ABSTRACT
The control of meningitis, meningococcemia and other infections caused by Neisseria meningitidis is a significant global health challenge. Substantial progress has occurred in the last twenty years in meningococcal vaccine development and global implementation. Meningococcal protein-polysaccharide conjugate vaccines to serogroups A, C, W, and Y (modeled after the Haemophilus influenzae b conjugate vaccines) provide better duration of protection and immunologic memory, and overcome weak immune responses in infants and young children and hypo-responsive to repeated vaccine doses seen with polysaccharide vaccines. ACWY conjugate vaccines also interfere with transmission and reduce nasopharyngeal colonization, thus resulting in significant herd protection. Advances in serogroup B vaccine development have also occurred using conserved outer membrane proteins with or without OMV as vaccine targets. Challenges for meningococcal vaccine research remain including developing combination vaccines containing ACYW(X) and B, determining the ideal booster schedules for the conjugate and MenB vaccines, and addressing issues of waning effectiveness.
Introduction
Neisseria meningitidis causes large epidemics of meningitis as well as smaller outbreaks, clusters of cases and endemic disease worldwide.Citation1-3 In the pre-serum, pre-antibiotic era, meningococcal disease had an overall mortality rate of 70–85%. Current mortality remains 10–15% in developed countries, and ranges higher (∼20%) in the developing world.Citation1-13 Morbidity related to sequelae of meningococcal disease remains high, with up to 20% of survivors experiencing long-term disabilities including developmental delays, deafness, and loss of limbs.Citation2,Citation6,Citation7
While antibiotic therapy has decreased mortality, the continued 10–20% mortality and the long-term morbidity remain significant issues. Thus, the ideal strategy to manage N. meningitidis is through immunization for disease prevention. Since the early 20th century, efforts to produce successful vaccines against the different serogroups of N. meningitidis have faced numerous challenges. The A, C, Y, W polysaccharide vaccines first introduced in the 1970's, while a major advance, had significant limitations including a short-lived duration of protection, weak immune response in infants (a high-risk group for the pathogen) and the failure to induce immunologic memory. Serogroup B vaccine development was particularly challenging due to identity of the B capsule to human antigens.Citation14,Citation15
Following the model of the successful Haemophilus influenzae b capsular polysaccharide-protein conjugate vaccines, new meningococcal capsular polysaccharide-protein conjugate vaccines were developed that overcame limitations of polysaccharide-alone vaccines. Meningococcal capsular polysaccharide-protein conjugate vaccines for A, C, W, Y provide herd protection through interference with N. meningitidis transmission. In addition, advances in serogroup B meningococcal vaccine development have been achieved through “reverse vaccinology” strategies, using genomic sequencing to first identify protective, conserved outer membrane proteins as vaccine targets individually or with outer membrane vesicles, as opposed to polysaccharide capsule. Despite these advances, further work is needed to 1) address gaps in vaccine coverage (e.g. some B subtypes, serogroup X, nongroupable strains), 2) better define duration of protection and waning vaccine efficacy and effectiveness over time (e.g. is herd protection induced by the new serogroup B vaccines?), 3) understand the best use of these vaccines in high risk populations and in outbreaks, 4) introduce meningococcal vaccines globally, and 5) reduce the costs (increasing availability) of these vaccines.
Serologic and genotyping of N. meningitidis
Neisseria meningitidis is classified into serogroups based on the immunogenicity and structure of the polysaccharide capsule.Citation1-3 Major virulence factors include capsule, other surface structures including the outer membrane proteins (OMPs, e.g. PorA, PorB Opc, Opa, NadA, FetA, FHbp), pili, and lipooligosaccharide (LOS), as well as iron sequestration mechanisms and virulence factors specifically related to genotype.Citation3 Almost all meningococcal strains responsible for causing invasive disease are encapsulated. N. meningitidis benefits from molecular mimicry through incorporation of Neu5Ac, the most common form of sialic acid in humans, into the meningococcal capsule.Citation14,Citation16 The capsule provides resistance against antibody/complement-mediated killing and inhibits phagocytosis.Citation17 The serogroup B capsule, an α(2-8)-linked sialic acid homopolymer, is identical in structure to the human fetal neural cell-adhesion molecule (NCAM).Citation14,Citation15 This identity results in a particularly poor immune response against serogroup B capsule in humans.Citation18
In addition to serogroups, N. meningitidis can be further classified by molecular typing techniques.Citation19 Molecular typing, genomic sequencing typing (ST) and now whole genome sequencing (WGS), is now the favored approach for identifying related strains, clades, clonal groups– particularly those involved in outbreaks, and potentially predicting vaccine coverage. Multilocus sequence typing (MLST) has been the gold standard genomic technique.Citation19 Isolates are categorized into sequence types (ST) defined by specific combinations of unique sequences of the 7 conserved gene loci. Closely related sequence types are further grouped into categories of clonal complexes (CC) and fine typed using Porin A (PorA), Porin B (PorB), and Ferric enterobactin transport (FetA) alleles. Sequence types and clonal complexes are independent of meningococcal serogroups. In recent years, WGS has also been widely applied to determine, sequence type and clonal complex, and to study of meningococcal molecular epidemiology, providing additional insights into the extensive, but highly structured genetic diversity of the meningococcus.Citation20-22
Risk factors for invasive disease
Bactericidal antibodies and intact complement pathways are the key correlate of protection against invasive meningococcal disease, with opsonization and phagocyte killing secondarily contributing. Antibodies typically appear in serum 2 weeks after meningococcal nasopharyngeal colonization.Citation2,Citation23 Of note, Goldschneider et al.Citation24 found that age specific incidence of meningococcal disease is inversely proportional to the prevalence of serum bactericidal antibodies (SBA) to the meningococcus. Individuals with congenital or acquired deficiencies of either immunoglobulins or complement, persons with anatomic or functional asplenia, and persons with HIV are at increased risk for invasive meningococcal disease.Citation25,Citation26
Environmental risk factors for meningococcal transmission and disease include tobacco exposure (active or passive), low humidity, winter months in temperate climates, the dry, dust exposure (Harmattan winds) of sub-Saharan Africa, and overcrowding.Citation27-31 Populations at increased risk include persons living in close quarters such as military recruits or college students housed in dorms, men who have sex with men (MSM),Citation32 microbiologistsCitation33 who regularly work with meningococcal isolates, individuals receiving the complement inhibitor eculizumab,Citation34 and travelers to the meningitis belt in sub-Saharan Africa or to the Hajj or Umrah in Saudi Arabia.Citation6,Citation8
Clinical disease
The clinical spectrum of disease ranges from transient bacteremia to septic shock. The most common clinical syndrome is acute bacterial meningitis with fever, headache, neck stiffness, and photophobia.Citation1,Citation2,Citation35 A erythematous, petechial or purpuric rash accompanies clinical disease 28–77% of the time but can be subtle.Citation2 Ten to 20% of patients present with fulminant meningococcemia with associated multi-organ failure, disseminated intravascular coagulation (DIC), and up to 50% of these cases result in death. Long-term sequelae of meningococcal disease including neurologic deficits, developmental disability, or limb ischemia and necrosis occurs in 10 to 20% of patients with meningitis and meningococcemia.Citation2,Citation36
Epidemiology of N. meningitidis
Figure 1. 2017 world map with recent reported incidence and predominant serogroup by region/country. The majority of disease globally is caused by six serogroups: A, B, C, W, X and Y.Citation35 Epidemiology varies based on both geographic location and age group.
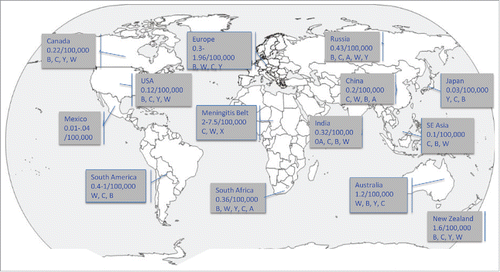
Meningococcal disease occurs worldwide but with significant variation in rates of disease based on circulating clonal complexes, serogroup, geographic location, population susceptibility and age. Disease can be sporadic, hyper-sporadic, or cause outbreaks or large epidemics. Since the 1990s in the US, the incidence has declined annually, a trend that started even before routine use of the meningococcal polysaccharide-conjugate vaccine in adolescents was recommended in 2005.Citation37 In 2015, the incidence in the US was 0.12 cases per 100,000 population.Citation7,Citation38
The explanation for this decline is multifactorial and includes advances in vaccine development, natural cyclic changes in incidence, and widespread use of antibiotics such as azithromycin and ciprofloxacin that eliminate meningococcal carriage. A published review of US antibiotic prescribing revealed that nearly a quarter of the US population is prescribed ciprofloxacin or azithromycin annually,Citation39 potentially indirectly contributing to the decline in meningococcal disease incidence.
Despite low incidence, the overall case-fatality ratio remains unchanged with an overall case-fatality ratio of 16.2% in the US in 2015.Citation7 Incidence also remains high in children under one year of age, 1.14 per 100,000 population, with the majority of disease being caused by serogroup B.
The highest burden of meningococcal disease, especially meningitis, occurs in sub-Saharan African across a region that includes 18 countries and parts of 8 others from Senegal in the west to Ethiopia in the east described as the “sub-Saharan African meningitis belt.” The overall incidence rate of meningococcal disease in sub-Saharan Africa in the years immediately pre–serogroup A conjugate vaccine (2004-2009) ranged from 10 per 100,000 to 26 per 100,000. In serogroup A epidemics, rates could approach 1000/100,000. There has been a dramatic decrease in meningococcal disease incidence and the elimination of massive outbreaks in the meningitis belt of Africa following the introduction of the serogroup A conjugate vaccine.Citation6 Serogroup A disease in the region has virtually disappeared. This meningococcal polysaccharide-tetanus toxoid conjugate vaccine (PsA-TT; MenAfriVac) was first introduced in 2010 in Burkina Faso; the overall incidence fell to less than 5 per 100,000 there in 2013. As the vaccine program has been extended across the countries of the belt (over 280 M doses given in the countries 2010–2017), in some areas of the belt meningococcal meningitis has fallen from 10–26/100,000 to 2–3/100,000. Overall the incidence of suspected meningitis cases fell an estimated 57% after MenAfriVac introduction.Citation11
In Europe since the introduction of first the serogroup C, and now the serogroup A, C, Y, W conjugate vaccines and the recent introduction of serogroup B vaccines, rates have fallen in most countries from up to 4.5 /100,000 to 1 or less/ 100,000.Citation9,Citation40,Citation41 Reporting is highly variable across countries in Latin America and Asia due to a number of factors including weak surveillance systems, lack of guidelines, inconsistent case definitions and varying awareness of healthcare providers,Citation10,Citation12,Citation13 but in some of these countries (e.g. Japan, Mexico) incidence of disease is <.04%.
