ABSTRACT
The longevity of antibodies induced by inactivated enterovirus 71 type (EV71) vaccine is not well studied. To estimate the immunity persistence following two-dose vaccination of EV71 vaccine, a five-year follow-up study was conducted as an extension of a Phase III clinical trial. In this study, a sub-cohort of volunteers who was eligible for enrollment and randomly administrated either 2 dose EV71 vaccine or placebo in the phase III clinical trial was selected, and then further observed 64 months post the 1st vaccination. 211 Subjects (106 vaccine subjects and 105 placebo subjects) who provided a full series of blood samples (at all the sampling points) were included in the final analyzed population. Seropositive rate (SR) and geometric mean titer (GMT) of the neutralizing antibodies (NAb) was calculated to detect the dynamic profiles of EV71 vaccine-induced immunogenicity. SR at the 5th year remained 94.34% in the vaccine subjects, with a GMT of 141.42. The SR was 71.43% in the placebo subjects, with a GMT of 71.83. Despite natural infection consistently promoted the NAb increase in the placebo subjects, the SR and GMT in vaccine subjects remained significantly higher than that in the placebo subjects at all the sampling points. The inactivated EV71 vaccine-induced immunity had a good persistence, within 5 years following the primary vaccination.
Introduction
Enterovirus type 71 (EV71) is the major etiological agent of hand, foot, and mouth disease (HFMD).Citation1-4 Infants and children less than 3 years old are especially prone to HFMD.Citation5 HFMD infections are usually mild and self-limiting, however, when the pathogen of HFMD is EV71, the disease can present with a high rate of neurological complications, including meningoencephalitis and pulmonary edema,Citation6,Citation7 which can occur fast and lead to death.
Since 1997, the prevalence of EV71 infection greatly increased in the Asia-Pacific region, especially in Southeast Asia. In the past two decades, a number of outbreaks of EV71-associated HFMD have been reported in Malaysia(1997,Citation8 2000, and 2005Citation9), Taiwan(1998Citation10), Singapore(2000Citation11), Vietnam(2005Citation12 and 2011Citation13), and mainland China (2008Citation14). In the outbreak of HFMD in 2008 in mainland China, nearly 500,000 cases were reported among children including more than 120 fatal cases, in which EV71 was confirmed as the major pathogen.Citation15 HFMD has been listed as a category “C” notifiable infectious disease since May, 2008. According to the statistic of the National Center for Disease Control and Prevention, from 2009 to 2016, over 15,000,000 HFMD cases and more than 3400 fatal cases were reported totally (www.nhfpc.gov.cn). Since 2009, number of yearly reported HFMD cases ranked first among notifiable infectious diseases every year.
Due to the tremendous burden on public health caused by EV71 outbreaks and the lack of effective drugs for treatment, the development of EV71 vaccines is of great significance for Asian countries. A Vero cell-based EV71 inactivated vaccine without aluminum hydroxide was developed by Sinovac Biotech (Beijing), and the vaccine strain is generated from a C4 genotype strain (GenBank:GU198367.1)
This Sinovac EV71 vaccine has shown evidence of safety, immunogenicity, and effectiveness in children of 6–35 months old, in phase I∼III clinical trials.Citation16-18 The phase III study was carried out in children aged 6–35 months in China since January, 2012. This study showed the one-year vaccine efficacy against EV71-associated HFMD or herpangina was 94.8%, and that against EV71-associated hospitalization and HFMD with neurologic complications were both 100%.Citation18 Further, an efficacy of 95.1% against EV71-associated HFMD was reported in extended 2-year follow-up after the initial study.Citation19 This vaccine was licensed for market in 2016 and thereafter supplied for the prevention of HFMD in several provinces in China, with associated post-market studies continuing.
We herein report a further observation conducted 64 months after the first vaccination in phase III clinical trial. This study aims to explore the dynamic changes of the vaccine-induced immunogenicity and answer whether a boost vaccination is needed on the basis of the two-dose schedule, to further provide guidance for vaccination strategy.
Results
Participants
343 subjects received the month-64 blood samplings and had the effective NAb assay results. 211 among them provided a full series of blood sampling (on month 2, month 8, month 14, month 26 and month 64) and had no history of HFMD/ herpangina /receipt of extra EV71 vaccine, thus were included in the per protocol set (PPS). There were 106 vaccine subjects and 105 placebo subjects in PPS. The demographic information of PPS subjects was listed in .
