ABSTRACT
The role of maternal vaccination in reducing neonatal morbidity and mortality is expanding but uptake remains suboptimal. While the barriers to uptake have been well described, women from minority groups have not been well represented in previous studies. In this study we examine the facilitators and barriers to uptake of antenatal vaccination by women from culturally and linguistically diverse backgrounds in Melbourne, Australia. 537 women attending antenatal care completed a survey; 69% were born overseas. 63% had or intended to receive pertussis vaccine and 57% had or intended to receive influenza vaccine during their pregnancy. On multivariable analysis, predictors of uptake of pertussis vaccine were healthcare provider recommendation (OR 10, 95% CI 5–21, p < 0.001) and belief maternal pertussis vaccination is safe (OR 36, 95% CI 18–70, p < 0.001). For influenza vaccine, predictors of uptake were previous receipt of influenza vaccine (OR 8, 95% CI 5–15, p < 0.001) and healthcare provider recommendation (OR 30, 95% CI 16–56, p < 0.001). Lack of healthcare provider recommendation was the main reason for non-vaccination (17/46, 37%). While most women were aware of and intended to receive recommended vaccinations, recently arrived migrant women (resident in Australia for less than two years) were less likely to be aware of pertussis vaccine (15/22, 68% vs 452/513, 88%, p = 0.01) and less likely to believe it to be safe during pregnancy (4/22, 18% vs 299/514, 58%, p < 0.001). This highlights the important role of healthcare providers in recommending and educating women, particularly newly arrived migrant women, in their decisions about vaccination during pregnancy.
Introduction
Vaccination of pregnant women to protect their newborns from infectious diseases is not a new strategy. Tetanus vaccination has been recommended to eliminate maternal and neonatal tetanus for more than 30 years.Citation1 The potential benefit of a maternal immunisation strategy has subsequently been recognised for other infections such as Bordetella pertussis and influenza virus, and in the future may include Group B streptococcus and respiratory syncytial virus.Citation2
In recent years, maternal pertussis and influenza vaccination have been widely implemented in high-income countries. Pertussis vaccination during pregnancy using the adult combined diphtheria-tetanus-acellular pertussis vaccine (dTPa) has been demonstrated to reduce pertussis infections by 91% in infants less than three months of age,Citation3,Citation4 and maternal influenza vaccination to reduce laboratory-confirmed influenza by two thirds in infants under six months of age.Citation5 In Australia, influenza vaccine has been funded for all pregnant women during the influenza season, irrespective of gestation, since 2010. Government funded maternal pertussis vaccination has been more recently introduced, with Victoria (the jurisdiction where this study took place) introducing this in 2015. National guidelines recommend maternal pertussis vaccination between 28 and 32 weeks in each pregnancy.Citation6
Despite evidence supporting the effectiveness and safety of pertussis and influenza vaccination during pregnancy, uptake varies considerably with reported rates of 14–75% in the United States (US),Citation7-9 30–60% in the United Kingdom (UK),Citation3,Citation10-12 and 26–70% in series from Australia.Citation13-15
Understanding the barriers and facilitators that contribute to such variability in uptake is central to implementing a successful and effective vaccination program. Most studies on women's attitudes toward maternal vaccination pertain to influenza vaccine given the recommendation for pertussis is more recent. In addition, women who did not converse or read in the dominant language have frequently been excluded from published studies. Of minority groups, the experiences of Black and Hispanic women in the US and UK have been describedCitation11,Citation16,Citation17 but pertinent to the Australian context, the experiences of women from Asia and recently arrived migrants and refugees are not as well understood. In this study we aim to address these knowledge gaps by surveying women attending for pregnancy care in a public healthcare network known to service a culturally diverse population.
Results
Between September and December 2016, 537 surveys were completed. 370/537 (69%) women were born overseas from 62 different countries. The majority (275/370, 74%) of overseas-born women were from Asia; 161/370 (43.5%) from South Asia, 64/370 (17%) from Southeast Asia, 45/370 (12%) from East Asia. 31/370 (8%) women were born in New Zealand and the Pacific Islands and the remaining 69/370 overseas-born women hailed from various other regions. The most common countries of birth were India (71/537, 13%), Afghanistan (54/537, 10%), China (38/537, 7%), Vietnam (26/537, 5%), and New Zealand (18/537, 3%). The majority (209/370, 57%) of overseas-born women had lived in Australia for more than five years but 138/370 (37%) had resided in Australia for 2–5 years and 22/370 (6%) for less than 2 years. Eleven (2%) of women identified as Aboriginal or Torres Strait Islander.