Incidence of invasive meningococcal disease is highest in three specific age groups: children under 5 years old, adolescents and young adults aged 16 through 21 years, and adults aged 65 and older.Citation7,Citation37 Certain serogroups are more likely to cause disease in specific age groups.Citation37,Citation42 Approximately 60% of meningococcal disease in US children under 5 years is caused by serogroup B. Serogroups C, W, and Y cause two thirds of disease in adolescents and young adults. The majority of disease in adults aged 65 and older is caused by serogroup Y or W. WGS techniques have provided additional insight into variations in invasive meningococcal disease based on age group. WGS data reveals that specific lineages and clonal complexes are strongly associated with invasive disease age groups.Citation43
Meningococcal vaccines
History of vaccine development
Attempts to create an effective vaccine against N. meningitidis date back to the early 20th century with the first (unsuccessful) attempt at a meningococcal vaccine prepared from heat killed bacterial cultures in the 1910s.Citation44 The first successful polysaccharide vaccines targeting serogroups A and C were developed based on the work of Gotschlich and colleagues between 1969 and 1971.Citation45 Clinical trials revealed vaccine efficacy of 89.5% against serogroup C disease.Citation46 These studies also established the basis for the serologic correlate of protection against meningococcal disease, the serum bactericidal assay, SBA.Citation47 The quadrivalent A, C, Y, W-135 meningococcal polysaccharide vaccine was first approved for use in 1978. Major limitations of meningococcal polysaccharide vaccines are that they are, except for the serogroup A polysaccharide, poorly immunogenic in children under age 2 and they are unable to induce long term immunologic memory since they behave as T-cell independent antigens and generate a predominant IgM antibody response.
Late in the 20th century meningococcal vaccine development efforts became focused on improving the T-cell mediated immune response through conjugation of the polysaccharide to a protein. The success of the Haemophilus influenzae type b (Hib) protein-polysaccharide conjugate vaccines provided a model for meningococcal conjugate vaccine development. Conjugation, covalently coupling the capsular polysaccharide to a protein carrier, changes the human immune response from T-cell independent to T-cell dependent engaging helper T cells in maximizing a predominant high affinity IgG immune response. Conjugation ultimately results in an improved primary antibody response, especially in young children, creates affinity maturation, immunologic B cell memory and an amnestic IgG response at re-exposure.Citation48
The first meningococcal conjugate vaccines targeted serogroup C and were introduced in the United Kingdom in 1999 as a broad-catch up campaign in adolescents (who have the highest meningococcal carrier rates). Serogroup C conjugates vaccines were subsequently incorporated into the routine vaccination program for infants and young children.Citation49 Serogroup C disease decreased dramatically in the first 2 years after vaccine introduction and virtually disappeared by 2005.Citation50 A major contributor (approximately 50% of effectiveness) to this rapid decline was herd protection created by the generation of mucosal immunity by these vaccines and the interference with transmission of serogroup C expressing meningococci.Citation51 Vaccine effectiveness for reducing nasopharyngeal carriage of serogroup C in adolescents was more than 75% (serogroup C specific carriage decline from 0.42% to 0.09%), and the reduction in serogroup C meningococcal disease has persisted now for almost two decades.
Similar MenC conjugate vaccine effectiveness was demonstrated recently in Brazil. Routine immunization with serogroup C meningococcal conjugate vaccines was started in 2010 in infants at 3 and 5 months, followed by a booster dose at 12 to 15 months. Unlike in the UK, a catch-up campaign was not implemented. After four years MenC invasive disease was reduced 64%-92% in the target population due to direct vaccine effects but overall population impact in older children and adults was not observed.Citation52,Citation53 Overall, the improved efficacy of meningococcal vaccines for serogroups A, C, W, Y in the last 15 years can largely be attributed to the success of protein-polysaccharide conjugation.
Serogroup B has presented significant challenges in vaccine development, as previously described. While some work has looked at vaccines based on unique structural aspects of the meningococcal serogroup B polysaccharide capsule,Citation54,Citation55 the targeting of N. meningitidis outer membrane proteins rather than the serogroup B capsule has been a major focus. However, meningococcal outer membrane proteins and lipoproteins demonstrate significant genetic and structural variability. Outer membrane vesicle (OMV) based vaccinesCitation56 such as “tailored” OMV vaccines were used successfully in outbreak settings such as in Cuba, Norway and New Zealand.Citation57 Recently, two meningococcal protein based serogroup B vaccines were licensed for routine and outbreak use in the US in persons aged 10–25 years old,Citation58 and in the UK, one is now used in infants and young children.Citation9
Vaccines
While meningococcal polysaccharide vaccines are still in use, meningococcal protein-polysaccharide conjugate vaccines and serogroup B OMP or OMP/OMV based vaccines are now the standard for meningococcal disease prevention and outbreak management. Polysaccharide vaccines are being phased out; the A, C, W, Y polysaccharide vaccine will soon no longer be available in the US. details the current available US and WHO prequalified meningococcal vaccines composition, dosage, administration, and adverse effects.Citation59-64
Table 1. US, European, and Globally-Licensed and WHO prequalified meningococcal vaccines.
Vaccine efficacy and immunogenicity
Correlates of protection – serum bactericidal antibodies
The gold standard correlate of immunity to predict meningococcal vaccine efficacy is the serum bactericidal antibody (SBA) titer. The SBA assay measures the titer of antibodies present in serum and able to lyse strains of meningococci in the presence of a complement source.Citation65 Studies have revealed that a SBA titer of 1:4 or greater using human complement is a valid correlate of both short- and long-term protection against meningococcal disease.Citation47,Citation66,Citation67 When using the rabbit complement SBA, the valid correlate titer has been defined at 1:8 or greater; though some older studies used 1:128 as a cut off. The SBA has been deemed to be a conservative estimate of vaccine efficacy.Citation65
Meningococcal polysaccharide vaccines: MPSV4
Antibody response after vaccination with MPSV4 is serogroup specific for each of the four polysaccharides included in the vaccine (A, C, Y, and W) and thus does not induce cross-protection.Citation37 Studies reveal an overall clinical efficacy of ≥85% using the MPSV4 vaccine against serogroup A and C disease.Citation68 Serogroup A antibody response to vaccine were documented in children as young as 3 months, but the response is not equivalent to that induced in adults until around age 4 to 5 years.Citation69,Citation70 The antibody response to serogroup C is reduced in children under 24 months. Vaccination with MPSV4 has been shown to induce production of bactericidal antibodies to polysaccharides.Citation71 but hypo-responsiveness with repeated vaccinations.Citation72
Polysaccharides alone are T-cell independent antigens and rely on inducing a humoral response via stimulation of IgM producing B lymphocytes. This response provides a short duration of protection and does not result in immunologic memory.Citation73 In order to maintain disease protection, repeat vaccinations were required every 3–5 years. Polysaccharide vaccines produce a particularly weak immune response in infants with very low documented SBA at one year post-vaccination.Citation74 The immunologic hypo-responsiveness with repeat dosing of serogroup A or C polysaccharide vaccine is also well documented.Citation75-78 The polysaccharide vaccines do not confer long-term herd protection.Citation79
Meningococcal conjugate vaccines
Conjugation refers to the covalent coupling of a meningococcal polysaccharide to a protein carrier. The success of the Haemophilus influenzae type b (Hib) conjugate vaccines in the late 1980s prompted a shift in meningococcal vaccine development towards conjugated vaccines with the first conjugate serogroup C vaccine introduced in the UK in 1999 and the first quadrivalent conjugate vaccine licensed for routine use in the US, MenACWY-D (Menactra), in 2010. Meningococcal conjugate vaccines are now in use globally with an excellent safety profile.
Conjugation changes the human immune response from T-cell-independent to T-cell-dependent, which results in an improved primary IgG response, especially in young children, and induces a stronger anamnestic response at re-exposure to encapsulated meningococci.Citation37,Citation48 While detectable SBA titers fall over time, immunologic memory persists. However, given the short incubation period from meningococcal acquisition to disease 1–10 days immunologic memory alone is not considered protective.
Studies of antibody persistence following vaccination with meningococcal conjugate vaccines show high SBA titers immediately following immunization followed by an initial decline in titers up to one year post-vaccination. In a study comparing conjugate to polysaccharide vaccine, the percentage of participants with rabbit SBA titers above 1:128 at 3 years post vaccination ranged from 71% to 95% in the conjugate vaccine group compared to 57% to 83% in the polysaccharide vaccine group.Citation80 One to five years post-vaccination titers remain relatively stable.Citation81 The decline in titers, while improved overall with conjugate vaccines, remains more pronounced in infants compared to older children.
