ABSTRACT
In an influenza pandemic, two vaccine doses administered 21 days apart may be needed for individuals of all ages to achieve seroprotection. Achieving dose-series completion can be challenging even for routinely recommended vaccines. To prepare for a two-dose influenza pandemic vaccine campaign and promote dose-series completion and correct dosing intervals, CDC and its partners developed a text message-based vaccine reminder system to remind persons who receive a first dose of pandemic influenza vaccine to receive the second dose. Taking advantage of the high prevalence of cell phones in the United States, the system sent second-dose text message reminders and hyperlinks to educational information. The system was pilot tested from November 2015 to April 2016 among graduate public health students enrolled at four United States universities. Universities were selected based on convenience, and each university used a different recruitment method. Among 59 volunteers who pilot tested the system and completed a survey, 57 (92%) felt the system would be helpful during a pandemic. Forty (68%) respondents felt the information included in the messages was informative. Volunteers recommended including actionable ways to stay healthy during a pandemic, though specific suggestions varied. With further development, text reminder systems could be used to promote adherence to a two-dose regimen in a future pandemic, although audience-specific messaging and other complementary systems will likely be needed. Public and private partners can adapt and implement this tool in conjunction with their routine patient information systems to improve dose-series completion and ensure optimal protection during an influenza pandemic.
Introduction
Vaccination against a pandemic influenza virus is the most effective intervention for reducing morbidity and mortality associated with an influenza pandemic.Citation1 However, pandemic vaccination recommendations may differ from recommendations for seasonal influenza vaccine, increasing the complexity of pandemic vaccination implementation. One dose of seasonal influenza vaccine is recommended annually for all persons >6 months old, with the exception that children 6 months-8 years of age should receive two doses the first year they receive influenza vaccine.Citation2 Similarly, for the 2009 H1N1 vaccine campaign, two doses were recommended for all persons 6 months through 8 years old.Citation3 However, in a future influenza pandemic, two vaccine doses administered 21 days apart with or without adjuvant may be needed for all ages to achieve seroprotection as suggested by clinical trials of vaccines against novel avian influenza A (H7N9) and A (H5N1) viruses that have caused human illnesses.Citation4–6 Experience with seasonal influenza dose-series completion among children <9 years old who needed two vaccine doses, and challenges with dose-series completion for other routinely recommended vaccines such as hepatitis B, human papillomavirus (HPV), and pneumococcal vaccines foretell difficulty in achieving high levels of dose-series completion during a pandemic response.Citation7–9 One method shown to increase vaccine series completion and improve immunization rates is use of short-message-service (SMS) messaging (i.e. “texts”) to facilitate communication with patients about health interventions.Citation10–14
In preparation for a possible future two-dose pandemic influenza vaccine program, the Centers for Disease Control and Prevention (CDC) and its partners developed a text message-based vaccine reminder system and piloted the system among a convenience sample of graduate public health students.
Methods
CDC partnered with the National Association of County and City Health Officials (NACCHO) to develop a prototype patient-centered text message-based reminder system for pandemic influenza vaccination purposes. The system, Flu Vax Alert, was designed to promote optimal two-dose vaccine series completion by sending automated messages to persons hypothetically vaccinated with a first dose to remind them about the need for and timing of the second dose. After message content and algorithm development, NACCHO identified and contacted a convenience sample of four schools of public health in the United States to pilot the system among their students. The objectives of the pilot test were to collect feedback on the system's capabilities and message content.
Pilot study recruitment
The protocol for conducting the pilot test was reviewed by human subjects advisors and declared non-research by the CDC. Institutional Review Boards at each of the four schools reviewed pilot study materials and found the study to be exempt from human subjects research requirements. Students were recruited to enroll in the pilot and provide feedback on their experience with the text message system. Each school of public health determined their own method of recruitment. Two schools distributed information relating to the pilot test and sent invitations for students to enroll via email listservs and newsletters. One school handed out information about the pilot test and instructions on how to enroll during class. The fourth school hosted an in-person enrollment event in conjunction with a student organization. This enrollment event was attended by CDC staff members. Students were offered pizza for attending and were invited to participate in the pilot study.
