 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: The nonavalent HPV (9vHPV) vaccine is indicated for active immunisation of individuals from the age of 9 years against cervical, vulvar, vaginal and anal premalignant lesions and cancers causally related to vaccine HPV high risk types 16, 18, 31, 33, 45, 52 and 58, and to the HPV low risk types 6 and 11, causing genital warts.
Objective: To estimate the lifetime risk (up to the age of 75 years) for developing cervical cancer after vaccinating a HPV naïve girl (e.g. 9 to 12 years old) with the 9vHPV vaccine in the hypothetical absence of cervical cancer screening.
Methods: We built Monte Carlo simulation models using historical pre-screening age-specific cancer incidence data and current mortality data from Denmark, Finland, Norway, Sweden and the UK. Estimates of genotype contribution fractions and vaccine efficacy were used to estimate the residual lifetime risk after vaccination assuming lifelong protection.
Results: We estimated that, in the hypothetical absence of cervical screening and assuming lifelong protection, 9vHPV vaccination reduced the lifetime cervical cancer and mortality risks 7-fold with a residual lifetime cancer risks ranging from 1/572 (UK) to 1/238 (Denmark) and mortality risks ranging from 1/1488 (UK) to 1/851 (Denmark). After decades of repetitive cervical screenings, the lifetime cervical cancer and mortality risks was reduced between 2- and 4-fold depending on the country.
Conclusion: Our simulations demonstrate how evidence can be generated to support decision-making by individual healthcare seekers regarding cervical cancer prevention.
Introduction
Human papillomavirus (HPV) is the most common viral infection of the reproductive tract. It is sexually transmitted and ubiquitous – most sexually active women will be infected at some point in their lives and some may be repeatedly infected.Citation1 Worldwide point prevalence is estimated at 10.4%.Citation2 Cervical cancer is caused by persistent infection with high-risk HPVCitation3 and is the fourth most common cancer in women worldwide and the second most common cancer in women 25–40 years.Citation4
Cervical cancers are largely preventable; primary prevention of infection through vaccination and secondary prevention to detect and treat pre-cancerous cervical lesions through screening. Screening impacts upon cervical cancer incidence only with no effect on the prevalence of HPV infection in the population, whereas vaccination prevents HPV infection and all stages of cervical cancer disease, from cervical pre-cancerous to invasive disease.Citation5 Vaccination also prevents the non-cervical HPV cancers.
The nonavalent HPV (9vHPV) vaccine (Gardasil9 ®, Merck/MSD), approved in Europe since June 2015,Citation6 covers the seven most common oncogenic HPV types (16/18/31/33/45/52/58) associated with cervical cancer, in addition to low-risk types 6 and 11 which are responsible for 90% of genital warts.Citation7 The 9vHPV efficacy was 97.1% [95% CI: 83.5%–99.9%] against high-grade cervical disease related to HPV-31, 33, 42, 52 and 58, based on clinical endpoints (disease, biopsy proved). The 9vHPV efficacy was similar to that of the quadrivalent HPV (4vHPV) vaccine against disease related to HPV-6, 11, 16 and 18, based on non-inferior immune response,Citation8,Citation9 with the 4vHPV efficacy being estimated to be 98.2% [95% CI: 93.5%–99.8%] against high-grade cervical disease related to HPV-16 and 18.Citation6
Among all vaccinations, HPV vaccination is the only vaccination implemented in a context where secondary prevention (cervical screening) is already in place. For at least 50 years, the main cervical cancer control measure has been secondary prevention through screening. Scandinavian countries were the earliest adopters of population-based screening in the 1960s.Citation10-12 In countries where organized cytological screening programmes are available, the incidence of squamous cell carcinoma of the cervix has declined.Citation13,Citation14 In some countries, cervical cancer screening programmes have recently shifted towards HPV DNA testing which is more sensitive, although less specific than cytology.Citation15
A vast body of literature is available on modelling the impact of screening and HPV immunization programmes on the HPV-related cancer burden at population level, accounting for both direct and indirect protection (i.e. indirect protection of non-immunized by immunized).Citation16,Citation17 Modelling studies focusing on the individual-level benefits of HPV vaccination are, to our knowledge, non-existing despite the value of such studies to inform decision-making by individual healthcare seekers.