Herd protection
A major advantage of meningococcal conjugate vaccines is the ability to induce herd protection through decreasing transmission and nasopharyngeal carriage. Several clinical studies have evaluated the effect of quadrivalent meningococcal conjugate vaccination on meningococcal nasopharyngeal carriage. Herd protection accounts for ∼one half of the effectiveness of conjugate vaccines at preventing disease, and has significantly enhanced their cost-effectiveness. A nationwide study conducted in the Netherlands examined the impact of herd protection in the 10 years following a successful mass immunization campaign with meningococcal serogroup C (MenC) conjugate (MCC) polysaccharide vaccine.Citation22 Comparing incidence rates of MenC disease in the 48 months prior to introduction of the vaccine to the 48-months at the end of the ten-year observation period, the authors found a 99% decline in MenC disease in the vaccinated population and a concomitant 93% decline in MenC disease in the unvaccinated population. Thus, herd protection plays a significant role in meningococcal conjugate vaccine impact and can have a durable impact lasting 10 years or longer after a mass vaccination campaign. In contrast to a mass vaccination campaign, the impact of a routine introduction into a immunization schedule on generating rapid herd protection is considerably reduced.Citation82
MenACWY-D
Studies of MenACWY-D (Menactra) showed immunologic non-inferiority compared to MPSV4. Compared to MPSV4, vaccination with MenACWY-D produces similar proportion of patients with a 4-fold rise or higher in SBA titers to each of the four serogroups.Citation83 Cases of serogroup C and Y disease decreased among early adolescents age 11 to 14 years five years following introduction of the MenACWY-D in adolescents in the United States, supporting clinical effectiveness.Citation84 The decrease in cases was less pronounced, however, in older adolescents age 15 to 18 years.
Waning immunity to MenACWY-D was observed in a case control study evaluating vaccine effectiveness.Citation84 Overall vaccine effectiveness for adolescents immunized in the prior 0 to 5 years was 78.0% (95% confidence interval [CI] 29%-93%), but varied significantly based on time since vaccination. Vaccine effectiveness was 95% (95% CI 10%-100%) and 91% (95% CI: 10–100%) for those vaccinated less than 1 year earlier and 1 year earlier, respectively. Vaccine effectiveness was only 58% (95% CI: 72–89%) for those vaccinated 2 to 5 years earlier. The evidence of waning immunity indicated the need for booster dosing, which was added to the US ACIP vaccine recommendations.
The overall safety of MenACWY conjugate vaccines is like the MPSV4 vaccines, excellent.Citation85,Citation86 Post licensure, several cases of Guillain-Barré Syndrome (GBS) were recorded as serious adverse events, suggesting a correlation between MenACWY-D and GBS.Citation37 Following these initial reports, a retrospective cohort study was performed which found the attributable risk of GBS with MenACWY-D vaccination to be around 1.5 cases per 1,000,000 doses.Citation87 However, a recent review of post-licensure surveillance safety data did not detect any new or unanticipated short or long-term safety concerns following MenACWY-D vaccination.Citation88 The overall benefit of meningococcal disease prevention is felt to outweigh a possible small risk of vaccine-related GBS.Citation89 A history of GBS remains listed as a precaution on the package insert for MenACWY-D.Citation64
MenACWY-CRM
MenACWY-CRM (Menveo) was the second quadrivalent meningococcal polysaccharide vaccine to become licensed in the US and multiple other countries, following MenACWY-D. Comparative trials of MenACWY-CRM to both MenACWY-D and MPSV4 have shown favorable immune responses. Post-vaccination SBA titers have been higher in several studies after administration of MenACWY-CRM compared to MenACWY-D or MSPV4.Citation90,Citation91 A correlation between MenACWY-CRM and GBS has not been established. MenACWY-D and MenACWY-CRM are safe to be co-administered with other routine vaccinations.
MenACWY-T
A third quadrivalent meningococcal conjugate vaccine MenACWY-T (Nimenrix) is now licensed in Europe, Australia and other countries. It is as effective as the other meningococcal conjugate vaccines in stimulating an immune response against all four serogroups of N. meningitidis and induces immunological memory.Citation92
Booster dosing of MenACWY
Studies have shown that revaccination with the MenACWY quadrivalent conjugate vaccines was safe, well-tolerated, and resulted in substantially higher SBA titers than following initial vaccination.Citation37 The MenACWY quadrivalent conjugate vaccines can be used interchangeably for the booster dose, regardless of which vaccine was administered with initial vaccination.Citation93 Hib-MenCY-TT, a bivalent conjugate vaccine targeted for infants 2 to 18 months to cover serogroups C and Y meningococcal disease as well as Haemophilus influenzae type b94,95 has been discontinued in the US and will no longer be available for administration.
MenAfriVac
MenAfriVac (PsA-TT) is a serogroup A meningococcal conjugate vaccineCitation60 that was developed by the Meningitis Vaccine Project (MVP) for use in the meningitis belt in Africa following the devastating epidemics in 1996–1997. MVP was a collaboration between the World Health Organization (WHO) and PATH as well as other local and international partners and collaborators.Citation96 MenAfriVac implementation required significant resource mobilization. The cost per vaccinated individual was estimated at US $40 and the total cost of implementation from 2011 to 2014 was estimated at US $253 million.Citation96
MenAfriVac mass vaccination campaigns targeted persons between age 1 and 29 years.Citation13 The campaign was well received by local governments and communities, with an aggregate administrative coverage in the initial 15 countries of nearly 98%.Citation96 The vaccine was found to be highly effective and resulted in a 57% decrease in incidence in suspected meningitis cases in vaccinated compared to unvaccinated populations and a 99% reduction in incidence of confirmed group A meningococcal disease was observed in fully vaccinated populations.Citation11 Over 280 M doses have now been given in the countries of the African meningitis belt. Nasopharyngeal carriage studies in Chad following mass vaccination showed a 98% difference in prevalence in the post-vaccine era compared with pre-vaccine, OR 0.019 (95% CI 0.002 – 0.138).Citation97 Carriage rates decreased in those that were unvaccinated as well, indicating a significant herd protection effect. A low-cost pentavalent meningococcal conjugate (A, C, W, X, Y) is in development for Africa, projected for implementation in 2020 or 2021.
Serogroup B vaccines
History of meningitis serogroup B vaccine development
Poor immunogenicity observed in early clinical trials such as a candidate vaccine using conjugated serogroup B polysaccharide with tetanus toxoidCitation54 as well as concerns of inducing autoimmunity stalled development of serogroup B polysaccharide vaccines. Serogroup B vaccine development initially targeted outer membrane proteins/vesicles as a strategy but encountered the organism's significant phenotypic and genotypic diversity in protein expression.Citation56
Initial monovalent OMV vaccines were developed targeting the immunodominant meningococcal PorA porin. These vaccines offered strain-specific protection and were therefore useful in outbreak settings, but not for routine immunization.Citation98,Citation99 Further work in the 1990s focused on developing vaccines that contained multiple PorA proteins to overcome the strain-specific limitation, but these were unsuccessful.Citation100 Broadly cross-reactive recombinant protein antigens in serogroup B vaccines to overcome the limitation of strain-specificity has proven a successful strategy. Novel targets were identified through whole genome sequencing: Neisseria adhesin A (NadA), Neisseria heparin-binding antigen (NHBA), and factor H binding protein (FHbp).Citation101 There are two currently available serogroup B meningococcal vaccines that utilize these proteins: MenB-4C vaccine (BexseroR) and MenB-FHp vaccine (TrumenbaR).
MenB-4C
In clinical trials investigating the immunogenicity of MenB-4C, the majority of subjects achieved ≥4-fold SBA response to two out of three test strains (expressing FHbp and NadA) at one month following vaccination and 63–88% had a composite SBA response ≥lower limit of quantification for all three test strains.Citation63,Citation102 Co-administration of MenB-4C with MenACWY vaccines has been evaluated and demonstrated limited immunologic interference.Citation103 Safety of MenB-4C is similar to other meningococcal vaccines though increased reactogenicity, including febrile seizures, has been a concern.Citation104,Citation105 Safety data following the 2015 implementation of a national routine immunization program in the UK reveal higher rates of fever and local reactions, but no reported increase in seizure rates.Citation106 The increased reactogenicity did not appear to have a negative impact on compliance with subsequent vaccine doses. The two-dose vaccine effectiveness of MenB-4C among UK infants was estimated at 82.9% for all serogroup B cases.Citation105
MenB-FHbp
Clinical trials for MenB-FHbp (Trumenba) evaluated SBA titers one month following the third dose of the series. In US and European studies with a 3-dose schedule of 0, 2, and 6 months, 81.8-85.7% of participants had a composite SBA response ≥lower limit of quantification for all four primary meningococcal B strains tested.Citation62 Concomitant administration of MenB-FHbp vaccine with other routinely recommended vaccines for adolescents in the US including 4vHPV, Tdap or dTap/IPV, and MenACWY was evaluated in three clinical trials.Citation102,Citation107 No immunologic interference was observed for any of the vaccine antigens with co-administration with MenB-FHbp except for the antibody response to HPV type 18. For HPV type 18, noninferiority criteria were not met for the mean geometric titer ratio at 1 month after the third dose of 4vHPV vaccine was administered, but ≥99% of subjects still had seroconversion for all four HPV antigens.Citation102 Overall safety was similar to MenB-4C. Both vaccines have a higher incidence of pain at the injection site. Recently a two-dose series was approved for MenB-FHbp.Citation108
Significant questions remain regarding the need for and the timing of booster dosing for both serogroup B vaccines. Studies have shown a rapid decline in SBA titers at 12–18 months after completion of the initial vaccination series. A long-term follow up study to assess persistence of bactericidal antibodies up to 4 years after initial MenB-FHbp vaccination series revealed SBA titers above the correlate of protection in 50% of more patients to three of four meningococcal serogroup B test strains.Citation109 Additionally, cases of MenB following vaccination have not been reported in those at increased risk, with the exception of patients on eculizumab.Citation110 Further studies are currently underway to examine the persistence of immunity after the initial vaccine series and to explore immune responses following booster dosing.