Volunteer participants were sent a letter detailing the purpose of the project, a hypothetical pandemic scenario, instructions on how to use the messaging system, and postcards describing the hypothetical vaccination details for each student. The postcard included a hypothetical vaccine-adjuvant combination (e.g. Vaccine A/Adjuvant A), the date that the hypothetical dose was administered (e.g. today or yesterday), and whether or not the participant was to respond as if they received a second vaccine dose.
Messaging
Participants opted-in to the system by texting the keyword “Vaccine” to a pre-registered short code (). Participants were instructed to respond to the system enrollment message prompts with the mock vaccination information on their pilot test postcard. The system prompted participants to indicate which combination of vaccine and adjuvant (e.g. vaccine A/adjuvant A, etc.) they received at their first dose and when it was given. Throughout the recommended 21-day interval between doses, participants were sent texts reminding them of the required timing and vaccine/adjuvant combination for their second vaccine dose. Messages also included a link to HealthMap Vaccine Finder, a web-based tool for finding vaccination locations that was used to simulate pandemic vaccination locations.Citation15 Some participants were instructed to reply to the text-message reminder system indicating that they had “received” the second vaccine dose. Upon receiving a two-dose completion message, the system sent a final text message which included a link to an anonymous survey where participants could provide feedback on their experience with the system. Participants that did not indicate receiving the second dose were sent a link to the survey after they received the maximum number of messages.
Figure 1. Pandemic Influenza Vaccine Text Message Reminder System (Flu Vax Alert) Message Flow Chart, Figure Legend: This figure details each message received by the user of the Flu Vax Alert system. The “DAY” number in each box states the number of days since completing enrollment that message is received. This varies based on when the participant reported receiving their first flu shot. Pilot test participants received an additional message inviting them to take the follow-up survey (not shown).
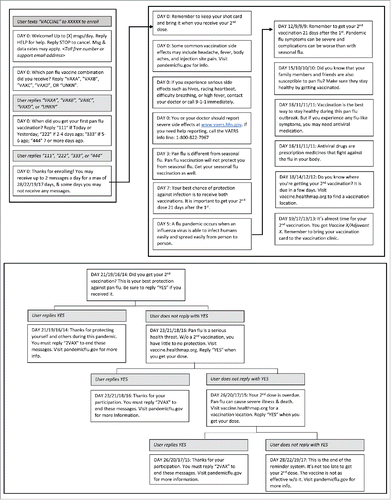
A user who enrolled in the system the same day they received their first vaccine dose would receive 20 messages over the course of 21 days: eight messages on the day of enrollment, and 12 staggered over the 21 day waiting period. The message algorithm included a maximum of 23 messages. Users who did not send the confirmation message upon receiving their second dose would receive the highest number of messages as the system sent three reminder messages after the vaccination due date. Participants also received one additional message containing a link to the survey.
Follow-up survey
The follow-up survey consisted of 11 questions. The questions asked the participants for their overall opinion about the system, and opinions about aspects that could be improved to increase usability and participation. Specific characteristics of the system that participants were asked about included the best time of day to send messages, the number of messages sent, and the quality of the message content. The survey also included fields for free-text responses so that respondents could explain the reasoning behind their opinions about the system. Finally, survey respondents were invited to suggest additional content that they felt would be appropriate to include in messages sent by the system during a pandemic. Results from the survey were analyzed in Microsoft Excel (Redmond, WA).
Results
Pilot test enrollment and completion
In total, 217 students volunteered to participate in the pilot test and sent the “opt-in” message to the short code to initiate the enrollment process (). The largest proportion of students participating in the pilot test were from the university which hosted the active enrollment event.
Figure 2. Pandemic Influenza Vaccine Text Message Reminder System (Flu Vax Alert) Enrollment Flowchart, Figure legend: This figure describes the number of participants enrolled in the study at a given point.
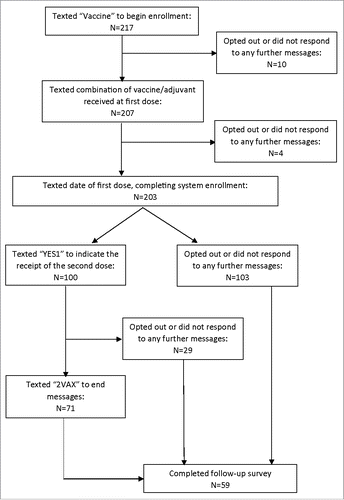
Of the 217 initial participants, 203 (94%) completed the reminder system enrollment process. Ten participants (5%) opted out of further participation by replying “STOP” or did not respond to any further messages after initiating the enrollment process. An additional four participants (2%) ceased participation after submitting the vaccine received at the first dose but prior to submitting the date of vaccination. After enrollment, participants received messages on the importance of completing the pandemic influenza vaccine series roughly every other day. One hundred participants (46%) responded “YES1” to indicate that they received their second dose of vaccine. One-hundred three participants (47%) opted out or did not respond to any further messages after completing the enrollment process. Fifty-nine participants (27%) completed the follow-up survey describing their experience.
