Introduction
Hepatitis B virus (HBV) infection is still a global health problem, with an estimated 248 million individuals worldwide that are persistently infected with HBV,Citation1 if without timely and effective treatment, about 25%-40% of whom would develop to liver cancer, cirrhosis or other HBV related end-stage liver diseases.Citation1, Citation2 Several studies have reported that HBV DNA and HBsAg are critical for predicting long-term outcomes of chronic HBV infection. Cohort data have shown that higher HBV DNA level is associate with higher Hepatocellular carcinoma (HCC) risk.Citation3 Meanwhile, a higher HBsAg level (≥1000 IU/mL) is associated with a higher risk of HCC in HBeAg-negative patients with low viral loads, and HBsAg level helps predict HCC risk in patients with intermediate viral loads.Citation4 The approved anti-HBV drugs, including interferon or nucleos(t)ide analogues, rare to achieve the ideal end point for anti-HBV therapy and have limited efficacy on blockade of HCC development.Citation2 Our previously study reported a unique antibody, named as E6F6, that recognizes HBsAg-aa119-125 and capable of prolonged suppression of HBsAg and HBV DNA levels in mice.Citation5 In this study, we evaluate the inhibitory effect of E6F6 on the development of HCC in HBV transgenic mice.
Materials and methods
The HBV-Tg mice were provided by Professor Pei-Jer Chen (National Taiwan University College of Medicine, Taipei, Taiwan) and maintained under specific pathogen-free conditions in the Laboratory Animal Centre of Xiamen University. The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals.
For tumor induction in HBV-Tg mice, males were treated with a low dose (2 mg kg−1) of DEN (Sigma, St. Louis, MO) at 2-week after birth as previously described.Citation6 This model closely resembles human HBV-induced HCC because chronic HBV carriers who are exposed to dietary carcinogens such as DEN and aflatoxins have a higher incidence of HCC. In this study, 28 DEN-treated male HBV-Tg mice were randomly assigned into two groups and treated with E6F6 (n = 14) or IgG (16G12, n = 14) as a control at 20 mg kg−1 every 2 weeks since the 6th week of age. This therapy lasted 30 weeks, and all animals received a total of 16 infusions of mAbs. Liver tumours were detected using 2D T2-weighted rapid-acquisition MRI. Relaxation enhancement (RARE) sequence images of the tumours were acquired using a 9.4-T dedicated animal MRI system (Bruker Biospin, Ettlingen, Germany). The images were exported, and the tumour volumes were measured using the ImageJ software package.
Results
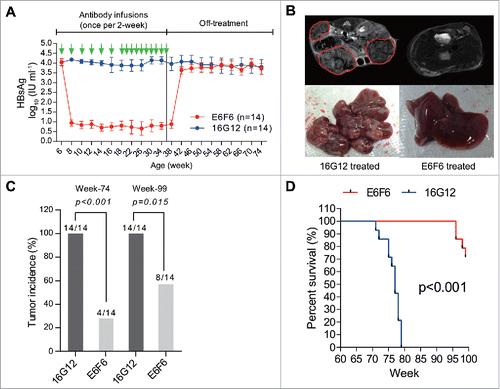
To examine whether persistent viraemia suppression by E6F6 can block HBV-induced hepatocarcinogenesis, male HBV-Tg mice were injected with a low dose of DEN at 2 weeks after birth and treated with E6F6 or 16G12 every 2 weeks starting from the 6th week after birth, the liver tumour in this mouse model was believed to be contributed by HBV, because wild-type mice treated with the same dose of DEN did not develop HCC.Citation6 All animals received a total of 16 infusions of mAbs. During the treatment period, E6F6 persistently suppressed the serum HBsAg to nearly undetectable levels, whereas control isotype IgG (16G12) did not induce significant changes (). After treatment discontinuation at week 36, the average serum HBsAg of the E6F6 group rebounded to a level that was similar to that of the 16G12 group after weeks 42. During follow-up observations, we performed magnetic resonance imaging (MRI) on surviving animals or autopsies on dead animals to examine liver tumour ( and Figure S1) at weeks 74 and 99. At weeks 74, liver tumors were detectable in all of 14 mice in the control group, whereas only 4 mice in the E6F6-treated group had tumors. In contrast to the placebo 16G12 (), E6F6 treatment significantly reduced the incidence of liver tumour at both weeks 74 (p<0.001) and 99 (p = 0.015). More importantly, as indicates, E6F6 greatly improved the lifespan of DEN-treated HBV-Tg mice.