342/537 (64%) of women spoke a language other than English (LOTE) at home with the most common languages being Dari, Mandarin, Punjabi, Vietnamese, Hindi, Khmer and Malayalam. These top seven languages accounted for 53% (180/342) of women speaking a LOTE. While only seven women (2%) elected to use translated surveys, one quarter (85/335) completed the survey using an interpreter.
presents demographic and pregnancy characteristics of women by whether they were Australian- or overseas-born. Women born overseas tended to be older and more likely to have a university qualification. There was no difference however in employment status. In terms of the current pregnancy, Australian- and overseas-born women did not differ in terms of gravidity or gestation at the time of completing the survey (mean 29 weeks, SD 7). However there was a significant difference in the number of antenatal visits Australian-born and overseas-born women had prior to completing the survey. Of 167 Australian-born women 142 (85%) had had at least two prior visits, 17 (10%) 1–2 prior visits, and 8 (5%) no prior visits. In the 370 overseas-born women, 291 (79%) had had at least 2 prior visits, 74 (20%) 1–2 visits, and 5 (1%) none (p = 0.002).
Table 1. Participant characteristics.
Knowledge of antenatal vaccines
Overseas born women were significantly less likely to have heard of dTpa than Australian-born women (86% vs 93%, p < 0.001) (). Furthermore there was marked variation when explored by region of birth. Women from Cambodia (5/10, 50%), Pakistan (5/13, 38.5%), Sudan (4/12, 33%), and Afghanistan (16/54, 30%) were the least likely to have heard of dTpa. Women were significantly more likely to have heard of dTPa if they were older than 25 years (p = 0.004), spoke English as their first language (p < 0.001), had lived in Australia for more than two years (p = 0.01), if they had completed more than primary school education (p < 0.001), were employed (p < 0.001), and were multiparous (p = 0.006). Awareness of influenza vaccine (IIV) was greater and did not differ by any of the variables examined. ()
Table 2. Knowledge of maternal vaccines.
Women derived knowledge of antenatal vaccines from a variety of sources. They reported hearing about vaccines from midwives (56% for dTpa and 45.5% for IIV) and general practitioners (44.5% for dTpa and 68.5% for IIV) more than obstetricians (24% for dTpa and 14% for IIV). Compared to IIV, more women reported hearing about dTpa through posters and information displayed in antenatal clinics (18% vs 4%), public health messages (18% vs 8%), within social circles (37% vs 27%) and on the internet (16% vs 6%). Conversely more women were aware of IIV through their workplace (12% vs 2%).
Despite recalling a recommendation from an obstetrician less often, more than a third of women (196/537, 36.5%) placed most trust in their obstetrician for vaccine advice. 30% reported trusting GPs most for vaccine advice, and 28.5% their midwife. Women reported less trust in the internet (0.4%) and family and friends (1%) compared to their maternity care providers.
Approximately 40% of women were unsure whether antenatal dTpa was safe for themselves or their baby. Factors associated with belief that maternal dTpa is safe for themselves of their baby were: age greater than 25 years (61% vs 46%, p = 0.003), being Australian-born (65% vs 53%, p = 0.008), residence in Australia longer than two years (90% vs 71%, p = 0.02), speaking English at home (64% vs 52%, p = 0.01), more than primary school education (61% vs 36%, p < 0.001), being employed (66% vs 47%, p < 0.001), having heard of (65% vs 0%, p < 0.001) or received dTpa previously (85% vs 8%, p < 0.001) and receiving a healthcare provider (HCP) recommendation for dTpa (76% vs 13%, p < 0.001).
Uptake
Overall 339/537 (63%) of women reported having already received or intention to receive dTpa during their pregnancy. Of the 204 women beyond 32 weeks gestation, 124 (61%) had been vaccinated against pertussis. A further 22/80 (27.5%) intended to be vaccinated prior to delivery of whom three were already 38 weeks gestation.
