ABSTRACT
Objective: To study the effect of enriched environment (EE) on cognitive function and pathological changes in Alzheimer's disease (AD) mice.
Method: 6-month-old of the male mice were divided into 3 groups (n = 8), the wild type C57BL/6 mice in the control group, the APP/PS1 transgenic mice of AD reared in either the standard environment or the EE. Mice were feeding for 8 weeks, and then moved into standard environment. The activity and cognitive function were measured by Open-field test and Morris-water Maze. Immunofluorescence was used to detect Aβ plaque, hydroxylamine colorimetric assay was used to detect the activity of Ach, ChAT and AchE, and ELISA was used to detect the Aβ protein and the inflammatory factors IL-1β, IL-6, TNF-α and IFN-γ in serum, as well as the trophic cytokines BDNF, NGF and IGF-1 in hippocampus.
Result: Compared with the controls, the behaviors of AD mice were degraded, the Aβ deposition could be detected in the brain, the activity of Ach and ChAT decreased while the AchE increased, and the inflammatory factors increased significantly in serum while the trophic factors decreased in hippocampus. By means of rearing in EE, the activity and cognitive functions of AD mice were improved, and the Aβ plaques were significantly reduced. Meanwhile, the inflammatory factors in serum were reduced while the trophic cytokines increased. Besides, the cholinergic system in the brain were improved without statistic difference. However, 3 months later, these improvements in AD mice which were previously raised in EE disappeared.
Conclusion: The EE can improve the behaviors of AD mice, reduce the Aβ deposition in the brain, regulate the levels of cytokines, and have benefit in pathological changes in AD, but these improvements are short-term.
Highlights
| 1. | EE improves the activities and the cognitive abilities in APP/PS1 mice. | ||||
| 2. | The Aβ plaques in AD mice feeding in EE are reduced with a lower levels of inflammatory factors. | ||||
| 3. | The neurotrophin factors in EE mice are increased and further to influence the cholinergic system. | ||||
| 4. | The improvements of EE are short-term and will be disappear within 3 months. | ||||
Introduction
Alzheimer's disease (AD) is a degenerative disease of the central nervous system and the most common type of senile dementia. The characterized by persistent deterioration in memory and cognitive function, accompanied by various mental and behavioral disorders.Citation1 Unfortunately, the exact pathogeny and pathogenesis are still not clear, and there are no specific treatments or effective drugs that can reverse the progression. The neuropathological features of AD are the deposition of β-amyloid proteins (Aβ), the formation of intracellular neurofibrillary tangles (NFTs) and progressive neuron loss in the brain.Citation2,Citation3 In addition, the cholinergic hypothesis proposes that there is a reduction in the activity of choline acetyltransferase (ChAT) andacetylcholine (Ach) levels in the cerebral cortex in elderly patients.Citation4
Currently, available nonpharmacological treatment strategies for patients with AD are associated with lifestyle interventions including exercise and socialization as well as a healthy diet.Citation5,Citation6 In recent years, based on the endogenous nerve regeneration theory, the continuous development of EE has brought new breakthroughs in the prevention and treatment of neurodegenerative diseases and rehabilitation nursing.Citation7 Accumulating studies showed that the brain can make obvious responses to environmental stimuli.Citation8 In the animal models of AD, a large number of experiments reported physical exercise and cognitive stimulation in form of EE could attenuate Aβ burdens, rescue impaired neurogenesis, enhance synaptic plasticity, and alleviate cognitive impairment.Citation9 However, there are also few reports report that EE exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease.
