were tested for baseline HBsAg, anti-HBs, and anti-HBc. The specific inclusion and exclusion criteria were described in detail in our previous report.Citation8 The eligible volunteers were randomized (1:1:1) into three vaccine groups, namely Group A [20 μg (0–1–6 month)], Group B [60 μg (0–1 month)] and Group C [60 μg (0–2 month)]. Blood samples for post-vaccination serologic testing were obtained from participants 1 month after the series vaccination, 12 months and 24 months following administration of the first dose. Non-responders (defined as vaccinated individuals who were seronegative for HBsAg before primary vaccination and with an anti-HBs titer ≤ 10 mIU/ml 1 month after receiving the last dose of primary vaccination) received one booster dose of a HB vaccine formulation containing 60 μg of antigen and were recorded separately. shows the flow chart of the study.
Written informed consent was obtained from all participants prior to enrollment. The Peking University Institutional Review Board approved the trial protocol. The design and procedure of the trial were in accordance with the Good Clinical Practice Guidelines and the ethical standards of the Helsinki Declaration. All relevant documents were approved by the ethical review committee of Guangxi Provincial Centers for Disease Control and Prevention (CDC).
Vaccines
The two types of hepatitis B vaccine containing 20 μg (batch number B201301004) or 60 μg (batch number A201301001) of HBsAg per dose (1.0 ml) were manufactured by Shenzhen Kangtai Biological Products Co., Ltd., and stored at 4°C and maintained within the cold chain during transportation as well. The vaccines were administered intramuscularly into the deltoid muscle of participants.
Serological tests
Baseline HBsAg, anti-HBs, and anti-HBc were tested qualitatively using commercially available ELISA (Enzyme-Linked Immunosorbent Assay) test kits (Shanghai Kehua Bio-engineering Co., Ltd.). Anti-HBs antibodies in vaccinees were measured quantitatively and performed by CMIA using the Architect i2000SR analyzer (Abbott Diagnostics Division). The accepted protective serum anti-HBs level was defined as detectable at a titer of anti-HBs ≥ 10 mIU/ml after vaccination in initially seronegative participants. Subjects with anti-HBs titers of less than 10 mIU/ml were considered to be non-protective. Anti-HBs ≥10 mIU/ml and < 100 mIU/ml were considered low response, and 100 ≤ anti-HBs < 1000 mIU/ml were medium or moderate response, and an anti-HBs level ≥1000 mIU/ml was considered a hyper response. Seroconversion rates and assay titers were reported overall and stratified by anti-HBs serostatus for each group.
Statistical analysis
Continuous variables were presented as Mean ± SD depending on the underlying normal distribution and Student's t-test or one-way ANOVA (analysis of variance) was used to analyze continuous data while Pearson Chi-square tests or Fisher's exact tests were used to compare categorical data. A P value of < 0.05 (2-tailed) was considered statistically significant. All statistical analyses were performed using SPSS 21.0 software (SPSS Inc., Chicago, IL, USA).
Abbreviations
| Anti-HBc | = | antibody against hepatitis B core antigen; |
| Anti-HBs | = | antibody against hepatitis B surface antigen |
| ANOVA | = | analysis of variance |
| BMI | = | body mass index |
| CI | = | confidence interval |
| CMIA | = | Chemiluminescent Microparticle Immunoassay |
| ELISA | = | Enzyme-Linked Immunosorbent Assay |
| GMC | = | geometric mean concentration |
| HBcAg | = | hepatitis B core antigen |
| HBsAg | = | hepatitis B surface antigen |
| HBV | = | hepatitis B virus |
| HB vaccine | = | hepatitis B vaccine |
| S/CO | = | sample signal-to-cutoff ratio |
| SD | = | standard deviation. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authorship
Dr. H Zhuang designed the study and critically proofread the manuscript. Dr. P Wang, L Wei, CP Xie, ZX Yang, J Lan, ZL Fang and Y Zeng vaccinated the subjects and collected blood samples. Dr. YH Gao and L Yan performed the experiments. Dr. ZZ Wang and Dr. YH Gao analyzed the data and wrote the manuscript. Dr. XE Liu revised the manuscript for important contents. All author approved the final version of the manuscript, including the authorship list.