On univariable analysis, factors associated with uptake (already or intended) of dTpa included age greater than 25 years, birth in regions other than Southern Asia, if overseas born living in Australia more than five years, speaking English as first language, completing more than primary school education, and being employed. In addition having heard of, previous receipt of, receiving a HCP recommendation for and believing dTpa is safe during pregnancy were significantly associated with uptake. On multivariable analysis, uptake of dTpa was significantly and strongly associated with receiving a HCP recommendation (OR 10, 95% CI 5–21, p < 0.001) and belief that the vaccine is safe during pregnancy (OR 36, 95% CI 18–71, p < 0.001). ()
Table 3. Univariable and multivariable analyses of uptake of maternal pertussis and influenza vaccines.
The survey commenced at the end of the 2016 influenza season in the southern hemisphere. 279/489 (57%) of women reported receiving an IIV during their current pregnancy. None of the women in the first trimester reported receiving an IIV, 63/159 (40%) in the second trimester and 189/317 (60%) in the third trimester.
On univariable analysis, factors associated with uptake of IIV were living in Australia for more than five years (if overseas-born), university education, being in the third trimester of pregnancy, and having heard of, previously received or reporting a HCP recommendation for IIV. On multivariable analysis university education, previous receipt of IIV and a HCP recommendation for IIV remained significantly associated with uptake. ()
Reasons for vaccination/ non-vaccination
Vaccination to protect their baby was the most common motivation reported by women for both vaccines ().
Figure 1. Reasons for vaccination/ non-vaccination Abbreviations: dTpa: Pertussis-containing vaccine (diphtheria, tetanus and acellular pertussis) IIV: Inactivated influenza vaccine HCP: Healthcare provider.
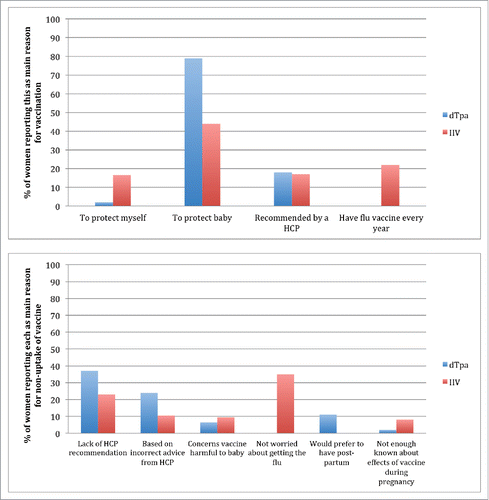
The primary reason cited for not intending dTpa vaccination was lack of HCP recommendation by 17/46 (37%) of these women. Women born overseas were significantly less likely to recall a HCP recommendation for dTpa than Australian-born women. () 25/537 (5%) of women reported that a HCP advised them not to have dTpa during pregnancy; 19/25 (76%) of women by a GP, and three each by a midwife or obstetrician. Among women not vaccinated against influenza, lack of concern about contracting influenza infection during pregnancy was reported by 33/95 (35%) and lack of HCP recommendation by 22/95 (23%). ()
A proportion of women were declining vaccines based on inaccurate advice from their HCP: 11/46 (24%) for dTpa and 10/95 (10.5%) for IIV. () Women in this category reported receiving advice to have pertussis vaccine post-partum rather than antenatally, that they retained adequate immune responses from previous vaccination, that it was too late in pregnancy for the vaccine to be administered or that the vaccination was not safe during pregnancy.
Discussion
We examined the attitudes to and knowledge of maternal vaccination of a large number of ethnically diverse women in an urban Australian setting. 70% of women surveyed were overseas-born, predominantly from Asia, representing the largest study published to date of pregnant women's attitudes to antenatal vaccination from this region. The key findings were (1) the majority of women had heard of both pertussis and influenza vaccines but women from culturally and linguistically diverse (CALD) backgrounds were less likely to be aware of and more likely to have concerns about the safety of dTpa in pregnancy; (2) a third of women did not recall receiving a HCP recommendation for either dTpa or IIV during their pregnancy and HCP recommendation was significantly less likely to be recalled by women born overseas; (3) a significant proportion of women declining vaccination appeared to be doing so based on incorrect advice from their HCP (4) Uptake of dTpa and IIV did not differ between Australian-born or women from CALD backgrounds but remains suboptimal. On multivariable analysis factors significantly associated with uptake of dTpa were HCP recommendation and belief in the safety of the vaccine during pregnancy and for IIV, HCP recommendation and previous receipt of IIV.