Research shows that the decrease of choline acetyltransferase (ChAT) activity and the increase of acetylcholinesterase (AChE) activity is one of the major biochemical changes in elderly patients with dementia, and also closely related to the intelligence of the elderly patients with dementia.Citation10 Meanwhile, in the AD disease, It is also accompanied by a slow inflammatory pathological process.Citation11 There are lots of immune molecules associated with inflammatory reactions in the brain of AD patients, and participate in the formation of AD amyloid plaques.Citation12
Considering the above facts, we used APP/PS1 mice to detect the behavior and morphology, including the learning and memory function and activity determination in the AD mice under the stimulation of EE, and its influence on Aβ plaques in the brain. Meanwhile, environmental-induced changes in neuroinflammation and cholinergic system in mouse models of AD have been, hitherto, almost completely neglected. Therefore, the present study aims to ascertain the effects on neuroinflammation and the cholinergic system in APP/PS1 transgenic mice exposed to EE. These studies may provide scientific basis for the improvement of clinical pathological condition of AD disease.
Results
EE improves the autonomous activity of AD mice in the Open-field test ()
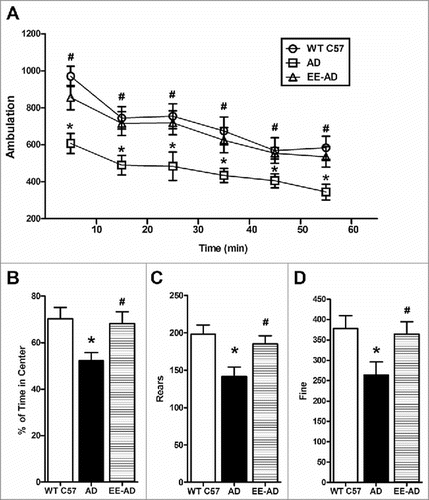
Compared with the wild-type mice, the activity of the APP/PS1 mice (Ambulation) in the open field were significantly decreased (P < 0.05), as well as Rears and Fine, while the mice in the EE group have a remarkably improvement in activities. The result shows that EE can enhance autonomy inquiry activities of AD mice.
Figure 1. EE significantly improved the activity of AD mice in the open field. In the 60 minutes open field test, the results were analyzed as a session every 10 minutes. The results showed that EE significantly enhanced the ambulation of AD mice during the whole experiment(A). EE significantly increased the time of central lattice movement (B), rears (C) and fine (D) of AD mice. *P < 0.05, compared with wild type C57; #P < 0.05, compared with AD mice.
EE improves the learning and memory function of AD mice in Morris water maze ()
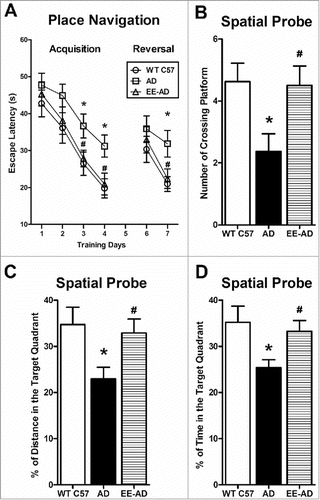
In the acquisition of place navigation, there is no difference of escape latency between the three groups in the 1–2 days. In the 3–4 days, compared with the AD group, the escape latency in EE and control groups are significantly less. On the 5th day of the spatial probe, the platform was removed from water maze equipment, the percentage of swimming time and distance in the target quadrant in EE mice were significantly higher than that of AD group, and the number of time through original platform quadrant position in EE mice is significantly more than AD group. In the 7th day, the reversal of place navigation, the escape latency in EE is decreased. It showed that EE improved the learning and memory abilities of AD mice.
Figure 2. EE significantly improved the learning and memory ability in AD mice. In the 3rd and 4th day, the acquisition of place navigation, wild type C57 and AD mice reared in EE used significantly less time to find the platform. In the 7th day, the reversal of place navigation, wild type C57 and AD mice reared in EE performed better (A). In the 5th day, the spatial probe, the AD mice reared in the EE spent significantly more times to through the target platform than the AD group (B), meanwhile the percentage of swimming time and distance in the target quadrant were significantly higher than the AD group (C, D). There was no difference in swimming speed between the three groups. *P < 0.05, compared with wild type C57; #P < 0.05, compared with AD mice.