Acknowledgments
The authors are grateful to Ms. Ellen Gant from State of Texas, United States of America for proofreading and editing the manuscript.
Additional information
Funding
References
- WHO. Global Hepatitis Report, 2017. Geneva: WHO, 2017. http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
- Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: The Chinese experience. Chin Med J (Engl). 2009;122:98–102. PMID:19187625.
- Cui F, Shen L, Li L, Wang H, Wang F, Bi S, Liu J, Zhang G, Wang F, Zheng H, et al. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. Emerg Infect Dis. 2017;23:765–72. PMID:28418296. https://doi.org/10.3201/eid2305.161477.
- WHO. Hepatitis B vaccines: WHO position paper. Wkly Epidemiol Rec. 2009;84:405–19. PMID:19817017.
- Zamani F, Fallahian F, Hashemi F, Shamsaei Z, Alavian SM. Immune response to hepatitis B vaccine in health-care workers. Saudi J Kidney Dis Transpl. 2011;22:179–84. PMID:21196642.
- Lin SY, Liu JH, Wang SM, Wang IK, Tsai CA, Liu YL, Lin HH, Chang CC, Huang CC. Association of response to hepatitis B vaccination and survival in dialysis patients. Bmc Nephrol. 2012;13:97. PMID:22935561. https://doi.org/10.1186/1471-2369-13-97.
- Psevdos G, Kim JH, Groce V, Sharp V. Efficacy of double-dose hepatitis B rescue vaccination in HIV-infected patients. AIDS Patient Care STDS. 2010;24:403–7. PMID:20586648. https://doi.org/10.1089/apc.2009.0340.
- Wang ZZ, Li MQ, Wang P, Yang ZX, Wei L, Zeng Y, Li YP, Yan L, Liu XE, Zhuang H. Comparative immunogenicity of hepatitis B vaccine with different dosages and schedules in healthy young adults in China. Vaccine. 2016;34:1034–9. PMID:26801063. https://doi.org/10.1016/j.vaccine.2016.01.018.
- China foundation for hepatitis prevention and control (CFFH). An investigation report on the current status of prevention and control of hepatitis B among college students in China. Beijing: CFFH; 2009.
- Potsch DV, Camacho LA, Tuboi S, Villar LM, Miguel JC, Ginuino C, Silva EF, Mendonca RM, Moreira RB, Barroso PF. Vaccination against hepatitis B with 4-double doses increases response rates and antibodies titers in HIV-infected adults. Vaccine. 2012;30:5973–7. PMID:22828589. https://doi.org/10.1016/j.vaccine.2012.07.028.
- Lin SY, Liu JH, Wang SM, Wang IK, Tsai CA, Liu YL, Lin HH, Chang CC, Huang CC. Association of response to hepatitis B vaccination and survival in dialysis patients. Bmc Nephrol. 2012;13:97. PMID:22935561; https://doi.org/10.1186/1471-2369-13-97.
- Tielemans CL, Vlasak J, Kosa D, Billiouw JM, Verpooten GA, Mezei I, Ryba M, Peeters PC, Mat O, Jadoul MY, et al. Immunogenicity and safety of an investigational AS02(v)-adjuvanted hepatitis B vaccine in patients with renal insufficiency who failed to respond or to maintain antibody levels after prior vaccination: Results of two open, randomized, comparative trials. Vaccine. 2011;29:1159–66. PMID:21167859. https://doi.org/10.1016/j.vaccine.2010.12.009.