While most women in our study were aware of influenza and pertussis vaccines, this varied considerably amongst ethnic groups (30% in women from Afghanistan compared with 93% in women from East Asia). This highlights that women from CALD backgrounds should not be regarded as a homogenous entity and consideration for the education and health literacy of each woman is important.
Two in five women overall but half of overseas-born women did not believe antenatal dTpa was safe for themselves or their baby. In addition, women resident in Australia for less than two years were less likely to believe in the safety of dTpa during pregnancy. Numerous studies have found an association between belief in the safety of vaccination during pregnancy and uptake.Citation2,Citation17,Citation18 HCP play a crucial role in addressing safety. Our study suggests this may be of particular importance for women who have more recently arrived from overseas countries and may therefore not have had access to information about the safety of maternal vaccination previously. Maternal vaccination in low- and middle- income countries focuses on tetanus and sometimes influenza but pertussis is rarely included in antenatal guidelines. This may account for less awareness and belief in safety of dTpa amongst women from these countries even if they have received pregnancy care in their country of origin previously.
As has been reported previously, women place trust in their HCP to provide information on vaccination during pregnancy but one third of women did not recall any HCP recommendation for either vaccine. Women from CALD backgrounds were even less likely to recall a recommendation. More than 80% of both Australian- and overseas-born women had had at least two antenatal appointments prior to completing the survey and therefore lack of contact or time with HCP was not likely to have contributed significantly to this. While recall bias and difficulties with language may contribute to these findings, they nevertheless suggest room for improvement as HCP recommendation has consistently been demonstrated to be a key driver of vaccine uptake.Citation11,Citation16,Citation19-21 It is incumbent on HCP to engage women from CALD backgrounds in timely, evidence-based, and culturally appropriate discussions about indications for, and safety of vaccines during their routine pregnancy care.
Also concerning was the number of women who reported declining vaccination based on incorrect advice from their HCP. This study commenced six months after the change to maternal dTpa recommendations and as described not all HCP were fully across the changes. HCP concern about safety of antenatal vaccination may also have contributed to inappropriate recommendation. While it is the duty of each HCP to keep abreast of guidelines, this also highlights the challenges faced by health departments in disseminating new information to such a diverse range of HCP.
Uptake of maternal vaccination amongst CALD women may be hampered by lack of familiarity with health services, language barriers and lack of interpreters, and competing priorities particularly for those who have only recently arrived.Citation22 Standing orders for vaccination within pregnancy care settings have been demonstrated to increase uptakeCitation23-25 and may be particularly useful for CALD women. By enabling vaccination during routine pregnancy care, standing orders would negate women having to navigate multiple healthcare services. In addition, given that intepreters are already engaged for the antenatal appointment, they could then also be utilised in the discussion and to consent women for vaccination by their antenatal care provider.
The strengths of our study are the large sample size and inclusion of women from a diverse range of backgrounds. Most studies published to date pertain to Black or HispanicCitation11,Citation16,Citation17 women who do not make up a large proportion of the population in Australia. Our survey captures the experience of women from Asia and Sub-Saharan Africa, which has been missing from the narrative until now. As women had access to a funded vaccination program cost could be excluded as a barrier. In addition by surveying women antenatally rather than post-partum the results are less likely to be influenced by recall bias.
There are several limitations of the study that need to be acknowledged. This was a study in a metropolitan university-affiliated healthcare network, women were relatively well educated and 50% were employed and therefore results may not be generalisable to other contexts. While we surveyed women from a large number of countries, this meant that there was only a small number to make inferences about each country. We were unable to confirm self-reported uptake due to the large number of immunisation providers and lack of a state-wide immunisation register at the time of the study. Finally, given that women of all gestations were surveyed, we included intention to be vaccinated, which may not equate with actually receiving vaccine and thereby may overestimate uptake.
Women from CALD backgrounds have been under-represented in maternal immunisation research to date. This study, conducted in a resource rich setting, focused primarily on these women's attitudes towards and uptake of maternal vaccines, without confounding by cost or access to vaccine. While in our study there was no difference in uptake, women born overseas, whose first language was not English and who had migrated less than two years earlier were less likely to have heard of pertussis-containing vaccines and were less likely to receive a HCP recommendation for vaccination. They were also more likely to have concerns about safety. Given HCP recommendation has consistently been demonstrated to be the most important factor contributing to uptake, and concerns about safety consistently reported as a barrier to uptake, new approaches to these areas with a focus on CALD women needs to be addressed.