Follow-up survey
Overall, 41 (69%) of 59 survey respondents felt that the quality of the text message reminder system was “outstanding” or “good” as presented, whereas seven survey respondents (12%) felt that the system “needs improvement” (). The vast majority (n = 54, 92%) of the survey respondents felt that during a pandemic, the text message reminder system would be helpful. Forty-three (73%) of 59 respondents who provided comments highlighted the utility that the system would have specifically in reminding vaccine recipients about the necessity of the second dose given the near-ubiquity of cell phones in the United States. As one respondent wrote, “The more you can remind people the better, and cell phones are an easy way to do it.”
Table 1. Participant Impressions of the Two Dose Pandemic influenza Vaccine Reminder Text Message System - Participant follow-up Survey, December 2015 — April 2016.
Most survey respondents preferred to receive messages either in the morning (36%) or at midday (42%) (). When asked to describe their opinions on the text message language by selecting applicable terms from a pre-defined list, the most frequent responses were “Informative” (n = 40, 68%), “Repetitive” (n = 31, 53%) and “Helpful” (n = 21, 36%). Only eight (14%) respondents felt that the language in the messages was “Persuasive.” The majority of respondents (92%) felt that the abbreviations used throughout the text message system were clear. With regards to message frequency, respondents were evenly divided. Given the scenario of an influenza pandemic where effective vaccination required two doses, 27 (46%) respondents felt that they received too many messages. Meanwhile, 28 (47%) felt that they received the right amount of text messages. One respondent felt that they did not receive enough messages.
Table 2. Participant follow-up survey regarding text reminder system.
Information that respondents reported would be helpful to add included tips on how to stay healthy during a pandemic (n = 30, 51%), information on potential adverse reactions from the vaccine (n = 27, 46%), and additional links to more resources for information (n = 28, 47%) (). One respondent suggested using the system to disseminate additional news related to the ongoing pandemic.
Discussion
The results of the pilot study and subsequent follow-up survey suggest that an automated system designed to remind individuals to receive a second dose of vaccine during an influenza pandemic may be helpful for some segments of the U.S. population. This conclusion is in agreement with other studies that have been conducted examining the impact text message reminder systems can have on vaccination rates.Citation10–12,Citation16,Citation17 However, disagreement among follow-up survey participants regarding the persuasiveness of the messages or the appropriate number of messages to send suggest that multiple versions of the system may need to be developed depending on the target audience.
Some survey respondents expressed a desire for messages to include actionable information to protect themselves from the pandemic. Messages did include a link to the HealthMap Vaccine Finder, which could be adapted for participants to identify vaccination sites in their area where they could receive their second dose.Citation15 The HealthMap link added utility to the system by allowing users to identify locations where they could receive a second dose of vaccine and adjuvant that was of the same variety as the first dose. This functionality would be of great importance, particularly early on in a pandemic when vaccine could be in short supply. It also reduced the likelihood of mixing and matching vaccine/adjuvant combinations between the first and second dose. The text messages sent via Flu Vax Alert could be customized during a pandemic to include information on how participants can best prevent disease or what steps to take if they felt that they were getting sick. Text messages have been previously shown to be effective means of disseminating health information to specific populations.Citation18 Using the Flu Vax Alert text message system to disseminate information promoting healthy behaviors as well as to remind users to get vaccinated could reduce the burden the pandemic places on public health and healthcare systems by alieving public concern over the pandemic and reducing the effort required for second-dose follow-up.
Over half of the survey respondents felt that the messages sent by the system were repetitive. Including a variety of information in messages to participants could help address this negative aspect of the system. However, including more text messages can lead to “message fatigue,” reducing the probability that they recall the content of a specific message. This phenomenon has been observed in healthcare providers during emergencies, where one study found that each additional public health message received per week reduced the odds of recalling the content of a specific message by 41%.Citation19 Including additional messages to include a wider variety of content could hinder participant recollection of the second vaccine dose reminder. Differences in participant preference further suggest that multiple versions of the reminder system may be needed.
