ABSTRACT
This study investigated the immunomodulatory influence of IL10 producing B regulatory cells, Bregs (CD19+CD24hiCD38hi) to standard Twinrix® vaccination. We also investigated HBsAg specific T-cell mediated IFN-γ responses to Twinrix® which in theory could provide effective immunity despite low anti-HBs titer. A total of 309 hepatitis B negative health care students and workers completed a standard Twinrix® vaccination schedule (0, 1 and 6 months). Depending on the vaccination response the participants were divided in to non-, low- and high responders according to anti-HBs titer (<10, <100 and >1000 mIU/mL respectively) two months after completed vaccination schedule. Blood samples from baseline and after vaccination from all non- and low-responders (23 participants) and the same number of high-responders were used for flow cytometric analyses of IL10 producing Bregs and T-cell mediated IFN-γ responses. A decrease in levels of IL10 producing Bregs was observed after vaccination in high responders compared to non- and low-responders. Compiling non-and low-responders against high-responders showed a lower T-cell mediated IFN-γ response at baseline in non-and low-responders when stimulated with Engerix® vaccine. In contrary no positive correlation between IL10 producing Bregs or IFN-γ positive T-cells and anti-HBs titer was observed. Hence this study cannot prove that levels of IL10 producing Bregs or IFN-γ positive T cell affect HBV vaccine response.
In this study we vaccinated 309 healthy adults against HBV with a combined hepatitis A and B vaccine (Twinrix®). We aimed to investigate if cellular parameters could predict serological response. Our study found no correlation between levels of IL10 producing B regulatory cells and anti-HBs titer. Furthermore we could not prove that levels of IFN-γ positive T cells were correlated to serological response.
Infection with HBV is a major global health problem affecting more than 240 million people.Citation1 Effective vaccines have been available since 1982 and have decreased the incidence of HBV infection and HBV-related morbidity and mortality.Citation2–5 In 1992, WHO recommended that all member states should include HBV vaccination in their national immunization programs.Citation5 However, this recommendation was not followed in Denmark where vaccination is recommended, but not routinely offered to health care workers.Citation6-7
Clinical studies have shown seroprotection (defined as anti-HBs >10mIU/mL) in 92.3 to 99.7% of vaccinees.Citation8–11 Older age, male gender, high BMI, smoking, vaccine administration route, human leucocyte antigen types as well as high levels of immunomodulatory lymphocytes and cytokines such as regulatory B cells (or Bregs) and IL10 have been associated with serological non-response to hepatitis B vaccination.Citation12–17
Measurements of anti-HBs titres are not always routinely offered after HBV vaccination, and consequently non-responders are not discovered. Undiagnosed non-responders might not take the necessary precautions in regards to HBV transmission and unawareness might delay diagnosis and ultimately treatment for HBV. The common interpretation is that serological non-responders are not protected against HBV transmission and hence revaccination is advised.Citation15 However, it is not clear to what extent serological non-responders may have persistent protection due to cell-mediated immunity (CMI). Several studies have examined this issue, but the results have been conflicting. This is mainly because it is difficult to establish whether a vaccine for HBV is protective in a non-endemic setting. One study found that 9% of serologic non-responders developed HBsAg-specific CMI, while another study reported 75%, as measured by antigen-specific proliferation of PBMCs.Citation13,Citation18 Recently Bauer et al. showed that despite vaccine recipients losing protective levels of anti-HBs they still had measurable HBsAg specific CD4 positive IFN-γ memory cells.Citation19 Low-responders (anti-HBs 10–100mIU/ml) are considered immunised but the duration of immunisation is reduced compared to high-responders. Grosso et al. showed that only 27% of low-responders had protective anti-HBs titres at a 30 year follow-up compared to 92% of high-responders.Citation20
The aim of this study was to investigate the influence of IL10 producing Breg subsets in serologic non- and low-responders to hepatitis B vaccination. Furthermore, we examined the CMI response as determined by an IFN-γ based flow cytometry assay in serologic non- and low-responders (anti-HBs <10 mIU/mL and <100 mIU/mL respectively) before and after receiving a standard course of HBV immunization.