EE reduces the Aβ plaques and the soluble/insoluble Aβ1–40/Aβ1–42 peptides in the brain of AD mice ()
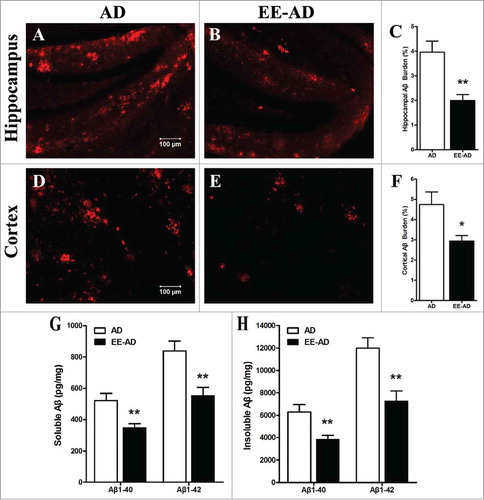
The content of Aβ plaques in brain of mice was detected by immunofluorescence staining, showed that compared with the control group, Aβ plaques could be observed in APP/PS1 mice. While the Aβ plaques of mice fed in EE decreased significantly (P < 0.05). We further examed the soluble/insoluble Aβ1–40/Aβ1–42 peptides in brain by ELISA, found the result is consistent with the deposition detection, that EE reduced the levels of Aβ peptides in AD mice (P < 0.05). It demonstrated that EE alleviates the Aβ plaques and proteins in AD mice.
Figure 3. EE significantly reduced the Aβ plaques and peptides in the brain of AD mice. Immunofluorescence showed that, compared with AD mice, most of the larger plaques in hippocampus (A, B) and cortex (D, E) area decrease in EE mice, while the deposition of some dispersion have also disappeared. Analysis of quantitative image showed the Aβ deposition in the hippocampus (C) and cortex (F) of AD mice in EE were significantly. The soluble/insoluble Aβ1–40/Aβ1–42 peptides (G, H) show EE reduced the levels of Aβ peptides in AD mice. *P < 0.05, **P < 0.01, compared with AD mice.
Changes in the activity of Ach, ChAT and AchE in the hippocampus and cortex of the mice ()
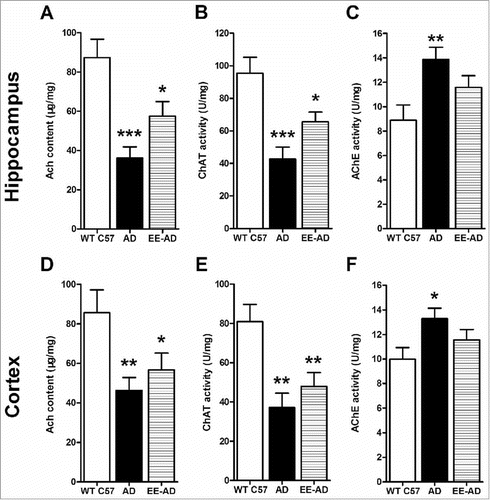
The activity of Ach and ChAT in hippocampus and cortex of AD mice was significantly lower than the control group, while the AchE was higher. It illustrated that the Ach in AD mice brain was not enough, caused central nervous system dysfunction. After reared in EE, there were still some differences with the controls, but the degrees were relived. Although there were no statistic differences between EE and AD mice, the former presents less inhibition of Ach and ChAT, and less activity of AchE.
Figure 4. Changes of the content of Ach, ChAT and AchE in the hippocampus (A, B, C) and cortex (D, E, F) of the mice. Compared with the control group, the Ach and ChAT in the brain of AD mice decreased significantly, while the content of AchE increased significantly. The AD mice fed in EE showed that these changes were relieved in varying degrees, but there wash no statistical difference compared with the AD mice. *P < 0.05, **P < 0.01, ***P < 0.001, compared with wild type C57.