Table 1. The demographic information of subjects in PPS (month 64).
Shift of immune levels in the 5-year follow-up
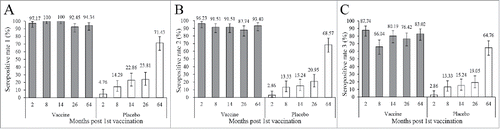
By the cutoff value of 1:8, 2 months, 8 months, 14 months, 26 months and 64 months post the 1st vaccination, the seropositive rates in vaccine group were 97.17% (placebo: 4.76%, P<0.0001), 100% (placebo: 14.29%, P<0.0001), 100%(placebo: 22.86%, P<0.0001), 92.45%(placebo: 23.81%, P<0.0001), and 94.34% (placebo: 71.43%, P<0.0001) respectively. Seropositive rates of vaccine group kept stable since immunized, while that of the placebo group experienced a noticeable increase. At all the observed time points, the seropositive rates of the vaccine group were significantly higher than that of placebo group. By the cutoff values of 1:16 and 1:32, the same results were found with all the P value <0.0001, except the seropositive rate comparison calculated by 1:32 on month 64 (P = 0.0025). The dynamic profiles of seropositive rates were displayed in .
Figure 1. Dynamic profiles of EV71-NAb seropositive rates during a 5-year follow-up period in healthy children immunized with EV71 vaccine or placebo. Blood samples from 211 participants, who provided a full series of blood samples within the 5-year follow up period were collected to evaluate the production of EV71 NAb. These children were 6–35 months old at the enrollment of the initial study and about 6–8 years at the last observed time point. (a) by the cutoff value of 1:8 (b) by the cutoff value of 1:16 (c) by the cutoff value of 1:32. The bar indicates the 95% confidence interval.
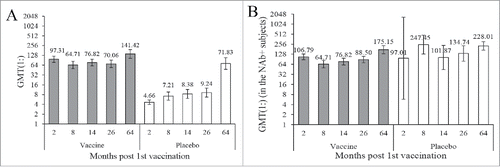
At the abovementioned time points, the NAb GMTs of vaccine subjects were 97.31(placebo: 4.66, P<0.0001), 64.71(placebo: 7.21, P<0.0001), 76.82(placebo: 8.38, P<0.0001), 70.06(placebo: 9.24, P<0.0001), and 141.42(placebo: 71.83, P<0.0001) respectively. The GMT in placebo group experienced a noticeable increase in the 5-year period while that in vaccine group was comparatively stable. However, the month-64 GMT increased compared with the month-26 in both groups, while the increase in the placebo group was dramatic. At all the observed time points, the NAb GMT of vaccine group was significantly higher than that of placebo group.
With regard to the seropositive (NAb≥1:8) subjects, the GMTs in vaccine group were 106.79(placebo: 97.01, P = 0.9296), 64.71(placebo: 247.45, P = 0.5274), 76.82(placebo: 101.87, P = 0.5075), 88.50(placebo: 134.74, P = 0.1481), and 175.15(placebo: 228.01, P = 0.1967). In the vaccine group, the month-64 GMT was higher than that at any former time points. In the placebo group, the month-8 GMT was the highest, closely followed by the month-64 GMT. With regard to the seropositive subjects, no significant GMT discrepancy was found between the vaccine and placebo group. The dynamic profiles of GMT were displayed in .
Figure 2. Dynamic profiles of EV71-NAb GMT during a 5-year follow-up period in healthy children immunized with the EV71 vaccine or placebo. Blood samples from 211 participants, who provided a full series of blood samples within the 5-year follow up period, were collected to evaluate the production of EV71 NAb. These children were 6–35 months old at the enrollment of the initial study and about 6–8 years at the last observed time point. (a) in all the 211 subjects (b) in the EV71-NAb seropositive (NAb≥1:8) subjects. The bar indicates the 95% confidence interval.