We included health care workers and medical students > 18 years of age at risk of HBV infection. Exclusion criteria were previous or chronic HBV infection, previous HBV immunization, pregnancy (or planned pregnancy within 6 months), and allergy to vaccine contents. The Danish national ethics committee approved this study and informed consent was obtained from all participants.
Participants received a standard course of immunization with a combined hepatitis A and B vaccine (Twinrix®, GSK) at 0, 1, and 6 months, administered intramuscularly in the deltoid muscle. Data on age, weight and height (BMI measurement), smoking status, alcohol consumption, medical co-morbidities, and medication were collected at baseline ().
Table 1. Characteristics of study population.
Blood was drawn at baseline (0 month) and at follow-up (8 months, i.e. 2 months after the last vaccination, +/- 2 weeks). Measurements of HBsAg (Architect, Abbott Diagnostics) were performed at baseline and measurements of quantitative anti-HBs (Architect, Abbott Diagnostics) were performed at both baseline and follow-up. PBMCs were isolated by density gradient centrifugation using Ficoll-Paque. PBMCs were cryopreserved at -179°C. Cryopreserved PBMCs from baseline and follow-up: 1. participants with anti-HBs titer below 100 mIU/mL (23 subjects) and 2. high-responders (anti-HBs titres ≥ 1000 mIU/mL) matched on a 1:1 basis by age, gender and smoking-status were used for flow cytometric analyses.
PBMCs at baseline were included as HBsAg naive controls.
To enable linear correlation analyses possible on flow cytometry results, we needed numerical titer-values for the 23 high-responders. Serum was diluted with Architect Anti-HBs specific diluent (Abbott) and quantitative anti-HBs titers were measured (Architect, Abbott Diagnostics).
For the IL10 Breg analysis PBMCs rested for one hour at 37°C, 5% CO2 in media (RPMI 1640, with 5% FBS, 2 mM L-glutamine, 100ug/ml Penicillin and 100U/ml Streptomycin). Every participant had 1,5 × 106 cells stimulated with 10 µg/mL CpG ODN2006 (Invivogen) and 1 µg/mL CD40L (RnD Systems) and 1,5 × 106 cells left unstimulated. The cells were incubated for 48 hours and during the last four hours a portion of cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA) and 1 µg/mL Ionomycin (both Sigma-Aldrich). All cells were treated with 1 µL BD GolgiPlug/mL (BD Bioscience) during these last four hours. After the final stimulation the cells were transferred to fluorescence-activated cell sorting (FACS) tubes.
The samples were first stained with LIVE/DEAD near-IR as recommended (Life Technologies) and thereafter surface stained with CD19-PE-Cy7 (Beckman Coulter), CD38-PerCP-Cy5.5 (Biolegend), CD24-APC (Biolegend) and CD27-PE (eBioscience) prepared as a mastermix. Prior to intracellular staining with IL-10-Alexa Fluor 488 (eBioscience). The cells were fixed and permeabilised with BD Cytofix/Cytoperm and centrifuged in BD Perm/Wash buffer as described by the manufacturer (BD Bioscience). All cells were acquired on a FACS Verse (BD Bioscience) flow cytometer.
IFN-γ positive CD4+ T cells: PBMCs rested for one hour at 37°C, 5% CO2 in media (RPMI 1640, with 5% FBS, 2 mM L-glutamine, 100ug/ml Penicillin and 100U/ml Streptomycin) prior to stimulation in 5 ml polystyrene tubes at 1 × 106 PBMCs/ml. We stimulated with Engerix® vaccine (1μL), and not the Twinrix® vaccine, in order to only activate the HBV specific immune response, as a negative control we used media alone. We acknowledge that both Engerix® and Twinrix® contain alum as an adjuvant and though we would have liked to stimulate cells with a alum free version- this was not available. As positive control we used PHA (10 mg/ml). In order to mimic HBV infection cells were stimulated with all envelope proteins HBsAg S M L, i.e. S, Pre-S1 and pre-S2, and conditioned media was used as a negative control.