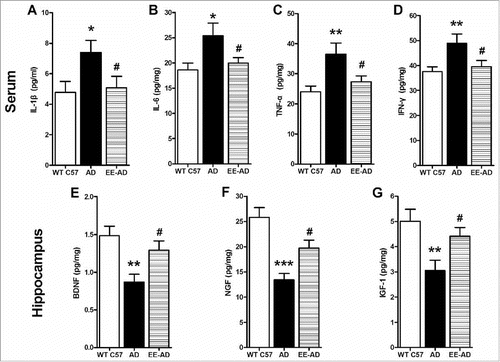
The inflammatory and trophic cytokines of mice ()
The levels of serum inflammatory factors IL-1β, IL-6, TNF-α and IFN-γ in AD mice were significantly higher than control group, while the EE suppressed the express of them, showed a significant decreased content (P < 0.05). At the same time, compared with the AD mice, the trophic factors BDNF, NGF and IGF-1 in the hippocampus of mice raised in EE increased significantly (P < 0.05).
Figure 5. Detection of inflammatory and neurotrophic cytokines level in mice. The results showed that the level of inflammatory factors IL-1β (A), IL-6 (B), TNF-α (C) and IFN-γ (D) in serum of AD mice were significantly higher than those of wild type C57 mice, while trophic factor BDNF (E), NGF (F) and IGF-1 (G) in hippocampus were lower. By reareing in EE, the inflammatory factors decreased while the trophic cytokines increased significantly. *P < 0.05, **P < 0.01, compared with wild type C57; #P < 0.05, compared with AD mice.
The improvements of cognition and cytokines in EE disappeared within 3 months (–)
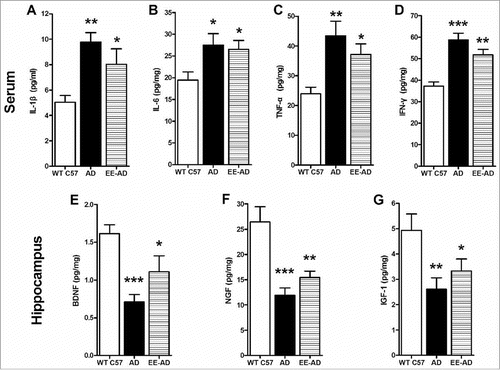
To detect the persistence of the improvements in EE, another experiment was carried out. After 8 weeks of feeding in EE, mice were all moved into standard environment. 3 months later, the Morris-water Maze was tested, and the mice previously raised in EE didn't represent better than the AD mice, demonstrated the improvement in cognition disappeared. Furthermore, we examed the inflammatory factors and trophic factors, and the changes by EE also had not remained. It illustrated that the benefit effect of EE is not last long. If the stimulate of EE is removed, the improvement in AD will gradually disappear, it is short-term.
Figure 6. The mice previously raised in EE represent no difference with the AD mice, In the place navigation (A), the wild type C57 spent significantly less time to find the platform. In the spatial probe (B-D), the wild type C57 spent more times and distance in the target quadrant than the other two groups. *P < 0.05, compared with wild type C57.
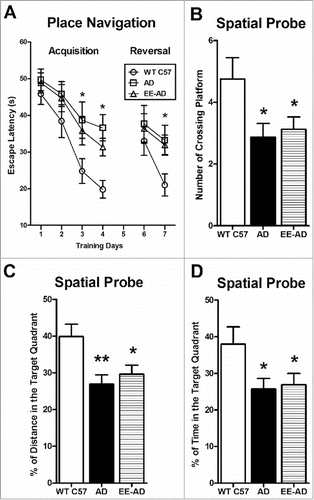
Figure 7. The inflammatory and neurotrophic cytokines in mice. The level of inflammatory factors IL-1β (A), IL-6 (B), TNF-α (C) and IFN-γ (D) in serum of AD mice which were previously reared in EE were higher than the controls, and the trophic factors BDNF (E), NGF (F) and IGF-1 (G) in hippocampus were lower. *P < 0.05, **P < 0.01, ***P < 0.001, compared with wild type C57.