Patients and methods
We recruited a convenience sample of pregnant women attending for antenatal care at Monash Health, Melbourne, Australia between September and December 2016. Monash Health is the largest public hospital network in Melbourne, providing maternity care to over 10 000 women per year across three hospitals. In Australia pregnant women are eligible for government-funded dTpa and IIV removing cost as a barrier to uptake.
All women attending for antenatal care were eligible. Researchers approached them in the waiting room and they were invited to complete an online or paper-based survey prior to their antenatal clinic appointment. The survey included information on demographics, pregnancy, attitudes towards and pregnancy care provider recommendation of whooping cough and flu vaccines during their pregnancy. The primary outcome of interest was uptake (already occurred or intended) of both vaccines. Secondary outcomes of interest were awareness of the vaccines and beliefs about safety of dTpa during pregnancy. The survey was translated into Dari, Vietnamese and Mandarin, the three most common languages requiring use of interpreting services in our antenatal clinics. Women whose first language was not English were able to complete one of the translated surveys where applicable or offered the use of an interpreter when available.
Statistical analysis was performed using Stata for Windows 14.2 (College Station, Texas). Differences between proportions was determined using Fisher's exact or Pearson chi-square tests. Logistic regression models were used to determine factors associated with uptake of vaccines. Independent variables were included if they answered the study question about the relationship between awareness of vaccines and uptake. Statistical significance was defined as p < 0.05.
Details of ethics approval
The study was approved by Monash Health and Monash University Human Research Ethics Committees on 26th July 2016 (Ref: 16254L) and 5th September (Ref: 0840).
Disclosure of potential conflicts of interest
SK received a Glaxo Smith Kline Small Project Grant for this research. The remaining authors report no conflicts of interest.
Acknowledgments
The authors wish to thank Karen Bellamy, Monash Health for her assistance with recruiting, and the women who so willingly participated.
Additional information
Funding
References
- Wold Health Organization. Maternal and Neonatal Tetanus Elimination 2017. [Available from: http://www.who.int/immunization/diseases/MNTE_initiative/en/.
- Wilson RJ, Paterson P, Jarretta C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: A literature review. Vaccine. 2015;33:6420–9. doi:10.1016/j.vaccine.2015.08.046. PMID:26320417.
- Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. Effectiveness of maternal pertussis vaccination in England: an observational study. The Lancet. 2014;384(9953):1521–8. doi:10.1016/S0140-6736(14)60686-3.
- Dabrera G, Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Fry NK, Ramsay M. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012–2013. Clin Infect Dis. 2015;60(3):333–7. doi:10.1093/cid/ciu821. PMID:25332078.
- Zaman K, Roy E, Arifeen S, Rahman P, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF. Steinhoff MC effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi:10.1056/NEJMoa0708630. PMID:18799552.
- Australian Technical Advisory Group on Immunisation (ATAGI). The Australian immunisation handbook 10th ed (2015 update). Council NHaMR, editor. Canberra: Australian Government Department of Health and Ageing; 2015.
- Koepke R, Schauer SL, Davis JP. Measuring maternal Tdap and influenza vaccination rates: Comparison of two population-based methods. Vaccine. 2017;35(18):2298–302. doi:10.1016/j.vaccine.2017.03.024. PMID:28341114.
- Ding H, Black C, Ball S, Donahue S, Fink R, Williams W, Kennedy ED, Bridges CB, Lu PJ, Kahn KE, et al. Flu Vaccination Coverage Among Pregnant Women – United States, 2015–16 Flu Season Centers for Disease Control and Prevention; 2016 [updated November 2 2017]. Available from: https://www.cdc.gov/flu/fluvaxview/pregnant-coverage_1516estimates.htm - data (accessed November 30 2017).
- Butler AM, Layton JB, Li D, Hudgens MG, Boggess KA, McGrath LJ, Weber DJ, Becker-Dreps S. Predictors of low uptake of prenatal tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis immunization in privately insured women in the United States. Obstet Gynecol. 2017;129(4):629–37. doi:10.1097/AOG.0000000000001927. PMID:28277354.
- Winslade CG, Heffernan CM, Atchison CJ. Experiences and perspectives of mothers of the pertussis vaccination programme in London. Public Health. 2017;146:10–4. doi:10.1016/j.puhe.2016.12.018. PMID:28404461.