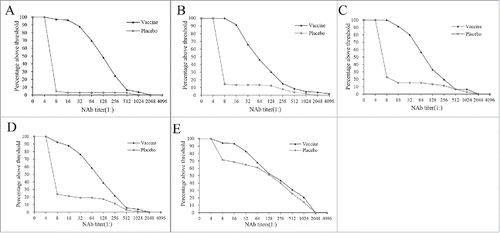
In the 5-year follow up period, the NAb titer distribution in placebo subjects experienced dramatic change, with the proportion of high titer increased. The titer distribution in the vaccine subjects did not experience noticeable change until the increase of high-titer proportion on month 64. The titer distribution in placebo subjects gradually approached to that of vaccine subjects as the time went on. The dynamic profiles of NAb titer distributions were displayed in .
Figure 3. The dynamic profiles of EV71 NAb titer distribution during a 5-year follow up period in healthy children immunized with the EV71 vaccine or placebo. Blood samples from 211 participants, who provided a full series of blood samples within the 5-year follow up period were collected to evaluate the production of EV71 NAb. These children were 6–35 months old at the enrollment of the initial study and about 6–8 years at the last observed time point. (a) month 2 (b) month 8 (c) month 14. (d) month 26. (e) month 64. Reverse distributions were displayed, the y axes indicate the proportion of subjects with a NAb titer no less than a certain titer.
The age-stratified immune levels 64 months post the 1st vaccination
In the vaccine subjects, month-64 seropositive rate (NAb≥1:8) was 96.97% (placebo: 86.67%, P = 0.1489) in the 6–11 month subgroup, 92.86% (placebo:69.81%, P = 0.0019) in the 12–23 month subgroup, and 94.12% (placebo:54.55%, P = 0.0181) in the 24–35 month subgroup. Overall, the seropositive rates decreased with the increasing age, particularly in the placebo group.
In the vaccine subjects, NAb GMT was 187.85 (placebo: 125.84, P = 0.3688) in the 6–11 month subgroup, 137.10(placebo: 75.40, P = 0.1130) in the 12–23 month subgroup, and 90.27 (placebo:29.74, P = 0.0607) in the 24–35 month subgroup. Overall, GMT decreased with the increasing age. The age-stratified immunogenicity on month 64 were listed in .
Table 2. The age-stratified EV71-NAb immunogenicity 64 months post 1st dose either EV71 vaccine or placebo.
Discussion
The phase III clinical trial suggested the investigated inactivated EV71 vaccine can induce good immunogenicity and be effective in the prevention of EV71 associated diseases in the 1-year follow up period.Citation18 A 2-year extended follow-up of a sub-cohort from the phase III clinical trial further provided evidence for the vaccine-induced immune persistence.Citation19 Based on the abovementioned historical studies, this study extended the follow-up period to 5 years to further explore the vaccine-induced immunogenicity persistence. Subjects who provided a full series of blood samples in the 5-year period were selected as the study population, to ensure the comparability between different time points and reduce potential bias.
We found that, 2 months post the 1st dose EV71 vaccine (28 days post the 2nd dose), good immunogenicity was induced in the vaccine subjects with a high seropositive rate (NAb≥1:8) of 97.17%. The seropositive rate generally kept stable in the further observation, and remained 94.34% five years later. Given the above evidence, boost vaccination may not be necessary as of the 5th year post immunization. NAb GMT reached 97.31 on month 2 and dropped to 64.71-76.82 during month 8–26, which may reflect the natural decay of the vaccine-induced antibodies.Citation20-23 Except for the investigated vaccine in this study, there are two EV71 vaccines currently available in China market, developed by the Institute of Medical Biology, Chinese Academy of Medical Science (CAMS) and Beijing Vigoo Biological Co., Ltd (Vigoo) respectively. Li et al reported that, 2 months after the 1st dose injection of CAMS EV71 Vaccine, a GMT of 64.37 and a seropositive rate (NAb ≥1:8) of 98.5% were observed.Citation24 According to another study of CAMS EV71 vaccine, the seropositive rate in the vaccine subjects remained 98.1% 2 years after the vaccination.Citation20 The CAMS EV71 vaccine manifested a good immunogenicity and 2-year immune persistence similar to that in our study. Besides, according to the phase III clinical trial, 1 year after the whole-schedule vaccination of Vigoo EV71 vaccine, the seropositive rate remained >99%,Citation25 which is also in accordance with our findings. However, to our knowledge, results of further investigation for immune persistence remain unavailable for the abovementioned two vaccines.