This study aims to demonstrate how evidence can be generated to support decision-making by individual healthcare seekers regarding cervical cancer prevention. To this end, we estimated the lifetime risks for developing cervical cancer (or the probability that an individual will develop the disease during a certain age span in the absence of competing causes of death) in vaccinated and unvaccinated females in a number of European countries as well as the corresponding lifetime mortality risks. Particularly, we estimated the current lifetime risk (up to the age of 75 years) of cervical cancer and related mortality in (i) the hypothetical absence of any HPV prevention measure and in (ii) the case of 9vHPV vaccination of HPV naïve girls assuming lifelong protection (assuming booster doses will be used if required). Lifetime risks were estimated for European countries with publicly available pre-screening cancer incidence data (e.g. Denmark, Finland, Norway, Sweden and the UK). We compared these estimates with the risk reductions observed after decades of repetitive screening in countries with a high uptake of cervical cancer screening. To facilitate the communication to individual healthcare seekers, we visually present the lifetime risks.
Results
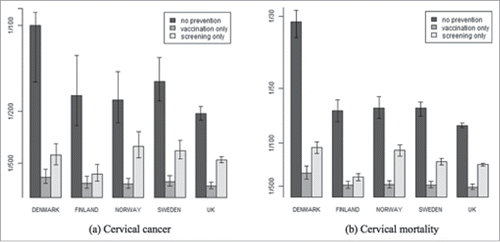
In the hypothetical scenario of no HPV prevention measures, cervical cancer lifetime risk (1/N) ranged from 1/76 (UK) to 1/31 (Denmark) and the lifetime mortality risk from cervical cancer (1/N) ranged from 1/205 (UK) to 1/117 (Denmark) (, see also Appendix A for table with lifetime risk estimates and 95% uncertainty intervals). Vaccination with 9vHPV, assuming lifelong protection, and in absence of additional prevention measures was estimated to reduce the lifetime cervical cancer risks to values ranging from 1/548 (UK) to 1/228 (Denmark) and the related lifetime mortality risk to values ranging from 1/1488 (UK) to 1/851 (Denmark). Overall, vaccination decreased the lifetime cervical cancer and related mortality risk by approximately 7-fold. The lifetime cervical cancer and the related mortality risks observed after decades of repetitive cervical screening decreased 2- to 4-fold, depending on the country (, Appendix A).
Figure 1. Lifetime risk (up to the age of 75) of cervical cancer (left) and related mortality (right) in the scenarios of either: no HPV prevention measures, after 9vHPV vaccination in the hypothetical absence of cervical cancer screening and after cervical cancer screening. The error bars reflect 95% uncertainty intervals. Risks are expressed as 1/N.
The sensitivity analyses () showed that the cervical cancer incidences based on the (country-independent) natural history model (42.5/100.000) fell in between the lowest (23.9/100.000) and the highest country-specific incidences (71.6/100.000) calculated based on the historical pre-screening data. The cervical cancer incidences derived from the CIN3 data were lower than the ones based on the historical data, likely explained by the impact of screening on the current CIN3 incidences. The cervical cancer incidences derived from Age-Period-Cohort (APC) models were comparable to the ones based on the historical data, implying that the estimated cohort effects were small.
Table 1. Cervical cancer incidence (/100.000) in the hypothetical absence of any HPV prevention measure and residual incidence in the case of 9vHPV vaccination of HPV naïve girls, in the hypothetical absence of HPV screening and assuming lifelong protection. Base-case results and results from thee different sensitivity analyses.
Discussion
This simulation study provides cervical cancer and related mortality lifetime risk estimates in the absence of any HPV prevention measures (i.e. in absence of both screening and vaccination) and after 9vHPV vaccination in the absence of screening. To our knowledge, this is the first modelling study on cervical cancer prevention from the perspective of the individual healthcare seeker as opposed to studies modelling the impact of HPV screening and immunization programmes on the disease burden at population level.Citation16,Citation17 Through Monte Carlo simulation, we estimated that the lifetime cervical cancer and related mortality risks reduced 7-fold after vaccination compared to 2- to 4-fold after decades of repetitive screening, a reduction that has not changed for several years. Although incidences were only obtained for five countries, we consider the findings regarding the lifetime risk reductions after vaccination generalizable to other geographical areas with comparable HPV infection incidence and genotype attributions.