Jurisdictions deploying a text message reminder system should carefully consider the number of messages that participants receive. Participants in the pilot test who did not opt out early could receive up to 23 messages within two to four weeks, depending on when they reported receiving the first vaccine dose. Twenty-seven survey respondents (46%) felt that they received too many messages during the pilot, while 28 (47%) felt that they received the right amount of messages. This sentiment from survey respondents, combined with the large number of pilot study participants that dropped out early, suggests that the system may have been too burdensome. However, there was still a large proportion of survey respondents who felt that they received the right number of messages. In a pandemic, the number of messages that an individual deems “the right amount” or “too much” will vary. Health departments or vaccine providers developing their own text message reminder systems could incorporate a function where the participant can select how frequently they would like to receive information, which may make the system more acceptable to a wider audience. However, adding such a feature would increase the complexity and may increase the cost to operate the system. The number of messages sent could be further reduced by simplifying the enrollment process or spacing out the reminder messages. Participants in the pilot study received eight messages on the first day, four of which were required for the system to function and four of which were informational. Combining the enrollment messages or waiting to send informational messages until a later date could reduce the initial burden the system places on the participant. Future studies could assess the feasibility and impact that these system modifications have on the acceptability of a text message based vaccine reminder system. The system could also be adapted to target parents of children, which would require slightly modified messaging and content.
Results of the pilot test of the Flu Vax Alert pandemic influenza text message vaccine reminder system suggest that many people may opt-in to using the reminder system if offered. However, text messages alone are not a simple solution to dose-series completion and redundant methods to optimize two dose completion should be concurrently utilized. Most survey respondents felt that the messaging was informative, however very few felt that the messaging was persuasive, which may have impacted completion of the pilot system by volunteers. One reason the messages may not have been persuasive is that the messages were very generic so as to be applicable to diverse populations. Distributing shot cards to patients, ensuring that providers remind patients about the necessity for a second dose, and enacting standing orders to schedule a patient's appointment for their second dose at the same visit where the first dose is given are all actions that can complement the deployment of a text message vaccine reminder system and personalize the recommendation in the mind of the user.Citation20, Citation21
Pre-pandemic communication and health education planning by public health programs and their partners should take into account the potential difficulty of ensuring dose-series completion for a two-dose pandemic influenza vaccine. Significant pre-pandemic planning by public health programs can maximize the impact of pandemic vaccination efforts by improving the number of persons who receive the correct number of recommended doses. Communications campaigns conducted by the CDC before and during a pandemic will also serve to reiterate the critical need for patients to receive the recommended number of vaccine doses to be protected from infection.
The results of this pilot test are subject to certain limitations. Low initial enrollment in the pilot test and low participation in the follow-up survey both serve to limit the generalizability of these results. Furthermore, the sample population that participated in the pilot test comprised of graduate level public health students from four U.S. schools of public health, who likely have different educational and literacy levels, as well as motivation for vaccination and perspectives on public health and healthcare unique from the rest of the U.S. population. The schools were not randomly selected, and each school used different recruitment strategies, potentially contributing to different participant characteristics among these students. Due to the method of recruitment, there is a significant likelihood of selection bias which should be taken into account when interpreting results of this pilot test. Furthermore, we do not know the total number of students approached and cannot calculate the proportion of those approached who chose to participate. We observed higher initial enrollment rates when an in-person event was conducted and staff were available to promote the system. This finding may have important implications for recruitment of patients into this or similar vaccine reminder systems during a pandemic, though determining optimal enrollment strategies was not an objective of this pilot test. Pilot testing did not occur during a real influenza pandemic and simulating interest and actions likely to occur in an actual pandemic may not be possible. This pilot test assumed that a pandemic vaccine was available when the system was activated. Pandemic vaccine may not be immediately available in the early stages of a pandemic, and public health jurisdictions should take this into account. The pilot test was designed to evaluate a number of potential vaccination scenarios, and therefore results should not be used to assess the likely rate of vaccine course completion in the event of a future influenza pandemic. All participants who completed the enrollment process were invited to complete the follow-up survey, regardless of whether they completed or opted out of the pilot test. Furthermore, responses to the follow-up survey was collected anonymously. As a result, some responses to survey questions may not be informed by full participation in the pilot study. The results of the follow-up survey previously discussed assume that survey respondents answered honestly.
