ABSTRACT
Interleukin-22 (IL-22) is a member of IL-10 family of cytokines. IL-22 induces proliferative and anti-apoptotic signaling pathways and production of anti-microbial molecules that enhance tissue regeneration and host defense. IL-22 has also been identified as a cancer-promoting cytokine since deregulation of the IL-22-IL-22R1 system is linked to different cancer entities including lung, breast, gastric, pancreatic and colon cancers. T cells and innate lymphoid cells are the main cellular sources of IL-22. Expression of its specific receptor IL-22R1 is restricted to the non-hematopoietic cells which makes the IL-22-IL-22R1 pathway an attractive target for anti-cancer therapy. For development of such therapies, a better understanding of IL-22 regulation in the tumor microenvironment is needed. We could recently decipher how cancer cells promote IL-22 production by memory T cells via induction of IL-1. Here we will discuss how this knowledge might contribute to developing therapies disregulating the IL-22 pathway for cancer immunotherapy.
Interleukin-22 (IL-22) belongs together with other members of the IL-20 subfamily (IL-20, IL-24 and IL-26) to the larger IL-10 family of cytokines that play an important role in tissue remodeling and wound healing.Citation2 As these functions are also central to many cancer types, it is not surprising that IL-22 has been implicated in cancer development and progression.
IL-22 acts via its heterodimer receptor complex composed of two distinct subunits: ubiquitously expressed IL-10R2 and IL-22 specific receptor 1 subunit (IL-22R1). IL-22R1 is exclusively expressed by epithelial and stromal cells of tissues that compose body barrier surfaces such as in the respiratory tract, skin and gastrointestinal tract.Citation3 With anecdotal exceptions, immune cells do not express IL-22R1, but are the only physiological cellular sources of IL-22. In addition to its functional receptor, for IL-22 there is an endogenous antagonist, IL-22-binding protein (IL-22BP, also known as IL-22RA2). IL-22BP binds with a 20–1000 fold higher affinity to IL-22 and is an endogenous inhibitor of IL-22 biological activity.Citation4 Upon binding to IL-22R1, IL-22 signals though the Janus kinase (JAK) – signal transducer and activator of transcription (STAT) pathway and activates principally STAT3, but also STAT1 and STAT5.Citation1,Citation5 IL-22 can also trigger nuclear factor kappa B (NF-κB), mitogen activated protein kinase (MAPK) and phosphatidylinositide 3-kinase-Akt-mammalian target of rapamycin (PI3-K-Akt-mTOR) signaling pathways. IL-22-mediated signaling enhances the expression of genes with anti-inflammatory, mitogenic, proliferative and anti-apoptotic effects, cellular effects that promote local tissue regeneration and host defense.Citation6
As IL-22 promotes cell survival and proliferation via activation of STAT3, it goes hand in hand with an involvement in the pathogenesis of various cancers. Similarly to other members of the IL-20 subfamily, IL-22 does not promote carcinogenesis by itself, as IL-22 transgenic mice do not develop tumors spontaneously. IL-22 acts as a tumor promoting cytokine in nascent tumors of epithelial origin such as lung, liver, pancreatic, colon and gastric cancers.Citation7 These cancers share the presence of IL-22 producing cells and proliferation of IL-22R1 positive cancer cells upon IL-22 binding. Apart from promoting cell survival and proliferation, IL-22 also induces cell mobility, matrix metalloproteinase (MMP) expression, angiogenesis and tissue dysplasia.Citation6 Although IL-22 transgenic mice fail to develop spontaneous tumors, they are more susceptible to chemically induced liver tumorigenesis. In inflammation driven cancer models such as the DSS-azoxymethane colorectal cancer model, IL-22 promotes epithelial proliferation and protection against chronic inflammation and therefore slows tumor development, while its presence after tumor initiation, accelerates cancer progression.Citation3 Despite these contrasting roles of IL-22 in cancer development, present data from human and mouse studies link deregulation of the IL-22-IL-22R1 signaling axis to increased susceptibility and poor prognosis in several cancers. This data further suggests that IL-22 targeting might be an attractive approach for anti-cancer therapies. Because of its pleotropic role in the body, a better understanding of the mechanisms that regulate the balance between protective and pathologic effects of the IL-22-IL-22R1 axis is needed.