For the conduct of this simulation study, we made several assumptions. First, we assumed lifelong vaccine protection. Although currently there is no evidence of waning immunity even 12 years after vaccination,Citation18 we assume that booster doses will be given to acquire lifelong protection if needed. Second, we focused on direct protection against vaccine-type HPV and do not account for herd protection or cross-protection as we deem the direct effects to be of the highest relevance for the perspective of the individual healthcare seeker. Third, we did not use historical mortality data to calculate the mortality risk in the hypothetical absence of screening as treatment for cervical cancer has substantially improved over the years.Citation19 Instead, we derived the mortality ratio using current (year 2012) cervical cancer incidence and mortality data, and applied this ratio to historical pre-screening cervical cancer incidences. This approach may have underestimated the mortality risk: without screening, cancers not prevented by the vaccine will be detected later and their treatment might be less successful, leading to more deaths. Fourth, screening practices and screening adherence are continuously evolving over time, implying that the future residual cervical cancer and -mortality risks after screening will be different from the currently estimated residual risks after screening. Fifth, the screening estimates refer to population-average screening as it is unknown whether the girl eligible for vaccination will comply with the screening recommendations when grown up. This population-averaged risk reduction is smaller than the risk reduction for a person fully compliant with the screening recommendations. Nevertheless, the population-averaged lifetime risk estimates after screening might provide a useful benchmark for the lifetime risks after vaccination. Sixth, historical pre-screening cervical cancer incidence data do not necessarily reflect what would have been the current HPV epidemiology in the hypothetical absence of screening. Indeed, indirect evidence suggests that HPV infection has become more prevalent over the last 50 years, mainly due to changes in sexual behaviour.Citation20,Citation21 This implies that the use of historical data would underestimate the current cervical cancer risk in the hypothetical absence of screening. We therefore conducted three sensitivity analyses using (i) a natural history model, (ii) current CIN3 incidences and (iii) results from APC models. The results of the sensitivity analyses were in line with the results based on the historical pre-screening incidence data, and therefore we opted for the latter approach for reasons of simplicity. Finally, we did not calculate the residual risk of the combined prevention strategy of vaccination and screening as it is unknown whether, at the time when the girl (and her parents/guardians) needs to take the decision to get vaccinated (i.e. around the age of the 10 years), the girl will adhere to the screening recommendations. In either case, the residual risk of the combined strategy will be less than the residual risk after vaccination or screening alone.
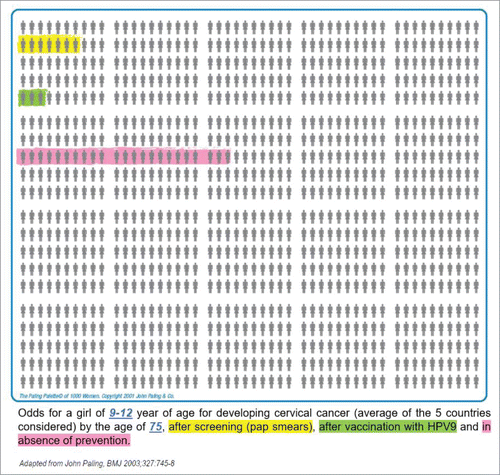
Despite these limitations, we believe that our simulations demonstrate how evidence can be generated to support decision-making regarding cervical cancer prevention by individual healthcare seekers (in this case, adolescent girls). Concepts like vaccine effectiveness and genotype attribution are difficult to explain to healthcare seekers. By translating these into individual lifetime risks, more tangible, easier to understand figures are obtained. The clarity of such figures can be further improved by means of visual aids, such as the Paling palette, designed to facilitate the communication of probabilities.Citation22 illustrates how a Paling palette could be used to communicate lifetime risk estimates without any prevention, after vaccination and after screening. We hope that our simulations demonstrated how the benefits of cervical cancer prevention strategies can be communicated to advising clinicians and healthcare seekers, with the objective of supporting decision-making and encouraging compliance to prevention measures. In this work, we focussed on the life-time risk of cervical cancer and related mortality after 9vHPV vaccination despite that the 9vHPV vaccine also prevents other HPV-related diseases, including anal, vaginal and vulvar cancer as per indication.Citation6 The same approach can be used to quantify the residual life-time risks after vaccination of these other HPV diseases and to quantify the risk of adverse events. To fully inform a healthcare seeker, additional analyses addressing all the benefits and risks of both vaccination and screening are needed.