The role of herd protection with the MenB vaccines is not established. Read et al. found reductions in nasopharyngeal carriage rates of all meningococcal strains 3 months after the second dose of MenB-4C compared with a control vaccination (Japanese Encephalitis vaccine).Citation111 Significant reductions in carriage rates for serogroups C, W, and Y were also seen at 1 month after a single dose of MenACWY-CRM compared with control vaccination.Citation112 Other studies have found no herd protection effect with the new serogroup B-focused vaccines.Citation113,Citation114 The MenB vaccines are relatively new and have not yet been widely implemented. Overall, initial data are conflicting and additional studies are underway or in development to evaluate the impact of MenB vaccines.Citation115
Current global meningococcal vaccine recommendations
Monovalent and multivalent meningococcal conjugate vaccines against C or A, C, W, and Y have been included in country specific vaccination programs since 1999, with wide variation in the vaccines selected and specific recommendations for use.Citation49 Serogroup C vaccines were first introduced in the United Kingdom in 1999.Citation50 In the next decade, the monovalent C conjugates vaccines were introduced in different public health strategies across Europe, Canada, Australia and Brazil, and in other countries, resulting in significant reductions in serogroup C disease.Citation9
In 2005 the United States adopted an A, C, W, Y conjugate vaccine strategy for adolescents which was subsequently expanded to infants, children and older adults at risk.Citation116 The increasing importance of serogroups W and Y in several countries has led to the replacement of monovalent C vaccines with quadrivalent conjugates in some vaccine programs. In Latin America, quadrivalent A, C, W, Y conjugate vaccine is available in Colombia, Chile, Brazil, and Argentina.Citation49 Mexico has a national response strategy that includes the use of vaccines, but currently only in the case of outbreaks or travel to high-risk countries.
Recommendations for vaccination in the Asia-Pacific region are highly variable.Citation12 China has routine mass immunization programs in place using polysaccharide Men A and bivalent MenA and C in infants and young children.Citation49 Quadrivalent meningococcal vaccines are available in Japan, but there are currently no standardized recommendations for vaccination. Several meningococcal vaccines are available for use in Australia, including serogroup B and quadrivalent conjugate vaccinesCitation117 but there is state and territory variation in the use of these vaccines.
US ACIP recommendations
Current US ACIP meningococcal vaccine recommendations by age group and risk category are summarized in .
Table 2. Recommendations for vaccination in the US by age group.Citation37,58,108,122,147
Special populations and persons at increased risk for meningococcal disease
Persons with HIV
Between 2010 and 2015 clusters of invasive serogroup C meningococcal disease were reported in men who have sex with men (MSM) in three major US cities: New York City (NYC), Los Angeles County (LAC), and Chicago.Citation118 This prompted a retrospective review of cases of meningococcal disease in men aged 18–64 years which revealed a significant increased risk of meningococcal disease in MSM compared to non-MSM, with HIV status as a potentially important factor contributing to increased risk.Citation32 HIV-infected MSM had 10.1 times the risk of meningococcal disease compared to HIV-uninfected MSM.
The ACIP updated meningococcal vaccine recommendations in June 2016 for persons with HIV based on the results from this review and from other studies conducted in the US,Citation119 EnglandCitation120 and South AfricaCitation121 that revealed a consistently elevated risk of meningococcal disease in HIV-infected individuals compared to HIV-uninfected individuals. The ACIP currently recommends that all persons aged ≥2 months with HIV infection should receive the meningococcal quadrivalent conjugate vaccine.Citation122
College students
First-year college students living in residence halls should have at least one dose of MenACWY administered in the 5 years prior to college entry. Ideally, the most recent dose (primary or booster) should have been administered after the 16th birthday. Some colleges and universities have policies requiring vaccination as a condition for enrollment and/or living in residence halls. Recent data also indicate and increased risk of serogroup B meningococcal disease in US college students.
Patients taking eculizumab (Soliris)
Eculizumab (Soliris) is a monoclonal antibody against complement component C5 licensed in the US for treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS).Citation123,Citation124 Meningococcal disease risk in patients on eculizumab is 300 to 2000-times higher than the baseline risk of meningococcal disease for healthy individuals in the US.Citation125,Citation126 Nongroupable N. meningitidis strains appear to cause significant disease in patients on eculizumab.Citation34
Current ACIP guidelines recommend that patients treated with eculizumab should be vaccinated with both MenACWY and MenB vaccines.Citation37,Citation102 This provides minimal protection, however, against the nongroupable meningococcal strains disease and there are vaccine failures; thus the ideal management for these patients remains challenging. One strategy is to treat eculizumab recipients with long-term penicillin prophylaxis as the majority, but not all, of the isolates remain susceptible to penicillin.Citation34 Providers, patients and families should maintain a high index of suspicion for meningococcal disease in all patients receiving eculizumab and rapid administration of agents like ceftriaxone with initial symptoms is also a consideration.
Additional high-risk population recommendations are summarized in .
Meningococcal serogroup B vaccine recommendations
Serogroup B OMV vaccines are now approved and in use in Europe, Canada, Australia, the United States and other countries but with wide variation in the recommendations for these vaccines. MenB-4C (Bexsero) was licensed in Europe in 2013 and in the United States in 2015. In the US adolescents and young adults aged 16 to 23 years (the age of greatest risk) may be vaccinated with a serogroup B vaccine.Citation58 In the United Kingdom, MenB-4C has been introduced in infants and young children as part of the routine immunization program.Citation127 Bexsero is now licensed for use in 35 countries. The second serogroup B vaccine was licensed in the United States in 2014 and Europe 2017, MenB-FHbp (Trumenba).
MenAfriVac vaccine recommendations in the African meningitis belt
The latest WHO recommendations for countries in the African meningitis belt were updated in a position paper in February 2015.Citation128 The WHO recommends that MenAfriVac should be introduced into the routine childhood immunization program in all meningitis belt countries within 1–5 years after completion of the mass vaccination campaign, along with a one-time catch-up campaign for birth cohorts born since the campaign who also would not fall within the age range targeted by routine childhood vaccination efforts.Citation128 In areas where vaccine coverage is less than 60% of the population, the WHO recommends periodic additional mass vaccination campaigns as herd immunity may be insufficient in areas with lower vaccine coverage rates. The WHO recommends a 1-dose schedule to be administered at age 9–18 months. In specific situations, vaccination may be needed in children less than 9 months. In this scenario, a 2-dose infant schedule is recommended starting at 3 months with doses separated by at least 8 weeks.
Recommendations for outbreak management
Community-based outbreaks
An outbreak is defined by the occurrence of at least three confirmed or probable primary cases of meningococcal disease caused by the same serogroup in ≤3 months with an associated primary attack rate of ≥10 cases per 100,000 population.Citation37 The at-risk population to target with a mass vaccination campaign in the setting of an outbreak is defined as the smallest geographically contiguous population that includes all patients. The vaccination group following a community outbreak usually includes the portion of the population at risk that is under age 30. In rare situations where multiple cases have occurred outside of this age group, the entire population may be administered vaccination. Vaccine choice depends on the serogroup responsible for the outbreak.
Organizational-level outbreaks – Universities and college campuses
Organizational-level outbreaks have been a topic of much recent discussion in the US; thirteen outbreaks of serogroup B meningococcal disease on college campuses have been reported between 2008–2017.Citation58 Prior outbreaks in Norway, France, Cuba and New Zealand had been effectively managed by mass vaccination campaigns with strain specific meningococcal OMV B vaccines.Citation129 Due to sustained disease transmission, the decision was made by the FDA to approve the use of a MenB-4C vaccine for use in two US college outbreaks prior to licensure in the US.Citation130 The two meningococcal serogroup B vaccines are now licensed in the United States and both are used in control of college outbreaks.
Organizational-level outbreaks remain an active issue, and the ideal treatment strategies for these outbreaks remain a topic of discussion among experts. The CDC recently published updated outbreak guidelines.Citation131 For A, C, W, or Y serogroup disease, a MenACWY conjugate vaccine is recommended in all persons aged ≥2 months at increased risk for meningococcal disease. For serogroup B disease, a MenB vaccine is recommended in all persons aged ≥10 years at increased risk for meningococcal disease. There are no current recommendations for booster dosing of MenB vaccine, but the need for re-vaccination is actively being evaluated, so this may change in the future.
A randomized, controlled trial was conducted recently in Canada to examine safety and immunogenicity of an accelerated vaccine schedule for MenB-4C (0, 21 days) compared to the standard schedule (0, 60 days).Citation132 The preliminary results from the trial show that SBA titers increased rapidly after vaccination and were higher earlier in the accelerated schedule group, without any significant change in adverse events. Potentially accelerated schedules could be implemented to control serogroup B outbreaks on college and university campuses.
Hajj and Umrah pilgrimages to Mecca
The annual Hajj or Umrah pilgrimages to Mecca poses public health issues, including the concern for outbreaks of meningococcal disease. The pilgrimages attract more than a million Muslim pilgrims yearly from around the world. Several epidemics have been linked to the Hajj – in 1987, a meningococcal serogroup A epidemic occurred during the pilgrimage and in 2000 and 2001 an outbreak of W meningococcal disease occurred.Citation133 In both epidemics, disease spread across multiple continents in returning pilgrims. Overcrowding contributes to disease spread and studies have shown increased rates of nasopharyngeal carriage of meningococcal strains among pilgrims and Saudi Arabian citizens living near the Holy Mosque.Citation134,Citation135
The latest Saudi Arabian health guidelines implemented in 2001 require all pilgrims and local at-risk populations to be vaccinated with quadrivalent polysaccharide vaccine prior to being issued a Hajj visa. With the new effective serogroup B OMV vaccines and changing meningococcal disease epidemiology, future guidelines may also require serogroup B vaccine prior to pilgrimage, but this is not currently mandatory.Citation135
Recent meningococcal disease updates and implications for future vaccine development
Meningococcal Urethritis
An increase in N. meningitidis urogenital infections has recently been documented in heterosexual men attending sexual health clinics in the US.Citation136 The presumed mode of acquisition is through oral sex (fellatio), given that N. meningitidis is a frequent colonizer of the nasopharynx.Citation137 To date ∼ 300 confirmed cases of meningococcal urethritis have been identified. Research has shown that all these cases are caused by the same nonencapsulated, nongroupable N. meningitidis clade, US_NmUC.Citation138 Furthermore, isolates from the US_NmUC clade have acquired gonococcal aniA and norB genes which allow the isolates to grow well anaerobically and help facilitate urogenital tract disease. These clusters of N. meningitidis urogenital disease raise concerns about emergence of nongroupable isolates in disease and support further research into pathogenesis and modes of sexual transmission of N. meningitidis.
