ABSTRACT
It is unclear whether simultaneous administration of a 23-valent pneumococcal polysaccharide vaccine (PPSV23) and a quadrivalent influenza vaccine (QIV) produces immunogenicity in older individuals. This study tested the hypothesis that the pneumococcal antibody response elicited by simultaneous administration of PPSV23 and QIV in older individuals is not inferior to that elicited by sequential administration of PPSV23 and QIV. We performed a single-center, randomized, open-label, non-inferiority trial comprising 162 adults aged ≥65 years randomly assigned to either the simultaneous (simultaneous injections of PPSV23 and QIV) or sequential (control; PPSV23 injected 2 weeks after QIV vaccination) groups. Pneumococcal immunoglobulin G (IgG) titers of serotypes 23F, 3, 4, 6B, 14, and 19A were assessed. The primary endpoint was the serotype 23F response rate (a ≥2-fold increase in IgG concentrations 4–6 weeks after PPSV23 vaccination). With the non-inferiority margin set at 20% fewer patients, the response rate of serotype 23F in the simultaneous group (77.8%) was not inferior to that of the sequential group (77.6%; difference, 0.1%; 90% confidence interval, −10.8% to 11.1%). None of the pneumococcal IgG serotype titers were significantly different between the groups 4–6 weeks after vaccination. Simultaneous administration did not show a significant decrease in seroprotection odds ratios for H1N1, H3N2, or B/Phuket influenza strains other than B/Texas. Additionally, simultaneous administration did not increase adverse reactions. Hence, simultaneous administration of PPSV23 and QIV shows an acceptable immunogenicity that is comparable to sequential administration without an increase in adverse reactions. (This study was registered with ClinicalTrials.gov [NCT02592486]).
Introduction
Pneumococcal and influenza infections can cause significant morbidity and mortality, particularly in older individuals.Citation1,Citation2 Mortality rates due to invasive pneumococcal disease (IPD) are highest in adults older than 65 years. Therefore, immunization with a 23-valent pneumococcal polysaccharide vaccine (PPSV23) is recommended to prevent IPD; the vaccine's protective efficacy rate is 74%.Citation3 In most developed countries, however, PPSV23 vaccination rates remain low (31.4–69.8%), whereas influenza vaccination rates are relatively high (50.0–82.0%) in individuals older than 65 years.Citation4–8 Thus, a global strategy for improving PPSV23 vaccination compliance rates is required.
Simultaneous administration of PPSV23 and influenza vaccines, defined as administering both vaccines on the same day, is a promising strategy for increasing PPSV23 coverage rates to levels similar to those of influenza vaccinations.Citation9 Furthermore, simultaneous administration facilitates immunization with both vaccines in older individuals who have difficulty accessing hospitals. Vaccination with both the PPSV23 and influenza vaccine, even if not on the same day, is more protective and cost-effective than the administration of either alone.Citation10,Citation11 However, previous studies that assessed the immunogenicity of simultaneous administration of the PPSV23 and quadrivalent influenza vaccine (QIV) in adults aged ≥65 years are limited.Citation12,Citation13
The objective of this study was to compare simultaneous vs. sequential administration of PPSV23 and QIV to test our hypothesis that simultaneous administration was not inferior to sequential administration as determined by the response rate (defined as a ≥2-fold increase of immunoglobulin G [IgG] concentrations in serotype 23F) 4–6 weeks after vaccination. We selected 6 pneumococcus serotypes that are highly associated with IPD, namely 23F, 3, 4, 6B, 14, and 19A, for immunogenicity analysis.Citation14–16
Results
Patient characteristics
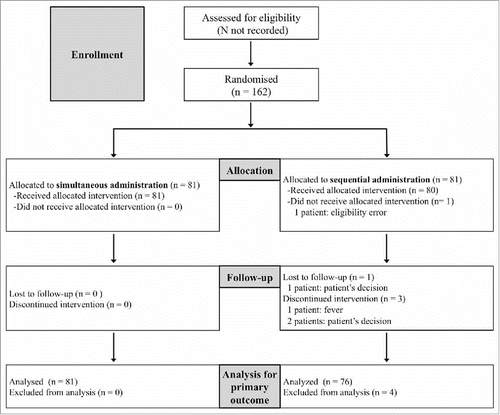
As shown in the CONSORT flowchart (), 162 patients were randomized to receive simultaneous (n = 81) or sequential administration (n = 81). After excluding 1 patient for ineligibility, the 81 patients in the former group received simultaneous vaccine administration while the 80 in the latter group received only QIV; the PPSV23 vaccine was administered 2 weeks later. The groups were well balanced upon randomization ().