Figure 1. E6F6 suppresses HBsAg and reduces hepatocarcinogenesis in HBV-Tg mice. (A) Serum HBsAg levels of DEN-induced HBV-Tg mice that received multiple mAb infusions. Twenty-eight male HBV-Tg mice were injected with a low dose (2 mg kg−1) of DEN 2 weeks after birth. Then, these animals were randomly assigned into two groups and treated with E6F6 (n = 14) or received a control treatment of IgG (16G12, n = 14) at 20 mg kg−1 every 2 weeks since the 6th week of age. This therapy lasted 30 weeks, and all animals received a total of 16 infusions of mAbs. (B) Representative axial 2D T2-weighted MRI images (top panel) and gross observations (bottom) of E6F6- or 16G12- treated mouse livers. The red lines highlight the tumours. Detailed MRI images for each enrolled mouse are presented in Figure S1. (C) The tumour incidence of animals after antibody treatment at weeks 74 and 99. P-values were calculated using a two-sided Fisher's exact test. (D) Survival curves of DEN-induced HBV-Tg mice treated with E6F6 and 16G12. Significance tests were conducted using the Cox-Mantel algorithm.
Discussion
In our previous study, we described the virus-clearance efficacies of antibodies primarily depend on their binding epitopes, E6F6-like antibodies, which recognize the evolutionarily conserved sA epitope, had more-profound and more-durable viral suppression effects than those that bind to other epitopes.Citation5 Among all mAbs, E6F6 exhibited the strongest suppression of the levels of HBV DNA and HBsAg: for the HBV-Tg mice, the levels decreased by more than 2 log10 for a time period of greater than 20 days.Citation5 Our results revealed that E6F6 mediated viral clearance through Fcγ-receptor-dependent phagocytosis. Moreover, E6F6-based immunotherapy could restore the HBV specific T cell response in HBV carrier mice. The most important advantage of E6F6 is its ability to greatly reduce the HBsAg level, but current approved drugs fail to do. Because a high level of HBsAg can exhaust HBsAg-specific T-cell response and is proposed as an important factor for viral immunotolerance in patients with chronic hepatitis B7, the lower HBsAg level predicts better outcomes.
In this study, we revealed the consecutive E6F6 treatments effectively reduced HBV-induced hepatocarcinogenesis in DEN-treated HBV-Tg mice. To our knowledge, this is the first report to indicate that antibody-mediated immunotherapy can block the HBV-induced tumour development. The anti-tumor effect of E6F6 treatment is likely contributed by the suppression of HBsAg in both peripheral blood and hepatocyte since the high expression of HBsAg in transgenic mice promotes the development of liver cancer.Citation8 Moreover, activating Fcγ receptors associated immunomodulation, which are induced by immune complex, might also partially contribute to E6F6-mediated anti-tumour effect.Citation9
Taken together, multiple-doses administration of E6F6 not only can effectively inhibit HBV viremia, but also can significantly reduce the incidence of HCC in mice. Thus, this E6F6 mAb-based immunotherapy, alone or in combination with current anti-viral therapies, may provide a promising pathway for the treatment of chronic hepatitis B.
Disclosure of potential conflicts of interest
None declared.
KHVI_A_1449553_Supplemental.pdf
Download PDF (377.7 KB)Funding
National Natural Science Foundation of China (81672023, 81702006), China Postdoctoral Science Foundation (174792) and Xiamen University President Fund Project (20720160063) support this work.
References
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55. doi:10.1016/S0140-6736(15)61412-X. PMID:26231459.
- Liaw YF, Chu CM. Hepatitis B virus infection. Lancet. 2009;373:582–92. doi:10.1016/S0140-6736(09)60207-5. PMID:19217993.
- Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH; REVEAL-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006;295:65–73. doi:10.1001/jama.295.1.65. PMID:16391218.
- Tseng TC, Liu CJ, Chen CL, Yang HC, Su TH, Wang CC, Yang WT, Kuo SF, Liu CH, Chen PJ, et al. Risk stratification of hepatocellular carcinoma in hepatitis B virus e antigen-negative carriers by combining viral biomarkers. J Infect Dis. 2013;208:584–93. doi:10.1093/infdis/jit209. PMID:23656977.
- Zhang TY, Yuan Q, Zhao JH, Zhang YL, Yuan LZ, Lan Y, Lo YC, Sun CP, Wu CR, Zhang JF, et al. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut. 2016;65:658–71. doi:10.1136/gutjnl-2014-308964. PMID:26423112.
- Zheng Y, Chen WL, Louie SG, Yen TS, Ou JH. Hepatitis B virus promotes hepatocarcinogenesis in transgenic mice. Hepatology (Baltimore, Md). 2007;45:16–21. doi:10.1002/hep.21445. PMID:17187428.
- Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris). 2010;58:258–66. doi:10.1016/j.patbio.2009.11.001. PMID:20116937.
- Huang SN, Chisari FV. Strong, sustained hepatocellular proliferation precedes hepatocarcinogenesis in hepatitis B surface antigen transgenic mice. Hepatology (Baltimore, Md) 1995;21:620–6. PMID:7875658.
- Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–93. doi:10.1084/jem.20130573. PMID:23897982.