IL-22 is exclusively produced by immune cells, mainly CD4+ helper T cells, but also by CD8+ cytotoxic T cells, γδ T cells, innate lymphoid cells (ILCs) comprising natural killer (NK) cells, lymphoid tissue inducer (LTi) like cells and NKT cells. Although IL-22 expression has been primarily associated with lymphoid cells, several myeloid cell subsets including CD11c+ dendritic cells, macrophages and monocytes secrete IL-22 during inflammation. These cellular sources may differ between different tissues, pathological conditions, stage of disease and species implying that these aspects might influence on the efficacy of IL-22-based anti-cancer therapy.Citation8
Increased numbers of intratumoral and blood circulating IL-22 secreting cells have been reported in lung, gastric, colorectal, pancreatic and hepatocellular carcinomas. Among IL-22 expressing cells, CD4+ T helper subsets of lymphoid lineage and the innate lymphoid cell 3 (ILC3) subset seem to be the main source of IL-22 in different cancers.Citation7,Citation9 IL-22 was first identified in activated human T cells, the first T helper cell subset shown to secrete IL-22 being human IFN-γ producing TH1 cells which make up 35% of IL-22 producing CD4+ T cells in humansCitation10 ( for overview). The biological function of IFN-γ IL-22 double positive cells in cancer remains to be identified. TH17 cells, best known for their secretion of IL-17A and F cytokines, represent the main cellular source of murine IL-22, while in humans only 10–18% of blood helper T cells co-express IL-22 and IL-17. However, not all TH17 cells produce IL-22 and this seems to be tightly regulated by other cytokines and transcriptional factors. While IL-6 and IL-1β induce IL-22+ TH17 cell differentiation from naïve CD4+ T cellsCitation9 and IL-23 promotes their proliferation in vitro, TGF-β and IL-6 enhance the generation IL-17 producing cells as IL-6 alone only generates IL-22 single positive cells.
Table 1. Summary of IL-22 producing human (and murine when different) T cells.
IL-22 induction further requires ligand-dependent activation of transcription factor aryl hydrocarbon receptor (AHR) and – to a lesser extent – of RORγt.Citation11 The “only IL-22” producing (TH22) cells are the primary source of IL-22 in humans. According to recent studies, between 37 – 63% of IL-22 producing human CD4+ T cells from peripheral blood belong to this subset. Although murine TH22 cells have been identified in vivo, they are still not defined as an individual subset. IL-6 and TNF-α are needed for the induction of human TH22 cells and vitamin D supports their proliferation while addition of IL-1β induces co-expression of IL-17 and IFN-γ resulting in inhibition of IL-22 secretion over time.Citation12
IL-22 secreting TH1, TH17 and TH22 effector T cells have been described in human pancreatic, gastric, colorectal, breast and lung cancers.Citation6 Increased levels of intratumoral and circulating IL-22 and IL-17 secreting CD4+ T cells have also been found in gastric cancer patients.Citation13,Citation14 The same trend has been reported for colon cancer patients where IL-22 expression correlated with poor survival outcome. Elevated counts of IL-22+ cells were observed in human hepatocellular carcinoma samples and transfer of IL-22+ TILs from HCC patients enhanced metastasis development in a lung cancer xenograft model.Citation15 Several studies reported an increased expression of IL-22 in malignant pleural effusion and primary tumors of non-small cell lung carcinomas. Pleural cytokines including TNF-α, IL-1β, IL-6, as well as chemokines have been shown to be responsible for the infiltration of TH22 cells into pleural spaces.Citation16 None of these studies have addressed if and how IL-22 production would be influenced by the cancer cells which might be of importance to target this axis.