Table 1. Patients' characteristics at allocation
Primary endpoint
and present the differences in the response rates to 23F between the 2 groups 4–6 weeks after PPSV23 vaccination. The response rate in the simultaneous group (63 of 81 patients [77.8%]) was not inferior to that of the sequential group (59 of 76 patients [77.6%]). The difference was 0.1% (90% confidence interval [CI], −10.8% to 11.1%), which was well above the −20% non-inferiority margin. The non-inferiority of simultaneous administration was maintained in both best-case and worst-case analyses (Supplementary Figure 1). The characteristics of sequential group patients who were analyzed after achieving their primary endpoint as well as those who dropped out are shown in Supplementary Table 1.
Figure 2. Differences in the response rates of 23F between the 2 groups (the rate in the simultaneous group minus that in the sequential group)
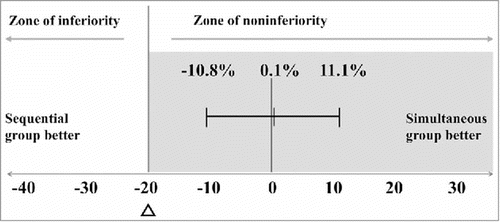
Table 2. Odds ratios for seroresponses 4–6 weeks post-vaccination with the 23-valent pneumococcal polysaccharide vaccine.
Secondary endpoints
shows the antibody titers to pneumococcal capsular polysaccharides in all serotypes. Before vaccination, the geometric mean concentrations (GMCs) with 23F, 4, 6B, and 14 were significantly higher in the simultaneous group. The GMCs 4–6 weeks after PPSV23 vaccination were not significantly different in any of the serotypes. At 6 months after PPSV23 vaccination, GMCs exhibited a significant difference for serotype 14 only. Multivariate analysis revealed that only serotypes 4 and 14 had significant reductions in seroresponse odds ratios (ORs) 4–6 weeks after vaccination in the simultaneous administration group (). According to post-hoc analysis, consistent results were observed on multivariate analysis using pre-vaccination pneumococcal IgG titers as a confounder in addition to age and sex (Supplementary Table 2).
Table 3. Antibody titers to pneumococcal capsular polysaccharides.
shows the comparisons between seroprotection rates 4–6 weeks post-vaccination with the QIV. The seroprotection rates against B/Texas and B/Phuket in the 2 groups were low (40.7–62.3%); however, the rates against H1N1 and H3N2 strains were 77.9–84.0%. There were no significant differences between the 2 groups in seroprotection against H1N1, H3N2, and B/Phuket strains of influenza on multivariate analysis, although significant reductions in the ORs for seroprotection against B/Texas were noted in the simultaneous administration group. There were no significant differences in ORs for seroprotection between the 2 groups with respect to any of the influenza antigens 6 months post-vaccination with the QIV on multivariate analysis.
Table 4. Odds ratios for seroprotection 4–6 weeks post-vaccination with the quadrivalent influenza vaccine.
Safety
shows the adverse events in the simultaneous and sequential groups. Simultaneous administration did not show any increase in systemic events and local reactions. However, fatigue was more frequent in the sequential group (24.1%) than in the simultaneous group (11.1%; P = 0.038).
Table 5. Adverse events in patients of the simultaneous and sequential groups.
Clinical events during the 6-month follow-up period
During the 6-month follow-up period, pneumonia and influenza-like illnesses were observed in 2 (2.5%) and 10 (12.3%) of the patients in the simultaneous group, respectively, and in 1 (1.3%) and 8 (10.7%) of the patients in the sequential group, respectively.
Discussion
We found that the response rate of serotype 23F following simultaneous administration was not inferior to that after sequential administration. There were no significant differences in GMCs 4–6 weeks after PPSV23 vaccination in any of the serotypes. Multivariate analysis revealed no significant differences in serotype 23F, 3, 6B, and 19A seroresponses in the simultaneous administration group, although serotypes 4 and 14 had significantly lower seroresponses. In the H1N1, H3N2, and B/Phuket strains of influenza, there were no significant differences in seroprotection between the 2 groups 4–6 weeks post-QIV administration, although seroprotection against B/Texas was significantly lower in the simultaneous administration group. Furthermore, there was no evidence of increased systemic events and local reactions with simultaneous administration.