Figure 2. Paling palette displaying the lifetime risk (per 1000 women) of developing cervical cancer by the age of 75 and after cervical screening (yellow), after 9vHPV vaccination in the hypothetical absence of cervical cancer screening (green) and in the absence of HPV prevention (pink). The risks are calculated using the mid-values of the range of the lifetime cervical cancer risks.
Materials and methods
Lifetime- and residual lifetime risk
We used lifetime risk to express the risk of disease and related mortality from the perspective of the individual healthcare seeker. If the lifetime risk is less than 10%, it is well approximated by the cumulative rate or the summation of the age-specific incidence rates over each year of age from birth to a defined upper limit.Citation23 Specifically, the lifetime risk of cervical cancer in absence of any HPV prevention measure was calculated as(1)
(1) with
being the annual age-specific cervical cancer incidence or mortality rate in the absence of any HPV prevention measure within the ith age group of width
, and where
is the number of cases and
the number of person years at risk within the ith age group. The standard errorCitation23 is then calculated as
(2)
(2)
Residual lifetime risk after vaccinating a HPV naïve girl with the 9vHPV vaccine in the absence of other HPV prevention measures was estimated as the lifetime risk without HPV prevention multiplied with the fraction of the disease not preventable through vaccination. Assuming lifelong protection, this equals(3)
(3) with
being the HPV attributable fraction,
the kth genotype contribution and with VEk being the genotype-specific vaccine efficacy (where k refers to 1 of the 7 oncogenic genotypes covered in 9vHPV vaccine). To obtain (residual) lifetime mortality risk estimates, we multiplied the lifetime cervical cancer risk with the corresponding mortality ratio λ. Finally, to facilitate the communication of the results to individual healthcare seekers, we presented lifetime and residual lifetime risk measures as 1/X (i.e. 1 person out of the X persons will develop the disease) and visually presented them using the Paling palette, designed to facilitate the communication of probabilities.Citation22
Monte Carlo simulation and sources
We built Monte Carlo simulation models to obtain estimates of the uncertainty in the (residual) lifetime risk estimates, reflecting the uncertainty in the parameters used to calculate these lifetime risks. An overview of the simulation model and input parameters is provided in . For every analysis, we generated 10.000 runs, based on which we calculated the median residual lifetme risks and the 95% uncertainty intervals (UI). All analyses were carried out in R version 3.4.0.Citation24 The sources of the input parameters are described below.
Table 2. Summary of the input parameters, distributions and sources.
Cancer and mortality incidences
Cervical cancer screening is widely implemented in the vast majority of the European countries. To estimate the effect of vaccination in the absence of screening, we searched for publicly available European historical pre-screening cancer incidence data. We obtained historical data for Denmark, Finland, Norway, Sweden and the UK. Details on the countries' HPV epidemiology and prevention policy are given in Appendix B. For the UK, we obtained data from 1989, immediately after the inception of the national screening programme in 1988,Citation25 whereas for the Scandinavian countries we obtained incidence data from 1960, well before the start of organized screening in the mid-1960s.Citation10-12 We did not use historical cervical cancer related mortality data, because of recent improvements in cancer treatment. Instead, we calculated the mortality ratio by dividing the number of cervical cancer related deaths by the number of cervical cancer cases for 2012Citation4 and applied this ratio to the historical cervical cancer incidence data ().