The HBsAg conditioned media was supernatant from hepatocarcinoma cell line “Huh7” cells transfected with different ratios of S, M or L in the pCI-CMV vector, all genotype D grown in Dulbecco's Modified Eagle Medium + 10% FBS. Cells were transfected with plasmid constructs at the following ratios: S:M = 19:1, S:L = 19:1 and S:M:L = 19:0.5:0.5.
After 18 hours of stimulation 1μL Golgiplug (BD Bioscience) was added to the cell-suspension and the cells incubated at 37°C, 5% CO2 for 6 hours. The cell-suspension was then washed and stained for surface markers including: CD45RO conjugated PE (BD Bioscience), CD4 conjugated Per-CP (BD Bioscience) and CD3 conjugated Pacific blue (BD Bioscience), and incubated at room temperature for 20 minutes in the dark. The cells were then fixed and permeabilised overnight at 4°C with BD Cytofix/Cytoperm and washed in BD Perm/Wash buffer (BD Bioscience). Intracellular staining was then performed using IFN-γ conjugated FITC (BD Bioscience). After 30 min incubation at 4° in the dark and washing, the cells were transferred to a 96 well plate and analysed on FACS Canto II flow cytometer (BD biosciences).
All data was analysed on Flow Jo v. 9.6.4 (TreeStar, Oregon). Before each analysis, the results were compensated using single stain compensation controls.
Gating IFN-γ positive CD4+ T cells: Lymphocytes were defined by gating on a forward- and side scatter plot and CD4+ T cells were defined on a CD3+/CD4+ plot. Memory T-cells was defined as CD4+ and CD45RO+. Each run included a sample, which had undergone same protocol as the rest of the samples except staining with IFN-γ conjugated FITC. In this sample we defined the gate for IFN-γ+ cells in both the CD4+ T-cells and memory cells to ensure consistency.
Gating IL10+ Bregs: Gating was influenced by the low number of these cells and that the morphology of the cells was altered after stimulation. Firstly, live cells were defined as negative for LIVE/DEAD near IR. Lymphocytes are usually gated on a tight forward-side scatter plot, but applying this would bypass the cells of interest, for which reason a broad forward-side scatter gate was used. For the same reason the following gate was a broad forward scatter -A/forward scatter-H gate. B lymphocytes were defined as CD19+, and the phenotype for Bregs were defined as CD24hiCD38hi. The number of CD19+CD24hiCD38hi cells producing intracellular IL10 was determined by gating IL10+ based on the IL10 expression in unstimulated cells.
All data were analysed in GraphPad prism version 6.0. A compiled group of non-/low-responders (cases) were compared to high-responders (controls). Fisher's exact test was used to compare the distribution of categorical variables such as sex, ethnicity, gender, smoking etc. For normally distributed variables, mean values (±standard deviation) were calculated and the t-test was used. To compare paired values e.g. high-responders at baseline vs. follow-up Wilcoxon Rank sum test was used. Pearsons correlation was used for linear evaluations. For unpaired values, e.g. non/low responders vs. high responders, Mann-Whitney test was used. For all tests, p-values below 0.05 were considered significant.
In order to normalize results we calculated ratios of Engerix and HBsAg results divided with results from our negative controls (background), media and conditioned media respectively. To standardize the number of Bregs, we used the number of IL10 producing CD19+CD24hiCD38hi cells of all CD19+ (B lymphocytes) (Bregs/CD19+) from the CD40L and CpG stimulated cells when analysing the data.
A total of 340 participants were included, of which 22 participants (6%) were excluded due to positive anti-HBs titre at baseline. Nine participants did not complete the full vaccination program: Five were lost to follow up, one withdrew consent, one emigrated, one discontinued due to mild side effects and one due to comorbidities.
On the basis of quantitative anti-HBs titres at follow-up, 4 (1%) of participants were categorized as serologic non-responders (anti-HBs < 10 mIU/ml), 19 (6%) of participants as serologic low-responders (anti-HBs 10–100 mIU/ml), 60 (19%) as intermediate responders (anti-HBs >100- and <1000 mIU/mL) and 226 (73%) as serologic high-responders (anti-HBs > 1000 mIU/ml) to the Twinrix® vaccine.