Rational of simultaneous administration of the PPSV23 and QIV
Pneumococcal pneumonia and influenza infections are both vaccine-preventable diseases. As PPSV23 vaccination rates remain low, specific strategies to increase PPSV23 immunization rates are required.Citation4–7 As influenza vaccinations are administered annually and PPSV23 revaccination is recommended 5 years after first vaccination in older individuals, influenza immunization schedules may provide ideal opportunities for older individuals to receive their primary and secondary PPSV23 administrations. Many countries that annually provide seasonal influenza vaccinations in older individuals also routinely provide PPSV23.Citation17
Several studies have reported the additive benefits of influenza and PPSV23 vaccinations.Citation10,Citation11,Citation17,Citation18 Large-scale cohort studies have also demonstrated the additive preventative effect of vaccination with both the PPSV23 and trivalent influenza vaccine (TIV) compared to either vaccination alone in elderly persons; the benefits included reductions in pneumonia rates, influenza infections, hospitalizations, morbidity, and mortality.Citation10,Citation18 Prior studies also found that administering both the PPSV23 and influenza vaccine reduced medical costs compared to the influenza vaccine alone.Citation11,Citation17
Immunogenicity of the pneumococcal antibody
Several randomized control studies have demonstrated that the immunogenicity of pneumococcal antibody in patients with simultaneous administration of pneumococcal polysaccharide and influenza vaccines is similar to that of separate administration.Citation12,Citation19,Citation20 In the present study, we evaluated the immunogenicity of 23F, 3, 4, 6B, 14, and 19A, which are the most prevalent serotypes associated with IPD.Citation14,Citation16 Moreover, these 6 serotypes represent a range of capsular polysaccharides, including serotype 3 that was previously shown to have a relatively weak antibody response.Citation15 We found no significant differences in the GMCs of any of the 6 serotypes 4–6 weeks after PPSV23 vaccination, and 4 of the 6 serotypes tested showed no significant changes in their seroresponses. Several studies have similarly demonstrated attenuated reductions in seroresponse rates in the serotypes of pneumococcal antibodies, such as serotypes 4 and 14, although most serotypes showed no significant differences.Citation12,Citation19,Citation21 Thus, the possibility of reduced response rates (≥2-fold IgG) in some serotypes appear to be reproducible; however, the clinical impact may be low, as pneumonia and invasive pneumococcal disease due to serotypes 4 and 14 are low worldwide.Citation14,Citation22 Additionally, immunogenicity should be assessed using multiple valuables, such as GMCs and seroresponse rates. We propose that the advantages of increasing the immunization rates for both vaccines outweigh the effects of a possible small reduction in seroresponse in certain serotypes of the pneumococcal antibody.
Immunogenicity of influenza antibody
We found no significant differences between the 2 groups with respect to seroprotection rates in the A/H1N1, A/H3N2, and B/Phuket strains of influenza 4–6 weeks after vaccination. However, seroprotection against B/Texas was significantly reduced in the simultaneous group. In a double-blind, randomized control study, 126 healthy participants aged 18–26 years who received simultaneous administration of 14-valent pneumococcal polysaccharide vaccine and TIV showed lower geometric mean titers of A/H1N1 and B strains than participants who received separate administrations, although there were no statistically significant differences in seroprotection rates (post-vaccination titer ≥1:40) in the 2 administration groups.Citation20 Other studies that assessed simultaneous administration of pneumococcal polysaccharide vaccine and TIV found no significant differences in geometric mean titers of the influenza strains between the simultaneous vs. sequential administration groups.Citation12,Citation19,Citation21 In our study, both groups exhibited lower B/Texas immunogenicity. Considering previous findings and our own results, there exists a possibility of a slight reduction in the immune response against certain strains of influenza with simultaneous administration of PPSV23 and QIV, although the clinical implications of this remain unknown.