Discussion
The exact pathogenesis of AD is still not clear, it is considered to be the common result of various factors such as aging, genetic and environmental.Citation13 There are many theories at present, the impact of the amyloid cascade hypothesis of Aβ is broader. The deposition of Aβ in the brain is the central link of the pathological changes of AD, which can cause a series of pathological process, thus forming a cascade amplification reaction.Citation14 These series of pathogenesis processes also includes the reduction of Ach and the process of chronic inflammation. AchE is a hydrolase of cholinergic neurotransmitter ACh, which directly involved in the autonomic regulation, memory and other important functions. Studies have confirmed that the central cholinergic system in Alzheimer's patients were damaged. On the other hand, the pathological characteristics of AD patients are slow inflammatory pathologic process, and causing cognitive dysfunction and other symptoms.
An EE is an environment with multiple intervention factors, it is a complex of inanimate objects and social stimulation.Citation15 The animals' breeding environment has increased, and the built-in objects are novel and rich, providing multiple sensory stimulation and exercise for animals. Many studies indicate that the EE plays a role in alleviating memory defects in many neurodegenerative diseases such as Alzheimer's disease and Down syndrome. Therefore, this study will combine the EE with AD transgenic mice to reveal the pathological characteristics of AD mice fed in it, and explore its possible mechanism.
The results of this study show that the activity and cognitive function of AD mice which fed in EE were significantly improved, Aβ plaques decreased, the Ach and its enzymes AchE and ChAT in brain were also regulated, while the levels of inflammatory cytokine were suppressed, and the trophic cytokines were increased. These results suggest that the EE improvement in the behavior of AD mice and the down-regulation of Aβ deposition may be achieved by inhibiting the inflammatory factors of AD mice and regulating the activity of ChAT and AchE in the brain to reduce the neurotoxicity of the protein. Whereas, these improvements were not last long. 3 months later, the AD mice previously raised in EE showed almost no difference with the others, which demonstrated that the effects of EE are short-term.
In animal behavioral studies, AD mice reared in EE showed stronger activity and better function of learning and memory, illustrated that EE can improve the behaviors of AD mice, relieve the symptoms of dementia, but this improvement gradually disappeared within 3 months.
It is generally believed that the amyloid cascade hypothesis of Aβ is the pivotal pathogenesis of AD. In the AD patients, senile plaques can be detected in the brain.Citation16 Aβ is the main constituent of the senile plaques, the Aβ plaque and protein in the AD mice fed in EE were significantly reduced, indicating that the course of the disease in AD mice has been alleviated.
Studies found that patients with AD have abnormal brain neurotransmitters, such as excitatory amino acids, norepinephrine, serotonin, etc. But the cholinergic system is the most serious, and it is most closely related to cognitive and behavioral disorders in patients.Citation17 ACh is the main neurotransmitter in hippocampus. The role of hippocampus in spatial working memory depends on the integrity of its cholinergic system. Fadda found that the improvement of animal performance in the learning process of the 12 days continuous radiation labyrinth task was proportional to the increase degree of ACh in the hippocampus.Citation18 Studies showed that the loss of memory in AD patients is associated with the cell death of the cholinergic neurons in the basal forebrain, and the degree of reduction is related to cognitive test. So the low activity of the cholinergic system may play an important role in the pathogenesis of AD. In this study, through the detection of Ach, ChAT and AchE activity, shows EE can regulate their content. Although there were no statistic differences between EE and AD mice, the former presents less inhibition of Ach and ChAT, and less activity of AchE, which may have benefit to restore the activity of the cholinergic system in AD mice.