The group of clinical interest was participants with non- or low response to the vaccine. Thus we mainly compared the serologic non-/low-responders (23 cases) to the serologic high-responders (226 controls), respectively (). In all groups being female gender and caucasian ethnicity was most frequent. In the intermediate group a bigger proportion were male, however this difference was not significant. Mean age at baseline was higher in the group of non-/low-responders compared to the high-responder group (36.8 years (±2.64) vs. 30,1 years (±0.73), p = 0.025). Accordingly, the intermediate group had higher age than the high-responders but lower than the non-/low-responders. Compared to serologic high-responders, the group of non-/low-responders had a significantly higher mean BMI (26.2 vs. 23.2, p = 0.025) and a significantly higher proportion was found to be smoking (22% vs. 5%, p = 0.011). No difference was found in the prevalence in history of co-morbidities or use of other medicines.
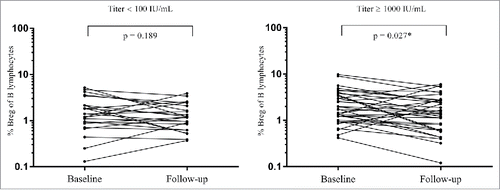
No differences were found between the proportion of Bregs/CD19+ at baseline or follow-up between serologic non-/low-responders and serologic high-responders (data not shown). However, in the high responder group, there was a significant decrease in Bregs/CD19+ number after vaccination (p = 0.027), which was not observed in the non-/low-responder group (p = 0.189) (). In the correlation analysis we found no significant correlation between anti-HBs titer and IL10+ Bregs including participants with serologic non-/low-response and high-responders.
Figure 1. Changes in proportion of Breg of total B lymphocytes from baseline to follow-up in low-responders and high-responders. A significant decrease in Bregs/CD19+ number after vaccination was found in the high-responder group (right graph), and not in the non-/low-responder group (left graph). *Significant p-values.
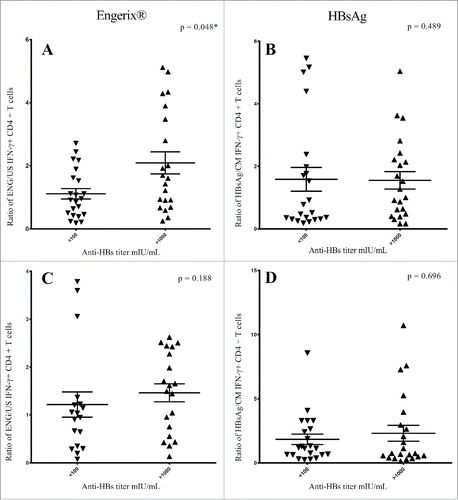
When comparing the stimulation ratio of IFN-γ positive CD4+ T cells at baseline, we found a significant higher ratio of IFN-γ positive CD4+ T cells (p = 0.048) in samples from high-responders stimulated with Engerix® compared to non-/low-responders (). No difference was found between the non-/low-responders and the high-responders in IFN-γ positive CD4+ T cells at baseline when stimulated with HBsAg or in follow-up samples stimulated with Engerix® or HBsAg (. Similarly, no difference was found when evaluating stimulation ratios of IFN-γ positive memory cells (data not shown).
Figure 2. Results from Engerix® (A) and HBsAg (B) stimulated baseline samples and Engerix® (C) and HBsAg (D) stimulated follow-up samples. Plots of the ratio of IFN-γ positive CD4+ T-cells for non-/low-responders and high-responders (Anti-HBs <100 mIU/ml and >1000 mIU/ml, respectively). Higher ratio of IFN-γ positive CD4+ T cells at baseline was found in samples from high-responders stimulated with Engerix® compared to non-/low-responders (). No difference was observed in ratios of IFN-γ positive CD4+ T cells in baseline samples when stimulated with HBsAg (). At follow-up, we observed no difference between the non-/low-responders and the high-responders in ratios of IFN-γ positive CD4+ T cells when stimulated with Engerix® or HBsAg (. *Significant p-values. A: ENG/US (Engerix®/Media), B = HBsAg/CM (HBsAg/Conditioned Media).