- Donaldson B, Jain P, Hodler BS, Lindsay B, Regan L, Kampmann B. What determines uptake of pertussis vaccine in pregnancy? A cross sectional survey in an ethnically diverse population of pregnant women in London. Vaccine. 2015;33(43):5822–8. doi:10.1016/j.vaccine.2015.08.093. PMID:26409139.
- Public Health England. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/615939/Weekly_national_influenza_report_week_21_2017.pdf: Public Health England; 2017 [Week 221 report]
- Andrews R. Maternal Immunisation in Australia- how are we going? South Australian Vaccinology Update Conference; 2015; Adelaide, Australia.
- Regan AK, Mak D, Gibbs R, Effler P. Uptake of pertussis and influenza vaccines among pregnant women in Western Australia. Public Health Association Australia 15th National Immunisation Conference; 2016; Brisbane, Australia.
- McHugh L, Andrews RM, Lambert SB, Viney KA, Wood N, Perrett KP, Marshall HS, Richmond P, O'Grady KF. Birth outcomes for Australian mother-infant pairs who received an influenza vaccine during pregnancy, 2012–2014: The FluMum study. Vaccine. 2017;35:1403–9. doi:10.1016/j.vaccine.2017.01.075. PMID:28190746.
- Kriss JL, Frew PM, Cortes M, Malik FA, Chamberlain AT, Seib K, Flowers L, Ault KA, Howards PP, Orenstein WA, et al. Evaluation of two vaccine education interventions to improve pertussis vaccination among pregnant African American women: A randomized controlled trial. Vaccine. 2017;35(11):1551–8. doi:10.1016/j.vaccine.2017.01.037. PMID:28216190.
- Chamberlain AT, Seib K, Ault KA, Orenstein WA, Frew PM, Malik F, Cortés M, Cota P, Whitney EAS, Flowers LC, et al. Factors associated with intention to receive influenza and tetanus, diphtheria, and acellular pertussis (Tdap) vaccines during pregnancy: A focus on vaccine hesitancy and perceptions of disease severity and vaccine safety. PLOS Curr Outbreaks. 2015. 1st Edition. doi:10.1371/currents.outbreaks.d37b61bceebae5a7a06d40a301cfa819.
- Taksdal SE, Mak DB, Joyce S, Tomlin S, Carcione D, Armstrong PK, Effler PV. Predictors of uptake of influenza vaccination. Aust Fam Physician. 2013;42(8):582–6. PMID:23971070.
- Laenen J, Roelants M, Devlieger R, Vandermeulen C. Influenza and pertussis vaccination coverage in pregnant women. Vaccine. 2015;33(18):2125–31. doi:10.1016/j.vaccine.2015.03.020. PMID:25796339.
- Reynolds G, Grant N, Thornley S, Hale M. Low uptake of maternal vaccination in notified pertussis cases aged less than 20 weeks. N Z Med J. 2017;130(1449):72–4. PMID:28178735.
- Ditsungnoen D, Greenbaum A, Praphasiri P, Dawood FS, Thompson MG, Yoocharoen P, Lindblade KA, Olsen SJ, Muangchana C. Knowledge, attitudes and beliefs related to seasonal influenza vaccine among pregnant women in Thailand. Vaccine. 2016;34(18):2141–6. doi:10.1016/j.vaccine.2016.01.056. PMID:26854910.
- Kpozehouen E, Heywood AE, Kay M, Smith M, Paudel P, Sheikh M, MacIntyre CR. Improving access to immunisation for migrants and refugees: recommendations from a stakeholder workshop. Aust N Z J Public Health. 2017;41(2):118–20. doi:10.1111/1753-6405.12602. PMID:27868296.
- Webb H, Street J, Marshall H. Incorporating immunizations into routine obstetric care to facilitate health care practitioners in implementing maternal immunization recommendations. Hum Vaccin Immunother. 2014;10(4):1114–21. doi:10.4161/hv.27893. PMID:24509790.
- Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clin Infect Dis. 2011;52(2):157–62. doi:10.1093/cid/ciq001. PMID:21288837.
- Krishnaswamy S, Wallace EM, Buttery J, Giles ML. Strategies to implement maternal vaccination: A comparison between standing orders for midwife delivery, a hospital based maternal immunisation service and primary care. Vaccine. 2018: pii: S0264-410X(18)30021-5. In Press. doi:10.1016/j.vaccine.2017.12.080. PMID:29395531.