A large numbers of immune molecules related to inflammation can be detected in AD patients, and many inflammatory factors harmful to normal body are released in AD patients, plaque develops from non inflammatory nerve to diffuse inflammatory nerve plaque, and aggravates nerve injury.Citation19 The most common inflammatory factors include IL-1β, IL-6, TNF-α and IFN-γ. IL-1β is a kind of proinflammatory cytokine, mediate all kinds of acute and chronic inflammation, involved in the destruction of human tissue and other pathological process extensively. Research suggested that IL-6 may have local effects on brain function, promoting the synthesis of Aβ precursor protein from neural cells and precipitating in brain tissue. TNF-α is a cytokine produced by activated macrophages and has a role in promoting inflammation. In the central nervous system, IL-1β and IFN-γ through inhibition of synaptic transmission approach to damage neurons function. IFN-γ was also found to be significantly increased in blood of AD patients. The results of this study show that compared to the control group, the level of IL-1β, IL-6, TNF-α and IFN-γ in AD mice serum were significantly increased. After EE feeding, the level of inflammatory cytokines in the serum of mice have been effectively controlled. Similar with the changes of behavioral tests, the inhibition of these inflammation factors could not be detected while the AD mice reared in standard environment for 3 months. It showed that EE reduced the inflammatory level in serum of AD mice, alleviates the cytotoxicity of inflammatory factors, but this improvement not last long when the EE removed.
As a kind of neurotrophic factor, BDNF plays an important role in the development, regeneration and function maintenance of neurons. It can regulate hippocampal synaptic plasticity and important in cognitive function. The expression of mRNA of BDNF in hippocampus of AD patients is significantly reduced.Citation20 Therefore, increasing the content of BDNF in the brain can improve the viability of neurons, promote the growth of neurons and improve their spatial memory ability. The other cytokine, NGF, also plays an irreplaceable role in the survival of the basal forebrain cholinergic neurons (BFCNs) in the central nervous system. A large number of experiments show that the decrease of NGF level in BFCNs can lead to the reduction of neurons in the cortex and hippocampus of the brain, and the AD like pathological changes gradually happened. In addition, IGF-1, as an important regulator of nerve cells, is found to be involved in many links of AD pathogenesis. This effect is not only expressed at the molecular level, but also at the cell and organism level. In this study, BDNF, NGF and IGF-1 in the brain of AD mice which fed in EE presented a significant increase, further showed a remission and improvement of AD pathological changes. Nevertheless, this effect is also transitory.
In the correlation study of AD patients and environmental factors, it is reported that the diversity of living environment plays an important role in the remission of the disease development process. For example, music therapy can help patients with AD to enhance speech ability, stimulate long and short-term memory, promote self-expression and communication skills, promote relaxation and relieve anxiety.Citation21 Besides, dance intervention therapy has significant effects on the physical-body, sensory, emotional, and social connections of AD patients, such as activate the patient's motor ability, arouse the patient's memory, open their own emotions, restore confidence, improve the patient's ability to communicate. In addition, both physical exercise and mental exercise can alleviate the disease process in AD patients. These interventions are alike the EE mentioned in this study. Compared with the traditional drug therapy and immune therapy, the change of the environment induces multi sensory stimulation, and provides a kind of free stress and entertaining environment for the dementia patients. It has a wide range of application, which can not only improve the mood, behavior, communication and cognition, but also reduce the use of drugs, and avoid the possible side effects to make the patients safer, relieve the economic burden on family and society. It improves the life quality of the AD patients, and may be a promising treatment.
In this study, after a series test of indicators, we found a number of pathological indexes in AD mice reared in EE were improved. The improvements probably related to the regulation of cytokines. The levels of inflammatory factors in AD mice reduced while the neurotrophic cytokines increased, further to regulate the cholinergic system in the brain, reduce the neurotoxicity of Aβ, and improve the behavior of AD mice. The EE plays a role in the treatment of AD, and the treatment without drugs is more safer and reliable. But this improvement can not be observed 3 months after animals leave the EE, and similar researches have reported, for example, Baker, etc found that the improvement observed during the intervention of multiple sensory stimulation therapy (MSS) disappeared after 1 months, while Milev and other studies showed that improvements in behavior still exist 12 weeks after intervention.Citation22 How long can this improvement lasts, and whether the effects can be strengthened or extended under the stimulation of EE repeatedly, these details need to be further explored.