In neither the non-/low-responder, nor the high-responder group, did we observe any difference in proportions of IFN-γ positive CD4+ or memory T cells when comparing samples from baseline and follow-up. This was also the case when testing for correlation of IFN-γ positive CD4+ or memory T cells and anti-HBs titer. This was true irrespective of the antigen stimulation (Engerix® or HBsAg) (data not shown).
We studied a large number (n = 309) of participants who completed an HBV vaccination programme. We identified 23 with serologic non- or low-response (anti-HBs < 100 mIU/ml) after vaccination. Surprisingly only 1% were serological non-responders after a standard course of Twinrix® vaccination, a proportion that is much lower than reported in previous studies.Citation8–11 This may be attributable to the young age and good health of the participants.Citation8
The non-/low-responder group were more likely to be older, smokers and have a higher BMI than vaccine responders. The high-responders used for flow cytometric analyses were matched to the non-/low-responder group on age, gender and smoking-status.
The impact of the finding that lower IL10 producing Bregs/CD19+ after vaccination is only observed in high responders is unclear. It may indicate that a decrease in immunomodulatory lymphocytes and cytokines after vaccination is a surrogate of immune activity and thus associated with higher anti-HBs titres. We conducted analyses to test for a positive correlation between IL10 producing Bregs/CD19+ at baseline and anti-HBs titer after vaccination. When focusing the analysis on the low and nonresponder group, we found a significant correlation (data not shown), but when we included serologic high responders, there was no correlation. Hence, our data do not provide any indication that the regulatory status of the immune system has any predictive value to vaccination response.
Garner-Spitzer et al. showed elevated levels of IL10 in supernatant and elevated levels of Bregs/CD19+ in HBV non-responders. However, this may be an antigen specific response since no decrease in levels of IL10 or Bregs/CD19+ was observed in HBV non-responders before and after vaccination against tick borne encephalitis (TBE), who produced adequate amounts of TBE antibodies after vaccination.Citation17
To our knowledge, no studies have compared cytokine level or presence of reactivity of T cells, in serological non-/low-responders and high-responders, prior to HBV vaccination. Our findings showed that serological low-responders had significantly lower stimulation ratios of reactive IFN-γ positive CD4+ T cells to Engerix® vaccine at baseline. However, we did not find a positive correlation between IFN-γ positive CD4+ T cells at baseline and anti-HBs titer after vaccination. Previous studies have reported that in vitro IFN-γ stimulation of PBMCs induced an increased level of anti-HBs producing B cells.Citation21 Thus lower numbers of IFN-γ positive T cells reactive to the Engerix® vaccine might explain decreased production of anti-HBs in non-/low-responders. This difference in IFN-γ positive CD4+ cells between the non-/low-responders and high-responders could not be found in samples stimulated with HBsAg. One potential explanation for this finding could be that components other than HBsAg in the vaccine preparation induced IFN-γ responses in CD4+ T cells in high-responders but not in non-/low-responders. The Engerix® vaccine preparation, that in addition to HBsAg, contains <5% yeast protein (after purification procedures), alum (as aluminium hydroxide), sodium chloride and phosphate buffer.
Velu et al, used an ELISA to measure IFN-γ, and showed a significantly higher IFN-γ response in high-responders compared to low-responders and non-responders after HBV vaccination.Citation22 In contrast, our results at follow-up showed no difference in proportions of IFN-γ + CD4+ or memory T cells between non-/low-responders and high-responders, indicating that the IFN-γ response is not produced by CD4+ T lymphocytes. However, Bauer et al. showed that after third HBV vaccination IFN-γ producing CD4+/CD45RA+ and CD4+/CD45R0+ T cells declined over time. Thus the 2-months time of follow-up might be too late for evaluation of cellular immune response.