HPV attribution
Virtually all cervical cancers are caused by HPV.Citation3
Genotype attribution
We obtained European genotype contribution data for the 7 oncogenic genotypes included in the 9vHPV vaccine.Citation26 For cervical cancer, HPV types 16–18 were detected in 71% of cases, the 5 additional HPV types being detected in 16% of cases.Citation26
Vaccine efficacy (VE)
VE for CIN2+ (proxy for cervical cancer) is 98.2% [95% CI: 93.5%–99.8%] for genotypes 16 and 18 and 97.1% [95%CI: 83.5%–99.9%] for the 5 additional high-risk types 31, 33, 45, 52 and 58.
Sensitivity analyses
The historical pre-screening cancer incidence data may not be representative of the cervical cancer epidemiology that would have been observed today in the hypothetical absence of screening. Hence, we conducted several sensitivity analyses, deriving cervical cancer incidences from; (i) a natural history model,Citation27 (ii) current pre-invasive lesion (CIN3) incidencesCitation28-31 multiplied with the cervical cancer progression riskCitation32 and (iii) cancer incidences derived from Age-Period-Cohort (APC) models projecting the cervical cancer incidence that would have been observed today in the absence of screening accounting for cohort effects (such as changes in sexual attitude).Citation33 As for most of these approaches age-specific data were not available, overall (residual) cervical cancer incidences (per 100.000) were calculated instead of lifetime risks (details in Appendix C).
Cervical screening
To put the residual lifetime risk estimates after vaccination into perspective, we also estimated the lifetime risk after cervical screening or,(4)
(4) with
being the current annual age-specific incidence or mortality rate within the ith age group of width
. Particularly, we used the age-specific cervical cancer incidence and mortality data from 2012,Citation25,Citation34 reflecting the impact of decades of screening.
KHVI_A_1450125_Supplemental.zip
Download Zip (78.7 KB)Acknowledgments
The authors would like to thank Marc Baay (P95) for support in collating cancer and mortality incidence data and Sally Jackson (P95) for writing services.
References
- Sellors JW, Karwalajtys TL, Kaczorowski J, Mahony JB, Lytwyn A, Chong S, Sparrow J, Lorincz A. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ. 2003;168(4):421–5. PMID:12591782.
- de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–9. doi:10.1016/S1473-3099(07)70158-5. PMID:17597569.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi:10.1002/(SICI)1096-9896(199909)189:1%3c12::AID-PATH431%3e3.0.CO;2-F. PMID:10451482.
- International Agency for the Research of Cancer. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in. 2012.
- Franco EL, Mahmud SM, Tota J, Ferenczy A, Coutlee F. The expected impact of HPV vaccination on the accuracy of cervical cancer screening: the need for a paradigm change. Arch Med Res. 2009;40(6):478–85. doi:10.1016/j.arcmed.2009.06.003. PMID:19853188.
- European Medicines Agency. Gardasil 9. summary of product characteristics. 2015.
- Garland SM, Cheung TH, McNeill S, Petersen LK, Romaguera J, Vazquez-Narvaez J, Bautista O, Shields C, Vuocolo S, Luxembourg A. Safety and immunogenicity of a 9-valent HPV vaccine in females 12–26 years of age who previously received the quadrivalent HPV vaccine. Vaccine. 2015;33(48):6855–64. doi:10.1016/j.vaccine.2015.08.059. PMID:26411885.
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED Jr, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. doi:10.1056/NEJMoa1405044. PMID:25693011.
- Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143–2159. doi:10.1016/S0140-6736(17)31821-4. PMID:28886907.
- Anttila A, Nieminen P. Cervical cancer screening programme in Finland. Eur J Cancer. 2000;36(17):2209–14. doi:10.1016/S0959-8049(00)00311-7. PMID:11072206.
- Bigaard J, Hariri J, Lynge E. Cervical cancer screening in Denmark. Eur J Cancer. 2000;36(17):2198–204. doi:10.1016/S0959-8049(00)00309-9. PMID:11072204.
- Dillner J. Cervical cancer screening in Sweden. Eur J Cancer. 2000;36(17):2255–9. doi:10.1016/S0959-8049(00)00320-8. PMID:11072216.
- Perez-Gomez B, Martinez C, Navarro C, Franch P, Galceran J, Marcos-Gragera R. The moderate decrease in invasive cervical cancer incidence rates in Spain (1980–2004): limited success of opportunistic screening? Ann Oncol. 2010;21(Suppl 3):iii61–68. PMID:20427362.