In this study, the seropositive rate of placebo subjects experienced a dramatic increase from 4.76% to 71.43% in 5 years, with the corresponding GMT increased from 4.66 to 71.83. These changes suggested the noticeable natural infections and the high risk of EV71-associated diseases among the unvaccinated subjects. Existing investigations carried out in different regions of mainland China reported diverse but generally high seropositive rates in children aged 6–10 years. Li et al carried out a serological investigation in four counties of Guangxi province in 2010, indicating the seropositive rate (NAb ≥1:8) in children aged 6–10 years was 87.5%.Citation26 Ding et al reported a seropositive rate of 77.5% in Lipu county of Guangxi province,Citation27 while a study in Ganyu county of Jiangsu province reported that of 97.5%.Citation28 The above recorded serum levels were similar to that in our study, revealing the vaccination coverage is still too limited to generate the herd immunity.
Despite the natural infection promoted the increase of NAb levels in placebo subjects, seropositive rates of vaccine subjects were consistently significantly higher than the placebo subjects. The results suggested a good persistence of vaccine-induced immunogenicity. Interestingly, after the 2nd year, the GMT noticeably increased not only in placebo but also in vaccine subjects. This finding illustrates the EV71 vaccine -induced immunogenicity in vaccinated population can be naturally strengthened in the epidemic region.
With regard to the seropositive subjects, it seems that the GMT in placebo group were higher than that in vaccine group, which may suggest people can produce relatively higher antibody titers through natural infection. This finding is consistent with the statement by Dai et al that, the antibody response and duration of immunity for the subclinical-infected children were intermediate between those caused by clinical infection and vaccine.Citation21 Despite the abovementioned, GMT of vaccine subjects was consistently higher than that of placebo group, further indicating the long duration of vaccine-induced antibodies.
From the result of month 64, for the placebo subjects, the seropositive rates decreased obviously with the increasing age. The seropositive rate was about 50% in 24–35 month old subgroup (aged approximately 8 years at blood sampling time). A study in Singapore suggested that, in samples from children >5 years old, the age-specific seroprevalence reached a steady state at approximately 50%,Citation29 which is in accordance with our findings. Whereas, for the vaccine subjects, the seropositive rates in different age groups were relatively stable. This illustrates the vaccine can alter the serum epidemiology of EV71 NAb among children.
In conclusion, inactivated EV71 vaccine-induced immunogenicity has a satisfactory persistency, and boost vaccination is not necessary till 5 years after the primary 2-dose immunization.
Methods
Vaccine and vaccination
The inactivated EV71 vaccine was developed in a good manufacturing practice-compliant facility, and was tested at the National Institutes for Food and Drug Control before the initial study. The vaccine was prepared from the EV71 H07 strain of sub-genotype C4, cultured in Vero cells for proliferation, and then inactivated by formalin-inactivated method and purified. Each dose of the vaccine contained 400U of inactivated EV71 viral antigen absorbed onto aluminum hydroxide, suspended in 0.5ml of buffered saline. Both the vaccine and placebo were administrated intramuscularly at the 0 and 28 days.
Study design and population
The initial study was a randomized, double-blind, placebo-controlled phase III clinical trial conducted in children aged 6–35 months from three counties (Ganyu, Sheyang, Taixing) of Jiangsu province in 2012. Besides, an extended one-year follow-up of this study was completed in 2014. On the basis of the above studies, this study is a further follow-up, in order to evaluate the EV71 immune levels 5 years after the primary vaccination.
A total of 10,077 participants (with 1293 in the immunogenicity group) were randomized in a 1:1 ratio to receive two doses Sinovac EV71 vaccine or placebo on a 0-, 28-day schedule, and then followed for a 12 month surveillance period (from month 2 to month 14).Citation18 Further, 1083 immunogenicity group participants completed the extended follow-up to month 26.
In this study, immunogenicity group participants in Sheyang county from the phase III clinical trial was chosen as the studied subjects and 343 subjects received the blood collection on month 64. A retrospective survey for the enrolled subjects involved with the history of HFMD, herpangina, and the extra vaccination of EV71 vaccine was conducted before blood collection. 3 ml venous blood were collected from each available subject for EV71 neutralizing antibody (NAb) testing. Thus, combined with the previous observation, the full series of sampling points in our report included day 0, month 2, month 8, month 14, month 26, and month 64. Since the unblinding was performed after the initial study completed, this study was an open-labelled detection for the persistence of the vaccine-induced EV71 NAb.