Adverse reactions
Several previous studies that evaluated the safety of simultaneous vaccine administration demonstrated acceptable adverse reactions in older individuals.Citation12,Citation19–21,Citation23 In a randomized control study assessing young individuals (18–26 years), simultaneous administration of the 14-valent polysaccharide pneumococcal vaccine and TIV did not result in increases in local or systemic reactions except for transient myalgias.Citation20 In a prospective cohort study assessing 861 elderly individuals, local erythema was reported more frequently by individuals who received simultaneous administration of PPSV23 and TIV; however, no significant differences in other adverse reactions were found between those immunized simultaneously with PPSV23 and TIV (n = 541) and those administered PPSV23 alone (n = 320).Citation23 In 2 other randomized control studies, simultaneous administration was not associated with an increase in local and systemic adverse reactions.Citation12,Citation19 Consistent with these findings, simultaneous administration did not show any increase in systemic events and local reactions in our study.
Clinical implications
Vaccination with both the PPSV23 and influenza vaccines is recommended worldwide for adults over 65 years.Citation17 Our results provide justification for clinicians to simultaneously administer PPSV23 and QIV, and for medical policymakers to recommend the same. The attrition rate in the sequential administration group is likely to be indicative of patients' inconvenience of having to return for a second vaccination in clinical practice. Clinicians should thus attempt to recommend the administration of PPSV23 at the same time as the QIV in adults over 65 years of age who were not previously vaccinated against pneumococci.
Limitations
First, we did not evaluate the opsonization index, which may be a more appropriate indicator for evaluating immune capability compared to quantitative IgG measurement. However, we employed a 2-fold increase in IgG concentrations as a measure of vaccine response based on previous studies.Citation24,Citation25 Second, our patients were predominantly enrolled at the Department of Pulmonology; therefore, chronic lung disease was the most frequent underlying disease, and a relatively low proportion of healthy subjects were enrolled. Therefore, our study should be interpreted with caution.
Conclusions
Simultaneous administration of PPSV23 and QIV showed an acceptable immune response that was comparable to that of sequential administration without an increase in adverse reactions. We propose that simultaneous administration of the 2 vaccines may be a promising strategy to increase PPSV23 coverage rates. Further studies assessing the opsonization index or clinical endpoints (such as all-cause pneumonia and all-cause mortality) following simultaneous administration should be performed to clarify the efficacy of simultaneous administration of PPSV23 and QIV.
Material and methods
Study design
This study was a randomized, open-label, non-inferiority trial conducted at Kameda Medical Center (Chiba, Japan) between October 2015 and August 2016. The protocol complied with the Helsinki Declaration and was approved by the Research Ethics Committee of Kameda Medical Center (#15-041-160127). Written informed consent was obtained from all participants. Additionally, CONSORT guidelines were followed during the development of the research plan and reporting of the results. This study is registered with ClinicalTrials.gov number: NCT02592486.
The primary endpoint was the percentage of patients with positive antibody responses (≥2-fold increase in IgG concentrations 4–6 weeks after PPSV23 vaccination) in serotype 23F of the pneumococcal antibody. This endpoint was compared between 2 groups of patients randomly allocated to receive either simultaneous administration (simultaneous injections of PPSV23 and QIV in 1 day) or sequential administration (injection of PPSV23 2 weeks after QIV vaccination). The 2-week gap was employed because of: 1) patients' ease-of-access to the hospital; and 2) in Japan, sequential vaccination of inactivated vaccine may be performed >7 days after the first vaccination. Serotype 23F was selected because it is a major causative serotype of pneumococcal pneumonia, a representative penicillin-resistant pneumococcus, and has a sufficiently high response rate.Citation24–26 Secondary endpoints included positive antibody responses in serotypes 3, 4, 6B, 14, and 19A as well as the GMCs of specific antibodies to 6 serotypes (23F, 3, 4, 6B, 14, and 19A) before vaccination, 4–6 weeks post-vaccination, and 6 months (24–27 weeks) post-vaccination with PPSV23. Another endpoint included the percentage of seroprotected patients (post-vaccination titer ≥1:40) 4–6 weeks and 6 months post-vaccination with QIV.
Data for patient characteristics including age, sex, previous influenza vaccination status in the last year, and lifetime history of pneumonia or influenza were obtained from questionnaires completed by the participants. Data for underlying diseases were obtained from existing medical charts at Kameda Medical Center. Patients were followed for 6 months post-vaccination. Pneumonia, influenza-like illness, hospitalization, and other medical events were recorded throughout the 6-month follow-up period. Supportive care, including cold medication and prophylactic antibiotic treatment, was administered.