Effectiveness of a group B outer membrane vesicle vaccine against Neisseria gonorrhoeae
Recently, Helen Petousis-Harris and others in New Zealand published a retrospective case-control study that suggested effectiveness of a group B outer membrane vesicle meningococcal vaccine (MeNZB) against clinical gonorrhea.Citation139 While the clinical syndromes are usually different, N. gonorrhoeae and N. meningitidis share 80–90% genetic homology in genome nucleotide sequences. The MenZB outer membrane vesicle vaccine is meningococcal strain specific vaccine with a major epitope, the VR2 epitope on the PorA P1.7-2.4 protein, a major porin protein PorA.Citation140 However, MenZB also contains other outer membrane proteins and lipooligosaccharide.Citation141
The Petousis-Harris et al. study examined subsequent rates of gonorrhea within the population that was eligible for vaccination following a mass vaccination program between 2004 to 2008 targeting infants aged 6 weeks up through adults up to age 20 years. The study population consisted of patients attending sexual health clinics and followed a case-control model where cases were individuals who were gonorrhea positive only and controls were chlamydia positive only. The study found that vaccinated individuals were much less likely to be cases of gonorrhea than controls, with an adjusted odds ratio (OR) of 0.67 (95% CI 0.61-0.79). The estimated vaccine effectiveness of MeNZB against gonorrhea was estimated at 31% (95% CI 21–39). This was the first time a vaccine has been shown to have a protective effect against gonorrhea. The outer membrane vesicle preparation is the same as that found in the licensed serogroup B vaccine Bexsero.
The same research group out of New Zealand also recently conducted a cohort study of hospitalizations due to gonorrhea between 2004 and 2015.Citation142 They found that MenZB vaccinated individuals in their early teens at the start of the mass vaccination program were significantly less likely to be hospitalized due to gonorrhea compared to those who were unvaccinated, with a hazard ratio of 0.53 and corresponding vaccine effectiveness estimate of 47%. Further, following the use of Bexsero in a Canada meningococcal outbreak, surveillance data suggests this vaccine may also have activity against N. gonorrhoeae. Additional research is needed to confirm these findings and understand the cross-reactive antigen.
Cost-effectiveness
As meningococcal vaccines continue to be developed and implemented worldwide, cost-effectiveness has emerged as a contentious issue.Citation143 Overall the public health burden of meningococcal disease has decreased, while the costs of developing and bringing new vaccines to market continue to rise.Citation144 Widespread licensure and population level immunization programs require significant financial support, with less cost effectiveness. However, vaccines remain very effective interventions and meningococcal disease, striking otherwise healthy infants, children and young adults and resulting in significant mortality and morbidity, is devastating to individuals, families and communities. Utilizing strategies that maximize cost-benefit such as vaccine campaigns that recognize and maximize her protection, developing programs such as MenAfriVac that produce low-cost effective meningococcal vaccines, and weighing the benefits of vaccines versus more costly medical interventions that provide little or no public health benefit are options to address the concern. Issues surrounding how vaccination implementation decisions are made at a governmental level will remain a challenge.
Vaccines in development
Other meningococcal vaccines in development include a low-cost ACYW(X) protein polysaccharide conjugate vaccine designed for use in Africa. The vaccine, NmCV-5, is being developed with support from the UK Department for International Development (DFID), the Serum Institute of India, and PATH and is expected between 2020 and 2022. A Phase 1 trial of the vaccine was recently conducted at the Center for Vaccine Development, University of Maryland, Baltimore.Citation145 The results were encouraging for both safety and immunogenicity with high rSBA titers elicited against all meningococcal serogroups.
Combination vaccines to cover serogroups ACYW and B are also in development. One approach combines the A, C, W, Y/CRM-197 conjugate with MenB-4C.Citation146
Conclusions
The control of meningitis, meningococcemia and other infections caused by Neisseria meningitidis continues to be a significant global health challenge. However, significant progress has occurred in the last twenty years in meningococcal vaccine development and global introduction. Immunization against N. meningitidis is the most effective means to prevent invasive disease. Meningococcal protein-polysaccharide conjugate vaccines to A, C, W, and Y (modeled after the Haemophilus influenzae b conjugate vaccines) were developed that provide better duration of protection and immunologic memory, and overcome the limitations of immune responses in infants and young children and the hypo-responsiveness to repeated vaccine doses seen with meningococcal polysaccharide vaccines. ACWY conjugate vaccines also interfere with transmission and reduce nasopharyngeal colonization and provide significant herd protection, expanding effectiveness. Advances in MenB vaccine development have occurred using conserved outer membrane proteins with or without OMV as vaccine targets. Future directions and challenges for meningococcal vaccine research include combination vaccines containing ACYW(X) and B, improving on the serotype coverage of the serogroup B vaccines, determining the ideal booster schedules for the conjugate (MenAfriVac) and MenB vaccines, tackling issues of waning effectiveness for a longer lasting immune responses (e.g. is there a herd protection effect for the new serogroup B vaccines?), understanding the best use of meningococcal vaccines in high risk populations and in outbreaks, introducing meningococcal vaccines globally to maximize effectiveness, and in reducing the costs (increasing the availability) of the vaccines. Meningococcal vaccine work may be providing important paths to previously unsuccessful gonococcal vaccine strategies.
Disclosure of potential conflicts of interest
The opinions expressed in this article are the authors' own and do not reflect the view of the Centers for Disease Control, the Department of Health and Human Services, or the United States government. No other potential conflicts of interest
Acknowledgment
We would like to thank Dianne Watson for help in the preparation of this paper.
Additional information
Funding
References
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–88. doi:10.1056/NEJM200105033441807. PMID:11333996.
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet (London, England). 2007;369:2196–210. doi:10.1016/S0140-6736(07)61016-2. PMID:17604802.
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20. doi:10.1007/978-1-61779-346-2_1. PMID:21993636.
- WHO. Initiative for Vaccine Research (IVR). Meningococcal Disease.
- Greenwood B. Manson Lecture. Meningococcal meningitis in Africa. Trans R Soc Trop Med Hyg. 1999;93:341–53. doi:10.1016/S0035-9203(99)90106-2. PMID:10674069.
- Lingani C, Bergeron-Caron C, Stuart JM, Fernandez K, Djingarey MH, Ronveaux O, Schnitzler JC, Perea WA. Meningococcal meningitis surveillance in the African Meningitis Belt, 2004–2013. Clin Infect Dis. 2015;61(Suppl 5):S410–5. doi:10.1093/cid/civ597. PMID:26553668.
- MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease-United States, 1996–2015. Clin Infect Dis. 2017. doi:10.1093/cid/cix993.
- CDC. Meningococcal Disease: Technical and Clinical information. 2017. https://www.cdc.gov/meningococcal/clinical-info.html.
- Whittaker R, Dias JG, Ramliden M, Kodmon C, Economopoulou A, Beer N, Pastore Celentano L; ECDC network members for invasive meningococcal disease. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004–2014. Vaccine. 2017;35:2034–41. doi:10.1016/j.vaccine.2017.03.007. PMID:28314560.
- Safadi MA, O'Ryan M, Valenzuela Bravo MT, Brandileone MC, Gorla MC, de Lemos AP, Moreno G, Vazquez JA, López EL, Taha MK, et al. The current situation of meningococcal disease in Latin America and updated Global Meningococcal Initiative (GMI) recommendations. Vaccine. 2015;33:6529–36. doi:10.1016/j.vaccine.2015.10.055. PMID:26597036.
- Trotter CL, Lingani C, Fernandez K, Cooper LV, Bita A, Tevi-Benissan C, Ronveaux O, Préziosi MP, Stuart JM. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis. 2017;17:867–72. doi:10.1016/S1473-3099(17)30301-8. PMID:28545721.
- Borrow R, Lee JS, Vazquez JA, Enwere G, Taha MK, Kamiya H, Kim HM, Jo DS; Global Meningococcal Initiative. Meningococcal disease in the Asia-Pacific region: findings and recommendations from the Global Meningococcal Initiative. Vaccine. 2016;34:5855–62. doi:10.1016/j.vaccine.2016.10.022. PMID:27780631.
- Borrow R, Caugant DA, Ceyhan M, Christensen H, Dinleyici EC, Findlow J, Glennie L, Von Gottberg A, Kechrid A, Vázquez Moreno J, et al. Meningococcal disease in the Middle East and Africa: findings and updates from the Global Meningococcal Initiative. J Infect. 2017;75:1–11. doi:10.1016/j.jinf.2017.04.007. PMID:28455205.
- Troy FA, 2nd. Polysialylation: from bacteria to brains. Glycobiology. 1992;2:5–23. doi:10.1093/glycob/2.1.5. PMID:1550990.
- Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol (Baltimore, Md: 1950). 1987;138:4402–7.
- Estabrook MM, Griffiss JM, Jarvis GA. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65:4436–44. PMID:9353017.
- Uria MJ, Zhang Q, Li Y, Chan A, Exley RM, Gollan B, Chan H, Feavers I, Yarwood A, Abad R, et al. A generic mechanism in Neisseria meningitidis for enhanced resistance against bactericidal antibodies. J Exp Med. 2008;205:1423–34. doi:10.1084/jem.20072577. PMID:18504306.
- Zimmer SM, Stephens DS. Serogroup B meningococcal vaccines. Curr Opin Investig Drugs (London, England: 2000). 2006;7:733–9.
- Jolley KA, Brehony C, Maiden MC. Molecular typing of meningococci: recommendations for target choice and nomenclature. FEMS Microbiol Rev. 2007;31:89–96. doi:10.1111/j.1574-6976.2006.00057.x. PMID:17168996.
- Mustapha MM, Marsh JW, Harrison LH. Global epidemiology of capsular group W meningococcal disease (1970-2015): multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine. 2016;34:1515–23. doi:10.1016/j.vaccine.2016.02.014. PMID:26876439.
- Harrison OB, Bray JE, Maiden MC, Caugant DA. Genomic analysis of the evolution and global spread of hyper-invasive meningococcal Lineage 5. EBioMedicine. 2015;2:234–43. doi:10.1016/j.ebiom.2015.01.004. PMID:25984558.