Materials and methods
Experimental animals and groups
6 month old of the male mice were divided into 3 groups, with 8 mice in each group, weight 24±2 g. One group is C57BL/6 mice of wild type, two groups are APP/PS1 transgenic mice of Alzheimer's model obtained from the Model Animal Research Center of Nanjing University. The APP/PS1 mice co-express human KM670/671L-mutated amyloid precursor protein (APP) and M146L-mutated presenilin 1 (PS1), which were on the C57BL/6 congenic background. One group of APP/PS1 transgenic mice were reared in the EE, which were in a large cage (60*40*40 cm), and placed a suitable amount of devices for mice activity, such as runners, pipes, colorful balls, etc. The other two groups were reared in the facilities of temperature control and light cycle control with free access to food and water. The experiment lasted for 8 weeks, and then the mice were all removed into standard environment. It was approved by the Institutional Animal Care and Use Committee of Wannan Medical College.
Detect mice activity by Open-field test
The open field equipment is made up of an open enclosed box with a size of 60* 60*45 cm. The bottom of the box is divided into 6*6 small lattices through the marking line. The center of the top is equipped with a video camera to record the behavior of mice in the open field. Each mouse records 60 min, and all the experiments are carried out in dim light.
Detect the learning and memory function of the mice by Morris-water Maze
The experiment lasted 7 days, the first 1–4 days is the acquisition of place navigation, the hidden platform placed in the first quadrant, and mice were placed into the pool from each quadrant to record the escape latency, which is the average time of the mice to find the platform, reflects the cognitive function of the mice.Citation23 The fifth day is the spatial probe, after removing the hidden platform, mice were placed into the pool from the third quadrant. They were requested to looking for the platform in memory, and the swimming time was 60s. Counted the number of times the mice passed the original platform, and the percentage of swimming time and swimming distance in target quadrant. It reflects the memory ability of the mice. The 6–7 days is the reversal of place navigation, the platform quadrant was placed in the opposite of the original platform quadrant, and repeat the navigation acquisition stage. It reflects the working memory ability.
Labeled Aβ plaques in the brain of the mice by immunofluorescent
Soaked in 4% polycondensation formaldehyde Solution fully fixed after the mice brain perfusion by normal saline, cut the brain tissue into 40 μm drifting slices from the coronal surface with frozen section machine, with 0.01 M PBS bleaching 3 times, and closed in 37°C water bath for 30 min with 1% BSA containing 0.25% Triton X-100, incubated 2 hours in Aβ42 (1:2000; Invitrogen), stay at 4 degrees overnight, take it out in the second day, then incubated in secondary antibodies Goat anti mouse 555 (1:400; Invitrogen) at 37°C water bath for 2 hours. Mount the slices of the brain on the slide proof slide after rinsing the slices, and mounting by glycerol/PBS (1:1), photographing with laser scanning confocal microscope.Citation24
Preparation of soluble and insoluble Aβ1–40 and Aβ1–42 peptides
The brains were removed, minced and then homogenized in 50 mM Tris-HCl buffer containing 2% SDS, and a mixture of protease inhibitors. Lysis buffer and proteinase inhibitor were used. For soluble Aβ, homogenates were centrifuged and supernatants were stored at −70°C for additional analysis. For insoluble, 70% formic acid was added to the pellets for extraction of SDS-insoluble Aβ. After sonication samples were centrifuged, supernatants were stored for analysis. Aβ40 and Aβ42 were analyzed using Aβ ELISA kits (Invitrogen).