Conclusion
This pilot test of a text message based pandemic vaccine dose reminder system suggests that such a system may be one of many effective strategies to improve appropriate vaccine uptake. State and local public health departments and healthcare providers need to consider how best to deploy such a system in the event of a severe pandemic. One method could be tying the system to jurisdiction's immunization information systems (IIS). Goal one of the 2018–2022 Immunization Information Systems Functional Standards state that IIS should support healthcare providers in delivering age- and risk-appropriate immunization services.Citation22 CDC could make available the message algorithm used in this pilot study as a starting point for future pilot studies or system roll-outs. Future pilots could be conducted by healthcare organizations and public health departments with their own patient populations. Organizations conducting pilot tests should consider how the number of messages and the inclusion of a wider variety of pandemic information could improve system use and vaccine dose series completion among their patients.
Participants' desire for actionable information must be balanced with the need to include enough information to maximize second dose uptake and provisions to avoid message fatigue. Public health programs and healthcare providers should consider how existing patient-centered healthcare reminder systems are currently incorporated into healthcare practice and identify parallels that may help to integrate the Flu Vax Alert text message reminder system into their pandemic influenza communication plans. One area of interest is the integration of vaccine reminder systems with other relevant elements of routine healthcare delivery, such as immunization information systems, electronic health records, and pharmacy prescription reminder systems. Further consideration should be given to how the integration of these systems and other educational resources and communication strategies could be used to increase dose-series completion during an influenza pandemic.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Disclosure of interest
The authors report no conflict of interest.
Acknowledgments
The authors would like to acknowledge Lisa Koonin and Anita Patel of the CDC Influenza Coordination Unit, colleagues at the National Association of County and City Health Officials, Compliant Campaign, and the staff and students who participated in the pilot test.
References
- Germann TC, Kadau K, Longini IM, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proceedings of the National Academy of Sciences 2006;103:5935–40. doi:10.1073/pnas.060126610310.1073/pnas.0601266103.
- Grohskopf LA. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2017–18 Influenza Season. MMWR Recommendations and Reports 2017;66:1–20. doi:10.15585/mmwr.rr6602a110.15585/mmwr.rr6602a1. PMID:28841201.
- Centers for Disease Control and Prevention (CDC). Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports 2009;58:1–8.
- Jackson LA, Campbell JD, Frey SE, Edwards KM, Keitel WA, Kotloff KL, Berry AA, Graham I, Atmar RL, Creech CB, et al. Effect of Varying Doses of a Monovalent H7N9 Influenza Vaccine With and Without AS03 and MF59 Adjuvants on Immune Response: A Randomized Clinical Trial. Jama 2015;314:237–46. doi:10.1001/jama.2015.791610.1001/jama.2015.7916. PMID:26197184.
- Beran J, Abdel-Messih IA, Raupachova J, Hobzova L, Fragapane E. A phase III, randomized, open-label study to assess the tolerability and immunogenicity of an H5N1 influenza vaccine administered to healthy adults with a 1-, 2-, 3-, or 6-week interval between first and second doses. Clinical Therapeutics 2010;32:2186–97. doi:10.1016/S0149-2918(11)00024-510.1016/S0149-2918(11)00024-5. PMID:21316535.
- Bresson J-L, Perronne C, Launay O, Gerdil C, Saville M, Wood J, Höschler K, Zambon MC. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. The Lancet 2006;367:1657–64. doi:10.1016/S0140-6736(06)68656-X10.1016/S0140-6736(06)68656-X.
- Williams WW. Surveillance of Vaccination Coverage Among Adult Populations—United States, 2015. MMWR Surveillance Summaries 2017;66:1–28. doi:10.15585/mmwr.ss6611a110.15585/mmwr.ss6611a1.
- Santibanez TA, Grohskopf LA, Zhai Y, Kahn KE. Complete Influenza Vaccination Trends for Children Six to Twenty-Three Months. Pediatrics 2016;137:e20153280. doi:10.1542/peds.2015-328010.1542/peds.2015-3280. PMID:26908692.