Eligibility of study subjects
Study participants included individuals aged ≥65 years with no history of a pneumococcal vaccination (PPSV23 or pneumococcal conjugate vaccine) or QIV during the 2015/2016 season. Participants were excluded if they exhibited the following: sensitivity to either pneumococcal or influenza vaccines; vaccination within 14 (inactivated) or 28 (live) days; conditions that impaired their response to pneumococcal vaccination; a diagnosis of cancer; an acute illness necessitating treatment with antibiotics or steroids within 30 days; using oral corticosteroids or immunosuppressive agents; prior history of splenectomy; an acute febrile illness or evidence of a severe acute illness at the timing of vaccination; a limited chance of surviving for 12 months, and other specific conditions as judged by the attending physicians.
Randomization
Randomization was performed at the Data Coordinating Center, Osaka University (Osaka, Japan). Participants were randomly assigned to 1 of 2 study groups: the simultaneous administration group (simultaneous injections of PPSV23 and QIV in 1 day) or the sequential administration group (injection of PPSV23 2 weeks after QIV vaccination). Randomization was stratified according to patient age (<70 and ≥70 years) and sex.
Vaccination
Commercially available PPSV23 (Pneumovax NP®, MSDKK, Tokyo, Japan), containing 25 μg of each of the 23 capsular polysaccharide types was used. Each patient received a single subcutaneous dose of the vaccine (0.5 mL) in their right upper arm. Using a FLUBIK HA syringe (Handai Biken Ltd, Osaka, Japan), the QIV (0.5 mL) containing inactivated A/California/7/2009 (H1N1) pdm09, A/Swiss/9715293/2013 (H3N2), B/Texas/2/2013, and B/Phuket/3073/2013 was administered as a single subcutaneous injection in the left upper arm. In Japan, subcutaneous administration of pneumococcal and influenza vaccinations is routine.
Serum sample collection and antibody measurements
Serum samples were collected at 3 time points: before vaccination; 4–6 weeks after vaccination; and 6 months after vaccination. We evaluated antibody titers at 6 months to assess the antibody titer decay of both the influenza and pneumococcal vaccines. All serum specimens were stored at −20˚C until analysis. Serotype-specific IgG antibody concentrations for the 6 pneumococcus serotypes (23F, 3, 4, 6B, 14, and 19A) were measured at PPD® Laboratories (Richmond, VA, USA) using the Merck Sharp & Dohme Corp. multiplex, electrochemiluminescence-based detection assay that was bridged to the World Health Organization reference ELISA.Citation27 Furthermore, serum antibody levels to influenza hemagglutinin were measured at the Research Foundation for Microbial Diseases of Osaka University using the standard microtiter hemagglutination inhibition method with the same antigens found in the vaccine.Citation28 Immunogenicity was assessed in patients who received the allocated intervention (i.e., received at least 1 dose of the study vaccine), and had a blood sample taken within the planned time period.
Safety
The safety profiles of the 2 groups were compared. Local reactions at the injection site as well as systemic reactions were monitored for 28 days in the group that received the simultaneous administration, and for 14 days after each injection in the sequential group, using case cards completed by the participants. The population in which safety was assessed consisted of study participants who received a minimum of 1 dose of the study vaccine.
Statistical methods
The primary endpoint was the inferiority/non-inferiority of the immune response following simultaneous administration to that following sequential administration, as measured by the percentage of patients with ≥2-fold increases in IgG concentrations in serotype 23F 4–6 weeks after administration. The non-inferiority margin was set at 20% fewer patients based on a previous study, given the probable benefits associated with simultaneous administration.Citation29 The lowest published response rate (≥2-fold rise) for serotype 23F was 55% in adults.Citation24,Citation25 The primary endpoint was tested using a 90% CI for differences in response rates. If the lower 90% confidence limit was within the non-inferiority region, non-inferiority was established. With α = 0.05, 154 patients (77 per group) were required to obtain 80% power (β = 0.20) for establishing non-inferiority.