Statistical analysis
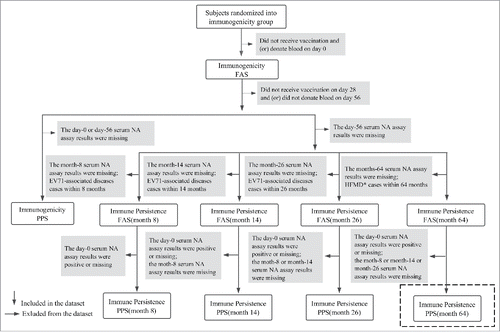
The determinations of different analysis set were shown in . The immune persistence per-protocol set (PPS) of month 64 was the final analyzed set in this study, referring to subjects meeting the following criteria:
| (1) | Included in the immunogenicity group of initial study and randomly into either vaccine or placebo group. | ||||
| (2) | Serum NAb assay was negative on day 0. | ||||
| (3) | Fulfilled the two doses vaccination following the protocol. | ||||
| (4) | Provided a full series of blood samples. | ||||
| (5) | Had no history of EV71 associated diseases within month 26 and had no history of HFMD between month 26 and 64. | ||||
| (6) | Had no history of extra EV71 vaccination. | ||||
Figure 4. Definitions of different analysis sets in the initial phase III clinical trial and its follow-up studies. FAS and PPS indicates Full Analysis Set and Per Protocol Set respectively. The dataset marked by the dashed box was the final analyzed set in this study. *During the extended follow-up period in this study (month26-month64), neither herpangina nor extra vaccination was found, and 16 HFMD case (8 in vaccine group and 8 in placebo group) were found through the retrospective survey, but no associated pathogen information was obtained.
Seropositive rates of EV71 NAb were calculated by the cutoff values of 1:8,1:16, and 1:32 separately. For the calculations of the Geometric mean titer (GMT) of EV71 NAb, the titer lower than 1:8 and higher than 1:1024 were assigned values of 1:4 and 1:1024 respectively. A chi-square test or Fisher's exact test was used to compare categorical data, while Student's T test was used to compare log-transformed NAb values. Hypothesis testing was two-sided with an alpha value of 0.05. Statistical analysis was conducted with SPSS20.0.
Limitations
There were several limitations in our study. First, during the extended follow up period in this study, 8 HFMD cases were reported in each group. However, we failed to obtain the pathogen information of these HFMD cases who was excluded from the PPS. As recorded, 41.4% of HFMD cases were caused by EV71, from January to May in 2017.Citation30 Moreover, number of the excluded HFMD cases was relatively small. Thus, we do not think the absence of pathogen information will have significant impacts on our major conclusions. Second, in the context of EV71 prevalence, we cannot avoid the influence of natural infection, which may partly contribute to the high immunogenicity 5 years after the primary immunization. However, associated bias was acceptable because of the presence of parallel control group. Despite the above limitations, this study provided preliminary evidence for the long-term immunity persistence within 1 year post marketing of the inactivated EV71 vaccine, which can provide evidence for the establishment of the immunization guidance. Besides, more post-marketing studies are still in need to confirm the immunity persistence of EV71 vaccine.
Ethical statements
Both the initial and the follow-up studies were approved by the ethics committee of the Jiangsu Provincial Center for Disease Control and Prevention (CDC) and the study was conducted according to Good Clinical Practices and the Declaration of Helsinki. Before enrollment, written informed consent was obtained from each subject's guardian.
Disclosure of potential conflicts of interest
Gang Zeng, Weixiao Han, Ying Zhang, and Jing Li are employees of Sinovac Biotech Co., LTD. All other authors: no conflicts.
Additional information
Funding
References
- Nasri D, Bouslama L, Pillet S, Bourlet T, Aouni M, Pozzetto B. Basic rationale, current methods and future directions for molecular typing of human enterovirus. Expert Rev Mol Diagn. 2007;7(4):419–34 doi:10.1586/14737159.7.4.419. PMID:17620049.