- Pan HX, Zeng Y, Song XF, Zhang YJ, Xu K, Liang ZL, Zhu FC. Immune response to hepatitis B vaccine with high antigen content in non-responders after standard primary vaccination in Chinese adults. Vaccine. 2014;32:3706–12. PMID:24681228. https://doi.org/10.1016/j.vaccine.2014.02.094.
- Ma FB, Tang WJ, Lu PS, Hu Y, Kang GD, Yang MW. Study on the Immunogenicity of one dose of 60 μg/1.0ml Recombinant Yeast Derived Hepatitis B Vaccine (Saccharomyces Cerevisiae) Among Health Population Older than 16 Years. Shiyong Yufang Yixue (Chinese). 2013;20(6):653–5.
- Stroffolini T, Guadagnino V, Caroleo B, De Sarro G, Foca A, Liberto MC, Giancotti A, Barreca GS, Marascio N, Lombardo FL, et al. Long-term immunogenicity of hepatitis B vaccination in children and adolescents in a southern Italian town. Infection. 2012;40:299–302. PMID:22173948. https://doi.org/10.1007/s15010-011-0233-2.
- Zanetti AR, Mariano A, Romano L, D'Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379–84. PMID:16226616. https://doi.org/10.1016/S0140-6736(05)67568-X.
- Scheiermann N, Gesemann M, Maurer C, Just M, Berger R. Persistence of antibodies after immunization with a recombinant yeast-derived hepatitis B vaccine following two different schedules. Vaccine. 1990;8 Suppl:S44-6, S60–2. PMID:2139282.
- Jilg W, Schmidt M, Deinhardt F, Zachoval R. Hepatitis B vaccination: How long does protection last? Lancet. 1984;2:458. PMID:6147519.
- Jilg W, Schmidt M, Deinhardt F. Decline of anti-HBs after hepatitis B vaccination and timing of revaccination. Lancet. 1990;335:173–4. PMID:1967468.
- Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis. 2008;197:1419–26. PMID:18444799. https://doi.org/10.1086/587695.
- Leuridan E, Van Damme P. Hepatitis B and the need for a booster dose. Clin Infect Dis. 2011;53:68–75. PMID:21653306; https://doi.org/10.1093/cid/cir270.
- Wang ZZ, Gao YH, Lu W, Jin CD, Zeng Y, Yan L, Ding F, Li T, Liu XE, Zhuang H. Long-term persistence in protection and response to a hepatitis B vaccine booster among adolescents immunized in infancy in the western region of China. Hum Vaccin Immunother. 2017;13:909–15. PMID:27874311. https://doi.org/10.1080/21645515.2016.1250990.
- Schonberger K, Riedel C, Ruckinger S, Mansmann U, Jilg W, Kries RV. Determinants of long-term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J. 2013;32:307–13. PMID:23249904. https://doi.org/10.1097/INF.0b013e31827bd1b0.
- Velu V, Nandakumar S, Shanmugam S, Shankar EM, Thangavel S, Kulkarni PS, Thyagarajan SP. Comparative efficacy of two dosages of recombinant hepatitis B vaccine in healthy adolescents in India. Pediatr Infect Dis J. 2007;26:1038–41. PMID:17984812. https://doi.org/10.1097/INF.0b013e3181342887.
- Levie K, Gjorup I, Skinhoj P, Stoffel M. A 2-dose regimen of a recombinant hepatitis B vaccine with the immune stimulant AS04 compared with the standard 3-dose regimen of Engerix-B in healthy young adults. Scand J Infect Dis. 2002;34:610–4. PMID:12238579. https://doi.org/10.1080/00365540110080881.
- Wang H, Cai B, Rao D, Liu M, Li Y, Liang X, Cui F, Zhang G, Wang F, Pang X, et al. Rapid immunization effects of a new type of 60 mug hepatitis B vaccine compared with traditional 20 mug hepatitis B vaccines in adults. Hum Vaccin Immunother. 2016;12:2921–6. PMID:27648684. https://doi.org/10.1080/21645515.2016.1206676.