Pneumococcal IgG concentrations were converted using natural log transformations and presented as a GMC. The percentage of patients with positive antibody responses (≥2-fold rise) was also calculated for all 6 serotypes of the pneumococcal antibody. Paired t-tests were used to assess the increase in serotype-specific IgG from pre-, to post-vaccination within study groups. Student's t-tests were used for between-group comparisons of pre- and post-vaccination IgG titers. We performed univariate and multivariate analyses using logistic regression to determine the relationship between age, sex, and pneumococcal antibody response.
Seroprotection rates (post-vaccination titer ≥1:40) 4–6 weeks and 6 months post-vaccination with QIV were calculated to assess the immunogenicity of influenza vaccination. Logistic regression was used to evaluate the independent effects that potential confounders may have on antibody induction. Seroprotection was used as the dependent variable in the constructed models, and the following potential confounders were used: age at vaccination (<70 and ≥70), sex, and pre-vaccination titer (<1:10 and ≥1:10, in H1N1, B/Texas and B/Phuket; ≤1:10 and >1:10, in H3N2) were considered explanatory variables. The ORs and 95% CIs were also calculated, and all tests performed were 2-sided. The proportions of subjects reporting systemic events or local reactions within 28 days were compared using Fisher exact test. For all tests, P<0.05 was considered significant. All analyses were performed using R version 3.2.2 (The R Project for Statistical Computing, Vienna, Austria).
Conflicts of interest
K.N. received a speaker's fee from MSD K.K. M.A. received a speaker's fee from MSD K.K. All the remaining authors report no potential conflicts. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp./MSD K.K.
KHVI_A_1455476_Supplemental.zip
Download Zip (365 KB)Acknowledgments
We would like to thank Nobuyuki Tamura, Midori Tada, Mieko Takahashi, and Noriko Maruyama from the Department of Nursing at Kameda Medical Center for their cooperation. We would also like to thank the entire staff of the Departments of Pulmonary Medicine, General Internal Medicine, Infectious Diseases, and Laboratory Medicine at Kameda Medical Center for their involvement in this study. We acknowledge the statistical analysis support provided by Hiroki Matsui (Clinical Research Support Division, Kameda Institute for Health Science, Kameda College of Health Sciences, Japan).
Additional information
Funding
References
- Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin Microbiol Infect. 2012;18(Suppl 5):7–14. doi:10.1111/j.1469-0691.2012.03937.x.
- Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep. 2010;59:1057–62. PMID:20798667.
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013:CD000422.
- Kohlhammer Y, Schnoor M, Schwartz M, Raspe H, Schafer T. Determinants of influenza and pneumococcal vaccination in elderly people: a systematic review. Public Health. 2007;121:742–51. doi:10.1016/j.puhe.2007.02.011. PMID:17572457
- Public Health England. Pneumococcal Polysaccharide Vaccine (PPV) coverage report, England, April 2014 to March 2015. Health Protection Report. 2015. Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/448406/hpr2615_ppv.pdf. ( Accessed Dec 21, 2017).
- National Center for Health Statistics. 2016. Early release of selected estimates based on data From the January–June 2016 National Health Interview Survey. https://www.cdc.gov/nchs/data/nhis/earlyrelease/earlyrelease201611.pdf ( accessed Dec 21, 2017).
- Poethko-Muller C, Schmitz R. Vaccination coverage in German adults: results of the German Health Interview and Examination Survey for Adults (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:845–57. PMID:23703506
- Organisation for Economic Co-operation and Development. 2017. Influenza vaccination rates (indicator) https://data.oecd.org/healthcare/influenza-vaccination-rates.htm. ( accessed Dec 21, 2017).
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization — recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1–64.