- Abubakar S, Chee HY, Alkobaisi MF, Xiaoshan J, Chua KB, Lam SK. Identification of enterovirus 71 isolates from an outbreak of hand, foot and mouth disease (HFMD) with fatal cases of encephalomyelitis in Malaysia. Virus Res. 1999;61(1):1–9 doi:10.1016/S0168-1702(99)00019-2. PMID:10426204.
- Shekhar K, Lye MS, Norlijah O, Ong F, Looi LM, Khuzaiah R, Marzuki I, Hussein I, Wong SL, Mohan J. Deaths in children during an outbreak of hand, foot and mouth disease in Peninsular Malaysia–clinical and pathological characteristics. Med J Malaysia. 2005;60(3):297–304 PMID:16379183.
- Gilbert GL, Dickson KE, Waters MJ, Kennett ML, Land SA, Sneddon M. Outbreak of enterovirus 71 infection in Victoria, Australia, with a high incidence of neurologic involvement. Pediatr Infect Dis J. 1988;7(7):484–8 doi:10.1097/00006454-198807000-00007. PMID:2841639.
- Wang Z, Lv H, Zhu W, Mo Z, Mao G, Wang X, Lou X, Chen Y. Epidemiologic features of enterovirus 71-associated hand-foot-and-mouth disease from 2009 to 2013 in Zhejiang, China. Inter J Env Res Pub Heal. 2017;14(1):33
- Xu J, Qian Y, Wang S, Serrano JM, Li W, Huang Z, Lu S. EV71: an emerging infectious disease vaccine target in the Far East? Vaccine. 2010;28(20):3516–21 doi:10.1016/j.vaccine.2010.03.003. PMID:20304038.
- Mcminn P, Lindsay K, Perera D, Chan HM, Chan KP, Cardosa MJ. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J Virol. 2001;75(16):7732
- Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, Ho KK, Han LL, Pallansch MA, Suleiman AB. Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: clinical and pathological characteristics of the disease. For the Outbreak Study Group. Clin Infect Dis. 2000;31(3):678 doi:10.1128/JVI.75.16.7732-7738.2001. doi:10.1086/314032. PMID:11462047.
- Chua KB, Chua BH, Lee CS, Chem YK, Ismail N, Kiyu A, Kumarasamy V. Genetic diversity of enterovirus 71 isolated from cases of hand, foot and mouth disease in the 1997, 2000 and 2005 outbreaks, Peninsular Malaysia. Malays J of Pathol. 2007;29(2):69–78
- An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341(13):929–35 doi:10.1056/NEJM199909233411301. PMID:10498487.
- Singh S, Chow VT, Phoon MC, Chan KP, Poh CL. Direct detection of enterovirus 71 (EV71) in clinical specimens from a hand, foot, and mouth disease outbreak in Singapore by reverse transcription-PCR with universal enterovirus and EV71-specific primers. J Clin Microbiol. 2002;40(8):2823–7 doi:10.1128/JCM.40.8.2823-2827.2002. PMID:12149336.
- Tu PV, Thao NT, Perera D, Huu TK, Tien NT, Thuong TC, How OM, Cardosa MJ, Mcminn PC. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam, 2005. Emerg Infect Dis. 2007;13(11):1733–41 doi:10.3201/eid1311.070632. PMID:18217559.
- Huu KT, Saraswathy S, Tan TT, Kim TLP, Chi TT, Hang VTT, Jeremy F, Tinh HT, Vinh CNV, Rogier VDH. Enterovirus 71–associated hand, foot, and mouth disease, Southern Vietnam, 2011. Emerg Infect Dis. 2012;18(12):2002–5 doi:10.3201/eid1812.120929. PMID:23194699.
- Yan Z, Zhen Z, Yang W, Ren J, Tan X, Yu W, Mao N, Xu S, Zhu S, Cui A. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of Hand Foot and Mouth Disease in Fuyang city of China. Virol J. 2010;7(1):94–102 doi:10.1186/1743-422X-7-94. PMID:20459851.
- Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao R, He Y, Bu G, Zhou S, Wang J. Enterovirus 71 Outbreak in the People's Republic of China in 2008. J Clin Microbiol. 2009;47(7):2351–2 doi:10.1128/JCM.00563-09. PMID:19439545.