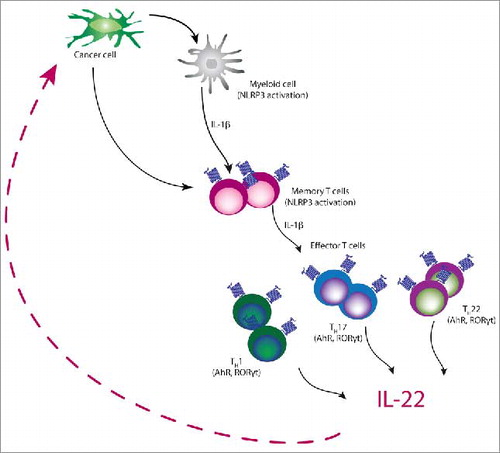
In our recent study, we were able to identify how IL-22 induction in human and murine lung and breast cancers is regulated. In both species, cancer cells drove IL-22 induction from memory CD4+ Th cells in an IL-1-dependent manner. In mice, IL-22 induction was mediated by IL-1α released from dying tumor cells, while human cancer cells induced NLP3-mediated secretion of IL-1β from myeloid and T cells. Activation of the IL-1 signaling cascade led to IL-22 induction via AHR and ROR-yt transcription factors. Blocking IL-1 activity by either IL-1R antagonist anakinra or anti-IL-1R neutralizing antibody led to a reduction in the number of IL-22 producing cells that correlated with slowing of tumor growth reduction in two breast cancer models. Our data indicates that blocking the IL-1 axis will disrupt IL-22 production and reduce its influence on cancer cells leading to enhanced tumor control ().Citation8
Along these lines, administration of IL-1R antagonist (anakinra) or anti-IL-1β neutralizing antibodies is considered to be safe based on more then 30 completed clinical trials.Citation17 On the other hand, increased IL-1 expression has been associated with poor prognosis in lung, skin, gastric, breast, head and neck cancers. This indicates that IL-1 is involved in cancer progression, potentially by inducing IL-22 but certainly also through other means.Citation17 The main advantage for using IL-1 neutralizing biologicals is their safety. Anakinra is a clinically approved drug in rheumatoid arthritis and clinical trials on breast and pancreatic patients are in progress. However, anakinra inhibits binding of IL-1α and IL-1β to IL-1R and this should be taken in consideration as several studies suggested for this two cytokines to have distinct roles in immune responses in inflammatory diseases and cancer development.Citation18 Further evidence for IL-1 neutralisation in cancer treatment comes from the recently published CANTOS trial, aiming at reducing myocardial reinfarction in a high risk population.Citation19 An unexpected but highly relevant finding of this study, utilizing the anti-IL-1β antibody canakinumab, was the reduced lung cancer incidence and lung cancer mortality in the treatment group.Citation20 While lung cancer incidence mortality were not predefined endpoints of the study, this finding raises the interesting possibility that blockade of cancer driving inflammation might effectively reduce cancer progression. Based on our own findings, it is tempting to speculate that blockade of IL-1 reduced IL-22 synthesis in these patients and ultimately prevented or slowed cancer development and spreading. Similarly, another way of influencing the IL-1-IL-22 axis would be to prevent processing of IL-1β through specific inhibitors of the IL-1-processing machinery such as the inflammasome. Development of such inflammasome inhibitors is currently under way for the treatment of inflammatory diseases. These compounds might also prove of use in cancer treatment when their in vivo efficacy in reducing IL-1β synthesis has been demonstrated.Citation21
Other promising approaches for targeting IL-22 in cancer include direct inhibition of IL-22-IL22-R1 signaling (e.g. by anti-IL-22 neutralizing antibodies or IL-22-BP), targeting of chemokines necessary for the infiltration of IL-22 producing cells or inhibition of transcriptional factors involved in IL-22 induction and signaling.Citation6,Citation7
Neutralizing antibodies against IL-22 already have shown promising results in psoriasis and rheumatoid arthritis and could therefore be used for the treatment of late stage tumors in order to increase responsiveness to chemotherapy and to reduce progression of metastatic cancers (studies NCT00883896, NCT00563524, NCT01941537 on http://clinicaltrials.gov/). Although IL-22BP is a natural antagonist of IL-22 and might therefore seem as an attractive candidate for targeting IL-22, some studies have shown that IL-22BP may even stabilize IL-22 to some extent and therefore increase its levels.Citation15 Development of a neutralizing antibodies that would block IL-22RA1 could also be beneficial in anti-cancer therapy, as this treatment would selectively and specifically affect receptor-bearing cells (including tumor cells), potentially also inhibiting the pro-tumoral effects of IL-22 sibling cytokines IL-20 and IL-24.
In summary, IL-22 plays an important role in host defense and tissue regeneration by promoting cell survival, migration, proliferation and angiogenesis, but these functions can also be exploited by cancer cell to enhance progression of various cancers. Therefore we believe that there is a need for development of anti-IL-22 therapies that will prevent tumor progression. Our studies add to current body of evidence that interfering with IL-22 regulation for example via IL-1 might provide similar and eventually also additional benefits to cancer immunotherapy.
IL-22 production is induced by cancer cell mediated activation of IL-1 signaling pathway. Cancer cells induce NLRP3 inflammasome with the subsequent release of IL-1β from both myeloid and T cells. IL-1 receptor signaling via the transcription factors AhR and RORγt induces IL-22 production by mixed T helper cell population comprised of Th1, Th17, and Th22 cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
Our studies are supported by grants from the international doctoral program “i-Target: Immunotargeting of cancer” funded by the Elite Network of Bavaria (to SK and SE), the Melanoma Research Alliance (grant number 409510 to SK), the Marie-Sklodowska-Curie “Training Network for the Immunotherapy of Cancer (IMMUTRAIN)” funded by the H2020 program of the European Union (to SE and SK), the Else Kröner-Fresenius-Stiftung (to SK), the German Cancer Aid (to SK), the Ernst-Jung-Stiftung (to SK), the LMU Munich‘s Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative (to SE and SK), the Bundesministerium für Bildung und Forschung VIP+ grant ONKATTRACT (to SE and SK) and the European Research Council Starting Grant (grant number 756017 to SK).