- Christenson B, Hedlund J, Lundbergh P, Ortqvist A. Additive preventive effect of influenza and pneumococcal vaccines in elderly persons. Eur Respir J. 2004;23:363–8. doi:10.1183/09031936.04.00063504. PMID:15065822
- Kawakami K, Ohkusa Y, Kuroki R, Tanaka T, Koyama K, Harada Y, Iwanaga K, Yamaryo T, Oishi K. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine. 2010;28:7063–9. doi:10.1016/j.vaccine.2010.08.010. PMID:20723631
- Grilli G, Fuiano L, Biasio LR, Pregliasco F, Plebani A, Leibovitz M, Ugazio AG, Vacca F, Profeta ML. Simultaneous influenza and pneumococcal vaccination in elderly individuals. Eur J Epidemiol. 1997;13:287–91. doi:10.1023/A:1007398606807. PMID:9258527
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8:81–88. doi:10.4161/hv.8.1.17623. PMID:22252006
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. doi:10.1016/S1473-3099(11)70090-1. PMID:21621466
- Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201:516–24. doi:10.1086/649839. PMID:20092407
- Chiba N, Morozumi M, Sunaoshi K, Takahashi S, Takano M, Komori T, Sunakawa K, Ubukata K, IPD Surveillance Study Group. Serotype and antibiotic resistance of isolates from patients with invasive pneumococcal disease in Japan. Epidemiol Infect. 2010;138:61–68. doi:10.1017/S0950268809990239. PMID:19538821
- Gilchrist SA, Nanni A, Levine O. Benefits and effectiveness of administering pneumococcal polysaccharide vaccine with seasonal influenza vaccine: an approach for policymakers. Am J Public Health. 2012;102:596–605. doi:10.2105/AJPH.2011.300512. PMID:22397339
- Hung IF, Leung AY, Chu DW, Leung D, Cheung T, Chan CK, Lam CL, Liu SH, Chu CM, Ho PL, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis. 2010;51:1007–16. doi:10.1086/656587. PMID:20887208
- Fletcher TJ, Tunnicliffe WS, Hammond K, Roberts K, Ayres JG. Simultaneous immunisation with influenza vaccine and pneumococcal polysaccharide vaccine in patients with chronic respiratory disease. BMJ. 1997;314:1663–5. doi:10.1136/bmj.314.7095.1663. PMID:9193290
- DeStefano F, Goodman RA, Noble GR, McClary GD, Smith SJ, Broome CV. Simultaneous administration of influenza and pneumococcal vaccines. JAMA. 1982;247:2551–4. doi:10.1001/jama.1982.03320430055032.
- Carlson AJ, Davidson WL, McLean AA, Vella PP, Weibel RE, Woodhour AF, Hilleman MR. Pneumococcal vaccine: dose, revaccination, and coadministration with influenza vaccine. Proc Soc Exp Biol Med. 1979;161:558–63. doi:10.3181/00379727-161-40596. PMID:39297
- Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011(1.). Emerg Infect Dis. 2013;19:1074–83. doi:10.3201/eid1907.121830. PMID:23763847
- Socan M, Frelih T, Janet E, Petras T, Peternelj B. Reactions after pneumococcal vaccine alone or in combination with influenza vaccine. Vaccine. 2004;22:3087–91. doi:10.1016/j.vaccine.2004.02.003. PMID:15297059
- Dransfield MT, Nahm MH, Han MK, Harnden S, Criner GJ, Martinez FJ, Scanlon PD, Woodruff PG, Washko GR, Connett JE, et al. Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:499–505. doi:10.1164/rccm.200903-0488OC. PMID:19556517
- Mori S, Ueki Y, Akeda Y, Hirakata N, Oribe M, Shiohira Y, Hidaka T, Oishi K. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann Rheum Dis. 2013;72:1362–6. doi:10.1136/annrheumdis-2012-202658. PMID:23345600
- Oishi K, Yoshimine H, Watanabe H, Watanabe K, Tanimura S, Kawakami K, Iwagaki A, Nagai H, Goto H, Kudoh S, et al. Drug-resistant genes and serotypes of pneumococcal strains of community-acquired pneumonia among adults in Japan. Respirology. 2006;11:429–36. doi:10.1111/j.1440-1843.2006.00867.x. PMID:16771912
- Marchese RD, Puchalski D, Miller P, Antonello J, Hammond O, Green T, Rubinstein LJ, Caulfield MJ, Sikkema D. Optimization and validation of a multiplex, electrochemiluminescence-based detection assay for the quantitation of immunoglobulin G serotype-specific antipneumococcal antibodies in human serum. Clin Vaccine Immunol. 2009;16:387–96. doi:10.1128/CVI.00415-08. PMID:19158284
- Hirst GK. The quantitative determination of influenza virus and antibodies by means of red cell agglutination. J Exp Med. 1942;75:49–64. doi:10.1084/jem.75.1.49. PMID:19871167
- Cordonnier C, Labopin M, Chesnel V, Ribaud P, De La Camara R, Martino R, Ullmann AJ, Parkkali T, Locasciulli A, Yakouben K. et al. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis. 2009;48:1392–401. doi:10.1086/598324. PMID:19368505