- Li YP, Liang ZL, Gao Q, Huang LR, Mao QY, Wen SQ, Liu Y, Yin WD, Li RC, Wang JZ. Safety and immunogenicity of a novel human Enterovirus 71 (EV71) vaccine: a randomized, placebo-controlled, double-blind, Phase I clinical trial. Vaccine. 2012;30(22):3295–303 doi:10.1016/j.vaccine.2012.03.010. PMID:22426327.
- Li YP, Liang ZL, Xia JL, Wu JY, Wang L, Song LF, Mao QY, Wen SQ, Huang RG, Hu YS. Immunogenicity, safety, and immune persistence of a novel inactivated human enterovirus 71 Vaccine: A Phase II, randomized, double-blind, placebo-controlled trial. J Infect Dis. 2014;209(1):46–55 doi:10.1093/infdis/jit429. PMID:23922377.
- Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. New Engl J Med. 2014;370(9):818–28 doi:10.1056/NEJMoa1304923. PMID:24571754.
- Li JX, Song YF, Wang L, Zhang XF, Hu YS, Hu YM, Xia JL, Li J, Zhu FC. Two-year efficacy and immunogenicity of Sinovac Enterovirus 71 vaccine against hand, foot and mouth disease in children. Expert Rev Vaccines. 2016;15(1):129–37 doi:10.1586/14760584.2016.1096782. PMID:26460695.
- Liu L, Mo Z, Liang Z, Zhang Y, Li R, Ong KC, Wong KT, Yang E, Che Y, Wang J. Immunity and clinical efficacy of an inactivated enterovirus 71 vaccine in healthy Chinese children: a report of further observations. BMC Med. 2015;13(1):1–10 doi:10.1186/s12916-015-0448-7. PMID:25563062.
- Dai B, Chen ZH, Liu QC, Wu T, Guo CY, Wang XZ, Fang HH, Xiang YZ. Duration of immunity following immunization with live measles vaccine: 15 years of observation in Zhejiang Province, China. Bull World Health Organ. 1991;69(4):415–23 PMID:1934235.
- Musher DM, Manoff SB, Liss C, Mcfetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201(4):516–24 doi:10.1086/649839. PMID:20092407.
- Fraser C, Tomassini JE, Xi L, Golm G, Watson M, Giuliano AR, Barr E, Ault KA. Modeling the long-term antibody response of a human papillomavirus (HPV) virus-like particle (VLP) type 16 prophylactic vaccine. Vaccine. 2007;25(21):4324–33 doi:10.1016/j.vaccine.2007.02.069. PMID:17445955.
- Li J, Liang Y, Lin HM, Zhang ZY, Che Y, Liao Y, Sheng JF, Zhao ZM, Cui PF, Long RX, et al. Dynamic monitoring of anti-poliovirus neutralizing antibody and anti-hepatitis A virus IgG antibody in healthy children immunized with inactivated enterovirus 71 vaccine (human diploid cell). Chin J Micro Immun. 2017;37(4):290–6. (in Chinese)
- Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381(9882):2024–32 doi:10.1016/S0140-6736(13)61049-1. PMID:23726161.
- Li Y, Zhu SL, Liu YM, Li RC, Yin DP, Xia W, Li YP, Qi ZJ, Wang DY, Li XL. Enterovirus type 71 neutralizing antibody levels among children 0–15 years old in four counties of Guangxi Zhuang Autonomous region. Chin J Vacc and Immun. 2015;21(3):273–7. (in Chinese)
- Li ZF, Liu TX, Yang GP, Xia XT. Serum epidemiology survey of EV71 in children in Lipu county of Guangxi province in 2010. J Appl Prev Med. 2013;19(5):281–3. (in Chinese)
- Ding Q, Zhu FC, LI L, Li DH, Liu YM. Serum epidemiology survey of HEV 71 among children in Ganyu county. J J Prev Med. 2011;22(5):4–6. (in Chinese)
- Ooi EE, Phoon MC, Ishak B, Chan SH. Seroepidemiology of human enterovirus 71, Singapore. Emerg Infect Dis. 2002;8(9):995–7 doi:10.3201/eid0809.010397. PMID:12194783.
- Chang ZR, Liu FF, Lv B, Wang ZC, Zeng LJ, Li ZJ, Liao QH. Analysis on surveillance data of hand, foot and mouth disease in China, January-May. Dis Surveil. 2017;32(6):447–52. (in Chinese)