- Bijlsma MW, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. A decade of herd protection after introduction of meningococcal serogroup C conjugate vaccination. Clin Infect Dis. 2014;59:1216–21. doi:10.1093/cid/ciu601. PMID:25069869.
- Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B71–7. doi:10.1016/j.vaccine.2009.04.070. PMID:19477055.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–48. doi:10.1084/jem.129.6.1327. PMID:4977281.
- Castagliuolo PP, Nisini R, Quinti I, Fattorossi A, D'Amelio R. Immunoglobulin deficiencies and meningococcal disease. Ann Allergy. 1986;57:68–70. PMID:3729084.
- Stephens DS, Hajjeh RA, Baughman WS, Harvey RC, Wenger JD, Farley MM. Sporadic meningococcal disease in adults: results of a 5-year population-based study. Ann Internal Med. 1995;123:937–40. doi:10.7326/0003-4819-123-12-199512150-00007.
- Fischer M, Hedberg K, Cardosi P, Plikaytis BD, Hoesly FC, Steingart KR, Bell TA, Fleming DW, Wenger JD, Perkins BA. Tobacco smoke as a risk factor for meningococcal disease. Pediatr Infect Dis J. 1997;16:979–83. doi:10.1097/00006454-199710000-00015. PMID:9380476.
- Moore PS, Hierholzer J, DeWitt W, Gouan K, Djore D, Lippeveld T, Plikaytis B, Broome CV. Respiratory viruses and mycoplasma as cofactors for epidemic group A meningococcal meningitis. JAMA. 1990;264:1271–5. doi:10.1001/jama.1990.03450100061026. PMID:2117679.
- Breakwell L, Whaley M, Khan UI, Bandy U, Alexander-Scott N, Dupont L, Vanner C, Chang HY, Vuong JT, Martin S, et al. Meningococcal carriage among a university student population – United States, 2015. Vaccine. 2018;36:29–35. doi:10.1016/j.vaccine.2017.11.040. PMID:29183735.
- Broome CV. The carrier state: Neisseria meningitidis. J Antimicrob Chemother 1986;18(Suppl A):25–34. doi:10.1093/jac/18.Supplement_A.25. PMID:3091563.
- Molesworth AM, Cuevas LE, Connor SJ, Morse AP, Thomson MC. Environmental risk and meningitis epidemics in Africa. Emerging Infect Dis. 2003;9:1287–93. doi:10.3201/eid0910.030182. PMID:14609465.
- Folaranmi TA, Kretz CB, Kamiya H, MacNeil JR, Whaley MJ, Blain A, Antwi M, Dorsinville M, Pacilli M, Smith S, et al. Increased risk for meningococcal disease among men who have sex with men in the United States, 2012–2015. Clin Infect Dis. 2017;65(5):756–763. doi:10.1093/cid/cix438. PMID:28505234
- Sejvar JJ, Johnson D, Popovic T, Miller JM, Downes F, Somsel P, Weyant R, Stephens DS, Perkins BA, Rosenstein NE. Assessing the risk of laboratory-acquired meningococcal disease. J Clin Microbiol. 2005;43:4811–4. doi:10.1128/JCM.43.9.4811-4814.2005. PMID:16145146.
- McNamara LA, Topaz N, Wang X, Hariri S, Fox L, MacNeil JR. High risk for invasive meningococcal disease among patients receiving Eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734–7. doi:10.15585/mmwr.mm6627e1. PMID:28704351.
- Rouphael NG, Zimmer SM, Stephens DS. Chapter 53 – Neisseria meningitidis A2 – Barrett, Edited by Alan D. T. In: Stanberry LR, ed. Vaccines for biodefense and emerging and neglected diseases. London: Academic Press;2009. p. 1061–79.
- Erickson LJ, De Wals P, McMahon J, Heim S. Complications of meningococcal disease in college students. Clin Infect Dis. 2001;33:737–9. doi:10.1086/322587. PMID:11477523.
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE; Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62:1–28. PMID:23515099.
- Enhanced Meningococcal Disease Surveillance Report, 2015. CDC, 2017.
- Hicks LA, Taylor TH, Jr., Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461–2. doi:10.1056/NEJMc1212055. PMID:23574140.
- 14th Congress of the EMGM. 14th Congress of the EMGM, European Meningococcal and Haemophilus Disease Society. Prague, Czech Republic; 2017.
- Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007;31:101–7. doi:10.1111/j.1574-6976.2006.00053.x. PMID:17168998.
- CDC. Meningococcal disease: surveillance. 2017.
- Hill DM, Lucidarme J, Gray SJ, Newbold LS, Ure R, Brehony C, Harrison OB, Bray JE, Jolley KA, Bratcher HB, et al. Genomic epidemiology of age-associated meningococcal lineages in national surveillance: an observational cohort study. Lancet Infect Dis. 2015;15:1420–8. doi:10.1016/S1473-3099(15)00267-4. PMID:26515523.
- Sophian A, Black J. Prophylactic vaccination against epidemic meningitis. JAMA. 1912;59:527–32. doi:10.1001/jama.1912.04270080209011.
- Gotschlich EC, Goldschneider I, Artenstein MS. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–84. doi:10.1084/jem.129.6.1367. PMID:4977283.
- Gold R, Artenstein MS. Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969–70. Bull World Health Organization 1971;45:279–82.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–26. doi:10.1084/jem.129.6.1307. PMID:4977280.
- Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165(Suppl 1):S49–52. doi:10.1093/infdis/165-Supplement_1-S49. PMID:1588177.
- Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, Head C, et al. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–28. doi:10.1080/14760584.2017.1258308. PMID:27820969.
- Borrow R, Abad R, Trotter C, van der Klis FR, Vazquez JA. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine. 2013;31:4477–86. doi:10.1016/j.vaccine.2013.07.083. PMID:23933336.
- Maiden MC, Ibarz-Pavon AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, Walker AM, Evans MR, Kroll JS, Neal KR, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197:737–43. doi:10.1086/527401. PMID:18271745.
- Andrade AL, Minamisava R, Tomich LM, Lemos AP, Gorla MC, de Cunto Brandileone MC, Domingues CMS, de Moraes C, Policena G, Bierrenbach AL, et al. Impact of meningococcal C conjugate vaccination four years after introduction of routine childhood immunization in Brazil. Vaccine. 2017;35:2025–33. doi:10.1016/j.vaccine.2017.03.010. PMID:28318769.
- Tauil Mde C, Carvalho CS, Vieira AC, Waldman EA. Meningococcal disease before and after the introduction of meningococcal serogroup C conjugate vaccine. Federal District, Brazil. Braz J Infect Dis. 2014;18:379–86. doi:10.1016/j.bjid.2013.11.012. PMID:24698710.
- Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine. 2004;22:1087–96. doi:10.1016/j.vaccine.2003.10.005. PMID:15003635.
- Beninati C, Arseni S, Mancuso G, Magliani W, Conti S, Midiri A, Biondo C, Polonelli L, Teti G. Protective immunization against group B meningococci using anti-idiotypic mimics of the capsular polysaccharide. J Immunol. 2004;172:2461–8. doi:10.4049/jimmunol.172.4.2461. PMID:14764718.
- Feavers IM, Maiden MCJ. Recent progress in the prevention of serogroup B meningococcal disease. Clin Vaccine Immunol: CVI. 2017;24(5).
- Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362:1511–20. doi:10.1056/NEJMra0906357. PMID:20410516.
- MacNeil JR, Rubin L, Folaranmi T, Ortega-Sanchez IR, Patel M, Martin SW. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171–6. doi:10.15585/mmwr.mm6441a3. PMID:26492381.
- WHO Prequalified Vaccines. WHO: WHO.
- MenAfriVac [package insert]. Pune (India): Serum Institute of India; 2011. http://www.who.int/immunization_standards/vaccine_quality/pq_197_menAconj_SII_PI_624-2.pdf.
- Menveo [package insert]. Cambridge (MA): Novartis Vaccines; 2013. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201349.pdf.
- Trumenba [package insert]. Philadelphia (Pennsylvania): Pfizer; 2014. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm421139.pdf.
- Bexsero [package insert]. Research Triangle Park (North Carolina): GlaxoSmithKline; 2015. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm431447.pdf.
- Menactra [package insert]. Swiftwater (PA): Sanofi Pasteur; 2016. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm131170.pdf.
- McIntosh ED, Broker M, Wassil J, Welsch JA, Borrow R. Serum bactericidal antibody assays – The role of complement in infection and immunity. Vaccine. 2015;33:4414–21. doi:10.1016/j.vaccine.2015.07.019. PMID:26187262.
- Holst J, Feiring B, Fuglesang JE, Hoiby EA, Nokleby H, Aaberge IS, Rosenqvist E. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine. 2003;21:734–7. doi:10.1016/S0264-410X(02)00591-1. PMID:12531351.
- Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff DM, et al. Neisseria meningitidis group B correlates of protection and assay standardization–international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006;24:5093–107. doi:10.1016/j.vaccine.2006.03.091. PMID:16838413.
- Rosenstein N, Levine O, Taylor JP, Evans D, Plikaytis BD, Wenger JD, Perkins BA. Efficacy of meningococcal vaccine and barriers to vaccination. JAMA. 1998;279:435–9. doi:10.1001/jama.279.6.435. PMID:9466635.
- Peltola H, Makela H, Kayhty H, Jousimies H, Herva E, Hallstrom K, Sivonen A, Renkonen OV, Pettay O, Karanko V, et al. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977;297:686–91. doi:10.1056/NEJM197709292971302. PMID:408682.
- Kayhty H, Karanko V, Peltola H, Sarna S, Makela PH. Serum antibodies to capsular polysaccharide vaccine of group A Neissera meningitidis followed for three years in infants and children. J Infect Dis. 1980;142:861–8. doi:10.1093/infdis/142.6.861. PMID:6780634.
- Griffiss JM, Brandt BL, Broud DD. Human immune response to various doses of group Y and W135 meningococcal polysaccharide vaccines. Infect Immun. 1982;37:205–8. PMID:6809627.
- Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011;10:307–22. doi:10.1586/erv.11.8. PMID:21434799.
- Vipond C, Care R, Feavers IM. History of meningococcal vaccines and their serological correlates of protection. Vaccine. 2012;30(Suppl 2):B10–7. doi:10.1016/j.vaccine.2011.12.060. PMID:22607894.
- Espin Rios I, Garcia-Fulgueiras A, Navarro Alonso JA, Vazquez Moreno J, Rodriguez Gonzalez T, Navarro Sanchez C, Pérez-Flores D. Seroconversion and duration of immunity after vaccination against group C meningococcal infection in young children. Vaccine. 2000;18:2656–60. doi:10.1016/S0264-410X(00)00062-1. PMID:10781851.
- Granoff DM, Gupta RK, Belshe RB, Anderson EL. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis. 1998;178:870–4. doi:10.1086/515346. PMID:9728562.
- Borrow R, Joseph H, Andrews N, Acuna M, Longworth E, Martin S, Peake N, Rahim R, Richmond P, Kaczmarski E, et al. Reduced antibody response to revaccination with meningococcal serogroup A polysaccharide vaccine in adults. Vaccine. 2000;19:1129–32. doi:10.1016/S0264-410X(00)00317-0. PMID:11137248.
- MacLennan J, Obaro S, Deeks J, Williams D, Pais L, Carlone G, Moxon R, Greenwood B. Immune response to revaccination with meningococcal A and C polysaccharides in Gambian children following repeated immunisation during early childhood. Vaccine. 1999;17:3086–93. doi:10.1016/S0264-410X(99)00139-5. PMID:10462244.
- Broker M, Veitch K. Quadrivalent meningococcal vaccines: hyporesponsiveness as an important consideration when choosing between the use of conjugate vaccine or polysaccharide vaccine. Travel Med Infect Dis. 2010;8:47–50. doi:10.1016/j.tmaid.2009.12.001. PMID:20188306.
- Hassan-King MK, Wall RA, Greenwood BM. Meningococcal carriage, meningococcal disease and vaccination. J Infect. 1988;16:55–9. doi:10.1016/S0163-4453(88)96117-8. PMID:3130424.
- Keyserling H, Papa T, Koranyi K, Ryall R, Bassily E, Bybel MJ, Sullivan K, Gilmet G, Reinhardt Al. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch Pediatr Adolesc Med. 2005;159:907–13. doi:10.1001/archpedi.159.10.907. PMID:16203934.
- Baxter R, Keshavan P, Welsch JA, Han L, Smolenov I. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum Vaccin Immunother. 2016;12:1300–10. doi:10.1080/21645515.2015.1136040. PMID:26829877.
- Ramsay ME, Andrews NJ, Trotter CL, Kaczmarski EB, Miller E. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ (Clinical research ed). 2003;326:365–6. doi:10.1136/bmj.326.7385.365. PMID:12586669.
- Zahlanie YC, Hammadi MM, Ghanem ST, Dbaibo GS. Review of meningococcal vaccines with updates on immunization in adults. Hum Vaccin Immunother. 2014;10:995–1007. doi:10.4161/hv.27739. PMID:24500529.
- Meningococcal conjugate vaccines policy update: booster dose recommendations. Pediatrics. 2011;128:1213–8. doi:10.1542/peds.2011-2380. PMID:22123893.
- Shao PL, Chang LY, Hsieh SM, Chang SC, Pan SC, Lu CY, Hsieh YC, Lee CY, Dobbelaere K, Boutriau D, et al. Safety and immunogenicity of a tetravalent polysaccharide vaccine against meningococcal disease. J Formos Med Assoc. 2009;108:539–47. doi:10.1016/S0929-6646(09)60371-5. PMID:19586827.
- Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54:1–21. PMID:15917737.
- Velentgas P, Amato AA, Bohn RL, Chan KA, Cochrane T, Funch DP, Dashevsky I, Duddy AL, Gladowski P, Greenberg SA, et al. Risk of Guillain-Barre syndrome after meningococcal conjugate vaccination. Pharmacoepidemiol drug Saf. 2012;21:1350–8. doi:10.1002/pds.3321. PMID:22807266.
- Hansen J, Zhang L, Klein NP, Robertson CA, Decker MD, Greenberg DP, Bassily E, Baxter R. Post-licensure safety surveillance study of routine use of quadrivalent meningococcal diphtheria toxoid conjugate vaccine. Vaccine. 2017;35(49 Pt B):6879–6884. doi:10.1016/j.vaccine.2017.09.032.
- Kimmel SR. Using the tetravalent meningococcal polysaccharide-protein conjugate vaccine in the prevention of meningococcal disease. Ther Clin Risk Manage. 2008;4:739–45. doi:10.2147/TCRM.S962.
- Reisinger KS, Baxter R, Block SL, Shah J, Bedell L, Dull PM. Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccin Immunol: CVI. 2009;16:1810–5. doi:10.1128/CVI.00207-09.
- Cooper B, DeTora L, Stoddard J. Menveo(R)): a novel quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135 and Y. Expert Rev Vaccin. 2011;10:21–33. doi:10.1586/erv.10.147.
- Assaf-Casals A, Dbaibo G. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT, Nimenrix): A review of its immunogenicity, safety, co-administration, and antibody persistence. Hum Vaccin Immunother. 2016;12:1825–37. PMID:26900984.
- Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease–Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018–9. PMID:21814165.
- Nolan T, Richmond P, Marshall H, McVernon J, Alexander K, Mesaros N, Aris E, Miller J, Poolman J, Boutriau D. Immunogenicity and safety of an investigational combined haemophilus influenzae type B-Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190–6. doi:10.1097/INF.0b013e3181fcb2bf. PMID:20948453.
- MenHibrix [package insert]. Rixensart, (Belgium): GlaxoSmithKline; 2012. https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm308577.pdf.
- Djingarey MH, Diomande FV, Barry R, Kandolo D, Shirehwa F, Lingani C, Novak RT, Tevi-Benissan C, Perea W, Preziosi MP, et al. Introduction and rollout of a New Group A Meningococcal Conjugate Vaccine (PsA-TT) in African Meningitis Belt Countries, 2010–2014. Clin Infect Dis. 2015;61(Suppl 5):S434–41. doi:10.1093/cid/civ551. PMID:26553672.
- Daugla DM, Gami JP, Gamougam K, Naibei N, Mbainadji L, Narbé M, Toralta J, Kodbesse B, Ngadoua C, Coldiron ME, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA–TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study. Lancet. 2014;383:40–7. doi:10.1016/S0140-6736(13)61612-8. PMID:24035220.
- Bjune G, Hoiby EA, Gronnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nøkleby H, Rosenqvist E, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet (London, England). 1991;338:1093–6. doi:10.1016/0140-6736(91)91961-S. PMID:1682541.
- Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B3–12. doi:10.1016/j.vaccine.2009.04.071. PMID:19481313.
- de Kleijn ED, de Groot R, Labadie J, Lafeber AB, van den Dobbelsteen G, van Alphen L, van Dijken H, Kuipers B, van Omme GW, Wala M, et al. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2–3 and 7–8 years of age. Vaccine. 2000;18:1456–66. doi:10.1016/S0264-410X(99)00423-5. PMID:10618543.
- Pizza M, Scarlato V, Masignani V, Giuliani MM, Arico B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science (New York, NY). 2000;287:1816–20. doi:10.1126/science.287.5459.1816.
- Folaranmi T, Rubin L, Martin SW, Patel M, MacNeil JR. Use of Serogroup B Meningococcal Vaccines in Persons Aged >/ = 10 Years at increased risk for serogroup B meningococcal disease: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:608–12. PMID:26068564.
- Findlow J, Bai X, Findlow H, Newton E, Kaczmarski E, Miller E, Borrow R. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine. 2015;33:3322–30. doi:10.1016/j.vaccine.2015.05.027. PMID:26025807.
- Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garces-Sanchez M, Martinón-Torres F, Beeslaar J, Szenborn L, Wysocki J, et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12:597–607. doi:10.1016/S1473-3099(12)70087-7. PMID:22569484.
- Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet (London, England). 2016;388:2775–82. doi:10.1016/S0140-6736(16)31921-3. PMID:28100432.
- Bryan P. Bexsero (4CMenB) vaccine – the UK safety experience. 14th Congress of the EMGM, European Meningococcal and Haemophilus Disease Society. Prague, Czech Republic, 2017.
- Vesikari T, Wysocki J, Beeslaar J, Eiden J, Jiang Q, Jansen KU, Jones TR, Harris SL, O'Neill RE, York LJ, et al. Immunogenicity, safety, and tolerability of Bivalent rLP2086 meningococcal group B vaccine administered concomitantly With Diphtheria, Tetanus, and Acellular Pertussis and Inactivated Poliomyelitis Vaccines to Healthy Adolescents. J Pediatr Infect Dis Soc. 2016;5:180–7. doi:10.1093/jpids/piv064.
- Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp Serogroup B meningococcal vaccine – advisory committee on immunization practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509–13. doi:10.15585/mmwr.mm6619a6. PMID:28520709.
- Marshall HS, Richmond PC, Beeslaar J, Jiang Q, Jansen KU, Garces-Sanchez M, Martinón-Torres F, Szenborn L, Wysocki J, Eiden J, et al. Meningococcal serogroup B-specific responses after vaccination with bivalent rLP2086: 4 year follow-up of a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2017;17:58–67. doi:10.1016/S1473-3099(16)30314-0. PMID:27745812.
- Meningococcal Vaccines. Meeting of the Advisory Committee on Immunization Practices (ACIP). Atlanta (GA): Centers for Disease Control and Prevention; 2017.
- Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJ, Pollard AJ, Turner DP, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet (London, England). 2014;384:2123–31. doi:10.1016/S0140-6736(14)60842-4. PMID:25145775.
- Taha MK, Deghmane AE. Meningococcal carriage: the dilemma of 4CMenB vaccine. Lancet (London, England). 2014;384:2088–90. doi:10.1016/S0140-6736(14)60935-1. PMID:25145776.