- Adab P, McGhee SM, Yanova J, Wong CM, Hedley AJ. Effectiveness and efficiency of opportunistic cervical cancer screening: comparison with organized screening. Med Care. 2004;42(6):600–9. doi:10.1097/01.mlr.0000128007.04494.29. PMID:15167328.
- Cuschieri K, Ronco G, Lorincz A, Smith L, Ogilvie G, Mirabello L, Carozzi F, Cubie H, Wentzensen N, Snijders P, et al. Eurogin Roadmap 2017: Triage strategies for the management of HPV-positive women in cervical screening programmes. Int J Cancer. 2018. doi:10.1002/ijc.31261. PMID:29341110. [Epub ahead of print]
- Brisson M, Benard E, Drolet M, Bogaards JA, Baussano I, Vanska S, Jit M, Boily MC, Smith MA, Berkhof J, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health. 2016;1(1):e8–e17. doi:10.1016/S2468-2667(16)30001-9. PMID:29253379.
- Mendes D, Bains I, Vanni T, Jit M. Systematic review of model-based cervical screening evaluations. BMC Cancer. 2015;15:334. doi:10.1186/s12885-015-1332-8. PMID:25924871.
- Kjaer K, Nygård M, Dillner J, Brooke Marshall J, Radley D, Li M, Munk C, Hansen BT, Sigurdardottir LG, Hortlund M, et al. A 12-Year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 nordic countries. Clinical Infectious Diseases. 2018;66(3):339–345
- Thomas GM. Improved treatment for cervical cancer–concurrent chemotherapy and radiotherapy. N Engl J Med. 1999;340(15):1198–200. doi:10.1056/NEJM199904153401509. PMID:10202172.
- Dillner J. Trends over time in the incidence of cervical neoplasia in comparison to trends over time in human papillomavirus infection. J Clin Virol. 2000;19(1–2):7–23. doi:10.1016/S1386-6532(00)00126-8. PMID:11091144.
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet. 2004;364(9430):249–56. doi:10.1016/S0140-6736(04)16674-9. PMID:15262102.
- Paling J. Strategies to help patients understand risks. BMJ. 2003;327(7417):745–8. doi:10.1136/bmj.327.7417.745. PMID:14512489.
- Day NE. Cancer Incidence in Five Continents. Cumulative rate and cumulative risk. IARC Sci Publ. 1992;(120):862–4.
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria; 2013.
- Trent Cancer Registry. http://www.empho.org.uk/tcr/aboutUs.aspx, Last accessed: November 27, 2015.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi:10.1016/S1470-2045(10)70230-8. PMID:20952254.
- Campos NG, Burger EA, Sy S, Sharma M, Schiffman M, Rodriguez AC, Hildesheim A, Herrero R, Kim JJ. An updated natural history model of cervical cancer: derivation of model parameters. Am J Epidemiol. 2014;180(5):545–55. doi:10.1093/aje/kwu159. PMID:25081182.
- Kitchener HC, Almonte M, Thomson C, Wheeler P, Sargent A, Stoykova B, Gilham C, Baysson H, Roberts C, Dowie R, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009;10(7):672–82. doi:10.1016/S1470-2045(09)70156-1. PMID:19540162.
- Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–72. doi:10.1016/S0140-6736(07)61450-0. PMID:17919718.
- Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, Rådberg T, Strander B, Johansson B, Forslund O, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–97. doi:10.1056/NEJMoa073204. PMID:17942872.
- Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Ghiringhello B, Girlando S, Gillio-Tos A, De Marco L, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249–57. doi:10.1016/S1470-2045(09)70360-2. PMID:20089449.
- McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, Skegg DC. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–34. doi:10.1016/S1470-2045(08)70103-7. PMID:18407790.
- Vaccarella S, Franceschi S, Engholm G, Lonnberg S, Khan S, Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. 2014;111(5):965–9. doi:10.1038/bjc.2014.362. PMID:24992581.
- NORDCAN. http://www-dep.iarc.fr/nordcan.htm, Last accessed: November 27, 2015.