References
- Kobold S, Völk S, Clauditz T, Küpper NJ, Minner S, Tufman A, Düwell P, Lindner M, Koch I, Heidegger S, et al. Interleukin-22 is frequently expressed in small- and large-cell lung cancer and promotes growth in chemotherapy-resistant cancer cells. J Thorac Oncol. 2013;8:1032–42. doi:10.1097/JTO.0b013e31829923c8. PMID:23774470.
- Kotenko SV. The family of IL-10-related cytokines and their receptors: related, but to what extent? Cytokine Growth Factor Rev. 2002;13:223–40.
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Ann Rev Immunol. 2011;29:71–109. doi:10.1146/annurev-immunol-031210-101312. PMID:21166540.
- Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53:465–74. doi:10.1007/s00535-017-1401-7
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–72. doi:10.1084/jem.20082683. PMID:19564350.
- Lim C, Savan R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 2014;25:257–71. doi:10.1016/j.cytogfr.2014.04.005. PMID:24856143.
- Sabat R, Ouyang W, Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Revi Drug Discovery. 2014;13:21–38. doi:10.1038/nrd4176.
- Voigt C, May P, Gottschlich A, Markota A, Wenk D, Gerlach I, Voigt S, Stathopoulos GT, Arendt KAM, Heise C, et al. Cancer cells induce interleukin-22 production from memory CD4(+) T cells via interleukin-1 to promote tumor growth. Proc Natl Acad Sci U S A. 2017;114:12994–9. doi:10.1073/pnas.1705165114. PMID:29150554.
- Tufman A, Huber RM, Völk S, Aigner F, Edelmann M, Gamarra F, Kiefl R, Kahnert K, Tian F, Boulesteix AL, et al. Interleukin-22 is elevated in lavage from patients with lung cancer and other pulmonary diseases. BMC Cancer. 2016;16:409. doi:10.1186/s12885-016-2471-2. PMID:27388918.
- Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4:669–77. doi:10.1016/j.intimp.2004.01.016. PMID:15120651.
- Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunolog Rev. 2013;252:116–32. doi:10.1111/imr.12027.
- Eyerich K, Dimartino V, Cavani, A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur J Immunol. 2017;47:607–14. doi:10.1002/eji.201646723. PMID:28295238.
- Liu T, Peng L, Yu P, Zhao Y, Shi Y, Mao X, Chen W, Cheng P, Wang T, Chen N, et al. Increased circulating Th22 and Th17 cells are associated with tumor progression and patient survival in human gastric cancer. J Clin Immunol. 2012;32:1332–9. doi:10.1007/s10875-012-9718-8. PMID:22760549.
- Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Guo G, Chen W, Liu XF, Zhang JY, Liu T, et al. Increased intratumoral IL-22-producing CD4(+) T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol Immunothe.: CII. 2012;61:1965–75. doi:10.1007/s00262-012-1241-5.
- Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao Y, Wang X, Sun B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology (Baltimore, Md.). 2011;54:900–9. doi:10.1002/hep.24486. PMID:21674558.
- Ye ZJ, Zhou Q, Yin W, Yuan ML, Yang WB, Xiang F, Zhang JC, Xin JB, Xiong XZ, Shi HZ. Interleukin 22-producing CD4+ T cells in malignant pleural effusion. Cancer Lett. 2012;326;23–32. doi:10.1016/j.canlet.2012.07.013. PMID:22809567.
- Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–29. doi:10.1007/s10555-010-9229-0. PMID:20422276.
- Song X, Voronov E, Dvorkin T, Fima E, Cagnano E, Benharroch D, Shendler Y, Bjorkdahl O, Segal S, Dinarello CA, et al. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol (Baltimore, Md.: 1950). 2003;171:6448–56. doi:10.4049/jimmunol.171.12.6448.
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–31. doi:10.1056/NEJMoa1707914. PMID:28845751.
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, CANTOS Trial Group. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2017;390:1833–42. doi:10.1016/S0140-6736(17)32247-X. PMID:28855077.
- Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55. doi:10.1038/nm.3806. PMID:25686105.