- McNamara LA, Thomas JD, MacNeil J, Chang HY, Day M, Fisher E, Martin S, Poissant T, Schmink SE, Steward-Clark E, et al. Meningococcal carriage following a vaccination campaign with MenB-4C and MenB-FHbp in response to a university serogroup B meningococcal disease outbreak-oregon, 2015–2016. J Infect Dis. 2017;216:1130–40. PMID:28968661.
- Soeters HM, Whaley M, Alexander-Scott N, Kanadanian KV, MacNeil JR, Martin SW, McNamara LA, Sicard K, Vanner C, Vuong J, et al. Meningococcal carriage evaluation in response to a Serogroup B meningococcal disease outbreak and mass vaccination campaign at a College-Rhode Island, 2015–2016. Clin Infect Dis. 2017;64:1115–22. doi:10.1093/cid/cix091. PMID:28158417.
- Wilkins AL, Snape MD. Emerging clinical experience with vaccines against group B meningococcal disease. Vaccine. 2017 Aug 1. pii:S0264-410X(17)30964-7. doi:10.1016/j.vaccine.2017.07.056. PMID:28778616
- Baccarini C, Ternouth A, Wieffer H, Vyse A. The changing epidemiology of meningococcal disease in North America 1945–2010. Hum Vaccin Immunother. 2013;9:162–71. doi:10.4161/hv.22302. PMID:23108355.
- Archer BN, Chiu CK, Jayasinghe SH, Richmond PC, McVernon J, Lahra MM, Andrews RM, McIntyre PB; Australian Technical Advisory Group on Immunisation (ATAGI) Meningococcal Working Party. Epidemiology of invasive meningococcal B disease in Australia, 1999–2015: priority populations for vaccination. Med J Aust. 2017;207:382–7. doi:10.5694/mja16.01340. PMID:29092704.
- Kamiya H, MacNeil J, Blain A, Patel M, Martin S, Weiss D, Ngai S, Ezeoke I, Mascola L, Civen R, et al. Meningococcal disease among men who have sex with men – United States, January 2012-June 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1256–7. doi:10.15585/mmwr.mm6444a6. PMID:26562570.
- Miller L, Arakaki L, Ramautar A, Bodach S, Braunstein SL, Kennedy J, Steiner-Sichel L, Ngai S, Shepard C, Weiss D. Elevated risk for invasive meningococcal disease among persons with HIV. Ann Intern Med. 2014;160:30–7. doi:10.7326/0003-4819-160-1-201401070-00731. PMID:24166695.
- Simmons RD, Kirwan P, Beebeejaun K, Riordan A, Borrow R, Ramsay ME, Delpech V, Lattimore S, Ladhani S. Risk of invasive meningococcal disease in children and adults with HIV in England: a population-based cohort study. BMC Med. 2015;13:297. doi:10.1186/s12916-015-0538-6. PMID:26654248.
- Cohen C, Singh E, Wu HM, Martin S, de Gouveia L, Klugman KP, Meiring S, Govender N, von Gottberg A; Group for Enteric, et al. Increased incidence of meningococcal disease in HIV-infected individuals associated with higher case-fatality ratios in South Africa. AIDS (London, England). 2010;24:1351–60. doi:10.1097/QAD.0b013e32833a2520. PMID:20559040.
- MacNeil JR, Rubin LG, Patton M, Ortega-Sanchez IR, Martin SW. Recommendations for use of meningococcal conjugate vaccines in HIV-infected persons – advisory committee on immunization practices, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1189–94. doi:10.15585/mmwr.mm6543a3. PMID:27811836.
- Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, Gaya A, Coyle L, de Castro C, Fu CL, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–7. doi:10.1182/blood-2007-06-094136. PMID:18055865.
- Zuber J, Fakhouri F, Roumenina LT, Loirat C, Fremeaux-Bacchi V. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–57. doi:10.1038/nrneph.2012.214. PMID:23026949.
- Charneski L, Patel PN. Eculizumab in paroxysmal nocturnal haemoglobinuria. Drugs. 2008;68:1341–6. doi:10.2165/00003495-200868100-00001. PMID:18578555.
- Administration FaD. Soliris product insert. In: US Department of Health and Human Services FaDA, ed. US Department of Health and Human Services, Food and Drug Administration. MD: Silver Spring; 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/125166lbl.pdf.
- Ladhani SN, Campbell H, Parikh SR, Saliba V, Borrow R, Ramsay M. The introduction of the meningococcal B (MenB) vaccine (Bexsero(R)) into the national infant immunisation programme–New challenges for public health. J Infect. 2015;71:611–4. doi:10.1016/j.jinf.2015.09.035. PMID:26433141.
- WHO. World Health Organization position paper, Meningococcal A conjugate vaccine: Updated guidance, February 2015. Vaccine. 2017 Jul 28. pii:S0264-410X(17)30971-4.
- Oviedo-Orta E, Ahmed S, Rappuoli R, Black S. Prevention and control of meningococcal outbreaks: the emerging role of serogroup B meningococcal vaccines. Vaccine. 2015;33:3628–35. doi:10.1016/j.vaccine.2015.06.046. PMID:26093201.
- Basta NE, Mahmoud AA, Wolfson J, Ploss A, Heller BL, Hanna S, Johnsen P, Izzo R, Grenfell BT, Findlow J, et al. Immunogenicity of a Meningococcal B Vaccine during a University Outbreak. N Engl J Med. 2016;375:220–8. doi:10.1056/NEJMoa1514866. PMID:27468058.
- CDC. Guidance for the Evaluation and Public Health Management of Suspected Outbreaks of Meningococcal Disease. 2017:1–20. https://www.cdc.gov/meningococcal/downloads/meningococcal-outbreak-guidance.pdf.
- Langley J. Preparedness for and response to meningococcal outbreaks: preliminary results of a Canadian Immunization Research Network (CIRN) randomized controlled trial of two schedules of 4CMenB vaccine in adolescents and young adults. Meningitis Research Foundation Conference. London, UK, 2017.
- Taha MK, Achtman M, Alonso JM, Greenwood B, Ramsay M, Fox A, Gray S, Kaczmarski E. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet (London, England). 2000;356:2159. doi:10.1016/S0140-6736(00)03502-9. PMID:11191548.
- al-Gahtani YM, el Bushra HE, al-Qarawi SM, al-Zubaidi AA, Fontaine RE. Epidemiological investigation of an outbreak of meningococcal meningitis in Makkah (Mecca), Saudi Arabia, 1992. Epidemiol Infect. 1995;115:399–409. doi:10.1017/S0950268800058556.
- Memish ZA, Al-Tawfiq JA, Almasri M, Azhar EI, Yasir M, Al-Saeed MS, Ben Helaby H, Borrow R, Turkistani A, Assiri A. Neisseria meningitidis nasopharyngeal carriage during the Hajj: a cohort study evaluating the need for ciprofloxacin prophylaxis. Vaccine. 2017;35:2473–8. doi:10.1016/j.vaccine.2017.03.027. PMID:28343777.
- Bazan JA, Peterson AS, Kirkcaldy RD, Briere EC, Maierhofer C, Turner AN, Licon DB, Parker N, Dennison A, Ervin M, et al. Notes from the field: increase in Neisseria meningitidis-Associated Urethritis Among Men at Two Sentinel Clinics – Columbus, Ohio, and Oakland County, Michigan, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:550–2. doi:10.15585/mmwr.mm6521a5. PMID:27254649.
- Bazan JA, Turner AN, Kirkcaldy RD, Retchless AC, Kretz CB, Briere E, Tzeng YL, Stephens DS, Maierhofer C, Del Rio C, et al. Large Cluster of Neisseria meningitidis Urethritis in Columbus, Ohio, 2015. Clin Infect Dis. 2017;65:92–9. doi:10.1093/cid/cix215. PMID:28481980.
- Tzeng YL, Bazan JA, Turner AN, Wang X, Retchless AC, Read TD, Toh E, Nelson DE, Del Rio C, Stephens DS. Emergence of a new Neisseria meningitidis clonal complex 11 lineage 11.2 clade as an effective urogenital pathogen. Proc Natl Acad Sci U S A. 2017;114:4237–42. doi:10.1073/pnas.1620971114. PMID:28373547.
- Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, Black S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet (London, England) 2017;390(10102):1603–1610. doi:10.1016/S0140-6736(17)31449-6.
- Martin DR, Ruijne N, McCallum L, O'Hallahan J, Oster P. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin Vaccin Immunol: CVI. 2006;13:486–91. doi:10.1128/CVI.13.4.486-491.2006.
- Holst J, Oster P, Arnold R, Tatley MV, Naess LM, Aaberge IS, Galloway Y, McNicholas A, O'Hallahan J, Rosenqvist E, et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother. 2013;9:1241–53. doi:10.4161/hv.24129. PMID:23857274.
- Paynter J. Impact of MenZB on the incidence of gonorrhoea and potential future implications for cost effectiveness of teenage meningococcal vaccination. Meningitis Research Foundation Conference. London, UK, 2017.
- Moxon R, Snape MD. The price of prevention: what now for immunisation against meningococcus B? Lancet (London, England). 2013;382:369–70. doi:10.1016/S0140-6736(13)61572-X. PMID:23911365.
- Crowcroft NS, Deeks SL, Upshur RE. Do we need a new approach to making vaccine recommendations? BMJ (Clinical research ed) 2015;350:h308. PMID:25637290.
- LaForce M. Progress on MenACWYX vaccine: phase 1 trial results. Meningitis Research Foundation Conference. London, UK, 2017.
- Saez-Llorens X, Aguilera Vaca DC, Abarca K, Maho E, Han L, Smolenov I, Dull P. Persistence of meningococcal antibodies and response to a third dose after a two-dose vaccination series with investigational MenABCWY vaccine formulations in adolescents. Pediatr Infect Dis J. 2015;34:e264–78. doi:10.1097/INF.0000000000000822. PMID:26135245.
- Updated recommendations for use of meningococcal conjugate vaccines — Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal wkly Rep. 2011;60:72–6. PMID:21270745.