- Lin X, Rodgers L, Zhu L, Stokley S, Meites E, Markowitz LE. Human papillomavirus vaccination coverage using two-dose or three-dose schedule criteria. Vaccine 2017;35:5759–61. doi:10.1016/j.vaccine.2017.08.09010.1016/j.vaccine.2017.08.090. PMID:28890189.
- O'Leary ST, Lee M, Lockhart S, Eisert S, Furniss A, Barnard J, Eblovi DE, Shmueli D, Stokley S, Dickinson LM, et al. Effectiveness and Cost of Bidirectional Text Messaging for Adolescent Vaccines and Well Care. Pediatrics 2015;136:e1220–7. doi:10.1542/peds.2015-108910.1542/peds.2015-1089. PMID:26438703.
- Kempe A, Saville AW, Dickinson LM, Beaty B, Eisert S, Gurfinkel D, Brewer S, Shull H, Herrero D, Herlihy R. Collaborative centralized reminder/recall notification to increase immunization rates among young children: a comparative effectiveness trial. JAMA pediatrics 2015;169:365–73. doi:10.1001/jamapediatrics.2014.367010.1001/jamapediatrics.2014.3670. PMID:25706340.
- Bar-Shain DS, Stager MM, Runkle AP, Leon JB, Kaelber DC. Direct messaging to parents/guardians to improve adolescent immunizations. The Journal of adolescent health: official publication of the Society for Adolescent Medicine 2015;56:S21–6. doi:10.1016/j.jadohealth.2014.11.02310.1016/j.jadohealth.2014.11.023. PMID:25863550.
- Fjeldsoe BS, Marshall AL, Miller YD. Behavior Change Interventions Delivered by Mobile Telephone Short-Message Service. American Journal of Preventive Medicine 2009;36:165–73. doi:10.1016/j.amepre.2008.09.04010.1016/j.amepre.2008.09.040. PMID:19135907.
- Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemedicine journal and e-health: the official journal of the American Telemedicine Association 2009;15:231–40. doi:10.1089/tmj.2008.009910.1089/tmj.2008.0099. PMID:19382860.
- HealthMap. HealthMap Vaccine Finder. Available at: https://vaccinefinder.org/.
- Rand CM, Vincelli P, Goldstein NP, Blumkin A, Szilagyi PG. Effects of Phone and Text Message Reminders on Completion of the Human Papillomavirus Vaccine Series. The Journal of adolescent health: official publication of the Society for Adolescent Medicine 2017;60:113–9. doi:10.1016/j.jadohealth.2016.09.01110.1016/j.jadohealth.2016.09.011. PMID:27836533.
- Morris J, Wang W, Wang L, Peddecord KM, Sawyer MH. Comparison of reminder methods in selected adolescents with records in an immunization registry. The Journal of adolescent health: official publication of the Society for Adolescent Medicine 2015;56:S27–32. doi:10.1016/j.jadohealth.2015.01.01010.1016/j.jadohealth.2015.01.010. PMID:25863551.
- Stockwell MS, Fiks AG. Utilizing health information technology to improve vaccine communication and coverage. Human Vaccines & Immunotherapeutics 2013;9:1802–11. doi:10.4161/hv.2503110.4161/hv.25031.
- Baseman JG, Revere D, Painter I, Toyoji M, Thiede H, Duchin J. Public health communications and alert fatigue. BMC health services research 2013;13:295. doi:10.1186/1472-6963-13-29510.1186/1472-6963-13-295. PMID:23915324.
- National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory C Standards for Adult Immunization Practice. Public Health Reports 2014;129:115–23. doi:10.1177/00333549141290020310.1177/003335491412900203. PMID:24587544.
- McLean HQ, VanWormer JJ, Chow BDW, Birchmeier B, Vickers E, DeVries E, Meyer J, Moore J, McNeil MM, Stokley S, et al. Improving Human Papillomavirus Vaccine Use in an Integrated Health System: Impact of a Provider and Staff Intervention. The Journal of adolescent health: official publication of the Society for Adolescent Medicine 2017;61:252–8. doi:10.1016/j.jadohealth.2017.02.01910.1016/j.jadohealth.2017.02.019. PMID:28462786.
- Centers for Disease Control and Prevention (CDC). Immunization Information System Functional Standards, 2018–2022. Available at: https://www.cdc.gov/vaccines/programs/iis/functional-standards/func-stds-2018-2022.html.