Limitations and weaknesses in this study include: 1.Few non-/and low-responders. 2. We did not stimulate with HBV vaccine preparations without alum. 3. We only took one blood sample after completed HBV vaccination, thus examination on decline in immune response over time could not be performed. Future studies on this subject should include more cytokines i.e. IL4 as a determinant on Th2 response and its regulatory effect on Th1 response. Also adding more analyses on cytokines in the T regulatory response i.e. TGF-β could provide details of the mechanism involved in the reduced production of anti-HBs in non-/low responders.
In summary none of the immune parameters evaluated in this study were capable of predicting vaccination responses. We observed that high-responders showed a decrease in IL10 producing Bregs/CD19+ following vaccination and that they had greater numbers of IFN-γ producing CD4+ T-lymphocytes to the Engerix® vaccine at baseline. The implications of these findings are not clear. In non- and low-responders we found no evidence of a mobilized protective CD4+ T cell response. Thus there is a continued need for measurements of post-vaccination anti-HBs titres and booster vaccination to non-responders, especially in risk groups and in HBV endemic areas.Citation13 However our study underline that early vaccination in a young healthy population is likely to be successful.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- WHO. Hepatitis B. Fact sheet N°204. 2015 [accessed 2015 sep 09]; http://www.who.int/mediacentre/factsheets/fs204/en/.
- de la Hoz F, Perez L, de Neira M, Hall AJ. Eight years of hepatitis B vaccination in Colombia with a recombinant vaccine: factors influencing hepatitis B virus infection and effectiveness. Int J Infect Dis. 2008;12(2):183–9. Epub 2007/10/05. doi:10.1016/j.ijid.2007.06.010. PMID:17913535
- Odusanya OO, Alufohai E, Meurice FP, Ahonkhai VI. Five-year post vaccination efficacy of hepatitis B vaccine in rural Nigeria. Hum Vaccin. 2011;7(6):625–9. Epub 2011/04/22. doi:10.4161/hv.7.6.14990. PMID:21508678
- McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, Parkinson AJ. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54(3):801–7. Epub 2011/05/28. doi:10.1002/hep.24442. PMID:21618565
- WHO. Immunization, Vaccines and Biologicals 2013 [accessed 2015 sep 09]; http://www.who.int/immunization/topics/hepatitis_b/en/.
- Krogsgaard KGI, Plesner AM, Hansen KG, Larsen M, Keiding H, Smith E, Stellfeld M, Nielsen JO, Skinhøj P, et al. Introduction of hepatitis B vaccination as part of childrens vaccination programme in Denmark. A health technology assessment. Danish Health Authority 2003.
- Weis NNJO, Gerstoft J, Christensen PB, Krarup H, Cowan S, Poulsen S, Nørgård-Edmund M, Fouchard J. Guidance for HIV (Human Immunodeficiency Virus) and hepatitis B and C virus. The prevention of blood-borne infection, diagnosis and management in health care and other workplaces. Danish Health Authority 2013.
- Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, Wiesinger K, Kollaritsch H. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19(15-16):2055–60. Epub 2001/03/03. doi:10.1016/S0264-410X(00)00410-2. PMID:11228377
- Chlibek R, von Sonnenburg F, Van Damme P, Smetana J, Tichy P, Gunapalaiah B, Leyssen M, Jacquet JM. Antibody persistence and immune memory 4 years post-vaccination with combined hepatitis A and B vaccine in adults aged over 40 years. J Travel Med. 2011;18(2):145–8. Epub 2011/03/04. doi:10.1111/j.1708-8305.2010.00499.x. PMID:21366801
- Joines RW, Blatter M, Abraham B, Xie F, De Clercq N, Baine Y, Reisinger KS, Kuhnen A, Parenti DL. A prospective, randomized, comparative US trial of a combination hepatitis A and B vaccine (Twinrix) with corresponding monovalent vaccines (Havrix and Engerix-B) in adults. Vaccine. 2001;19(32):4710–9. Epub 2001/09/06. doi:10.1016/S0264-410X(01)00240-7. PMID:11535321
- Thoelen S, Van Damme P, Leentvaar-Kuypers A, Leroux-Roels G, Bruguera M, Frei PC, Bakasenas V, Safary A. The first combined vaccine against hepatitis A and B: an overview. Vaccine. 1999;17(13-14):1657–62. Epub 1999/04/09. doi:10.1016/S0264-410X(98)00421-6. PMID:10194819
- Van der Wielen M, Van Damme P, Chlibek R, Smetana J, von Sonnenburg F. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine. 2006;24(26):5509–15. Epub 2006/05/27. doi:10.1016/j.vaccine.2006.04.016. PMID:16725234
- Cardell K, Akerlind B, Sallberg M, Fryden A. Excellent response rate to a double dose of the combined hepatitis A and B vaccine in previous nonresponders to hepatitis B vaccine. J Infect Dis. 2008;198(3):299–304. Epub 2008/06/12. doi:10.1086/589722. PMID:18544037
- Egea E, Iglesias A, Salazar M, Morimoto C, Kruskall MS, Awdeh Z, Schlossman SF, Alper CA, Yunis EJ. The cellular basis for lack of antibody response to hepatitis B vaccine in humans. J Exp Med. 1991;173(3):531–8. Epub 1991/03/01. doi:10.1084/jem.173.3.531. PMID:1825504
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179(2):489–92. Epub 1999/01/07. doi:10.1086/314578. PMID:9878036
- Hohler T, Reuss E, Freitag CM, Schneider PM. A functional polymorphism in the IL-10 promoter influences the response after vaccination with HBsAg and hepatitis A. Hepatology. 2005;42(1):72–6. Epub 2005/05/27. doi:10.1002/hep.20740. PMID:15918171
- Garner-Spitzer E, Wagner A, Paulke-Korinek M, Kollaritsch H, Heinz FX, Redlberger-Fritz M, Stiasny K, Fischer GF, Kundi M, Wiedermann U. Tick-borne encephalitis (TBE) and hepatitis B nonresponders feature different immunologic mechanisms in response to TBE and influenza vaccination with involvement of regulatory T and B cells and IL-10. J Immunol. 2013;191(5):2426–36. Epub 2013/07/23. doi:10.4049/jimmunol.1300293. PMID:23872054
- Jarrosson L, Kolopp-Sarda MN, Aguilar P, Bene MC, Lepori ML, Vignaud MC, Faure GC, Kohler C. Most humoral non-responders to hepatitis B vaccines develop HBV-specific cellular immune responses. Vaccine. 2004;22(27-28):3789–96. Epub 2004/08/19. doi:10.1016/j.vaccine.2004.02.046. PMID:15315860
- Bauer T, Jilg W. Hepatitis B surface antigen-specific T and B cell memory in individuals who had lost protective antibodies after hepatitis B vaccination. Vaccine. 2006;24(5):572–7. Epub 2005/09/21. doi:10.1016/j.vaccine.2005.08.058. PMID:16171909
- Grosso G, Mistretta A, Marventano S, Ferranti R, Mauro L, Cunsolo R, Proietti L, Malaguarnera M. Long-term persistence of seroprotection by hepatitis B vaccination in healthcare workers of southern Italy. Hepat Mon. 2012;12(9):e6025. Epub 2012/10/23. doi:10.5812/hepatmon.6025. PMID:23087756
- Bocher WO, Herzog-Hauff S, Schlaak J, Meyer zum Buschenfeld KH, Lohr HF. Kinetics of hepatitis B surface antigen-specific immune responses in acute and chronic hepatitis B or after HBs vaccination: stimulation of the in vitro antibody response by interferon gamma. Hepatology. 1999;29(1):238–44. Epub 1998/12/24. doi:10.1002/hep.510290120. PMID:9862872
- Velu V, Saravanan S, Nandakumar S, Shankar EM, Vengatesan A, Jadhav SS, Kulkarni PS, Thyagarajan SP. Relationship between T-lymphocyte cytokine levels and sero-response to hepatitis B vaccines. World J Gastroenterol. 2008;14(22):3534–40. Epub 2008/06/21. doi:10.3748/wjg.14.3534. PMID:18567083