Detect the Aβ peptides and cytokines by ELISA
The Aβ peptides and cytokines were detect by ELISA kits. Each well is added to 200 μl sample, 37°C water bath for 2 hours, Washing by PBST and pat dry, then adding Goat anti mouse IgG HRP, 37°C water bath for 1 hour, Washing by PBST and pat dry, adding TMB substrate and avoid light for 10–15 minutes, and then adding stop solution, observed reading by enzyme-linked immunosorbent in 30 minutes, record the result at the wavelength of 490 nm, calculate the corresponding concentration according to the standard curve.Citation25
Detect the activity of Ach, ChAT and AchE in the hippocampus of the mice
The experimental animal were killed by decapitation, take out the brain quickly, rinse the surface blood by pre cold saline, dry with filter paper. Remove the pial vascular. Quickly remove the hippocampus and cerebral cortex tissue of the brain and weighed, respectively, place it into a mini type homogenizer, grinding with anhydrous ethanol, and adding normal saline water to make the homogenate fully.Citation26 Detect the activity of ChAT and AChE according to the Kit instruction.
Labeled Aβ plaques in the brain of the mice by immunofluorescent
Soaked in 4% polycondensation formaldehyde Solution fully fixed after the mice brain perfusion by normal saline, cut the brain tissue into 40 μm drifting slices from the coronal surface with frozen section machine, with 0.01 M PBS bleaching 3 times, and closed in 37°C water bath for 30 min with 1% BSA containing 0.25% Triton X-100, incubated 2 hours in Aβ42 (1:2000; Invitrogen), stay at 4 degrees overnight, take it out in the second day, then incubated in secondary antibodies Goat anti mouse 555 (1:400; Invitrogen) at 37°C water bath for 2 hours. Mount the slices of the brain on the slide proof slide after rinsing the slices, and mounting by glycerol/PBS (1:1), photographing with laser scanning confocal microscope.
Statistical analysis
The data were analyzed by SPSS19.0 statistical software. The data were statistically analyzed by one-way ANOVA. All the results were expressed as x±s. P < 0.05 indicating that the difference was statistically significant.
Abbreviations
| AD | = | Alzheimer's Disease |
| Aβ | = | Amyloid β |
| Ach | = | Acetylcholine |
| AchE | = | Acetylcholinesterase |
| ChAT | = | Choline Acetyltransferase |
| EE | = | Enriched Environment |
| IL-1β | = | Interleukin-1β |
| IL-6 | = | Interleukin-6 |
| TNF-α | = | Tumour Necrosis Factor-α |
| IFN-γ | = | Interferon-γ |
| BDNF | = | Brain Derived Neurotrophic Factor |
| NGF | = | Nerve Growth Factor |
| IGF-1 | = | Insulin-like Growth Factor-1 |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12(4):459–509.
- Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, et al. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7(9):940–6. doi:10.1038/sj.embor.7400784.
- Hickman RA, Faustin A, Wisniewski T. Alzheimer disease and its growing epidemic: risk factors, biomarkers, and the urgent need for therapeutics. Neurol Clin. 2016 Nov;34(4):941–53.
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011 Aug 19;10(9):698–712.
- Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006338.
- Contestabile A. The history of the cholinergic hypothesis. Behav Brain Res. 2011 Aug 10;221(2):334–40.
- Chakrabarti S, Khemka VK, Banerjee A, Chatterjee G, Ganguly A, Biswas A. Metabolic risk factors of sporadic alzheimer's disease: implications in the pathology, pathogenesis and treatment. Aging Dis. 2015 Aug 1;6(4):282–99. doi:10.14336/AD.2014.002.
- Mendiola-Precoma J, Berumen LC, Padilla K, Garcia-Alcocer G. Therapies for prevention and treatment of alzheimer's disease. Biomed Res Int. 2016;2016:2589276. doi:10.1155/2016/2589276.
- Laviola G, Hannan AJ, Macrì S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008 Aug;31(2):159–68. doi:10.1016/j.nbd.2008.05.001.
- Jungling A, Reglodi D, Karadi ZN, Horvath G, Farkas J, Gaszner B, Tamas A. Effects of Postnatal Enriched Environment in a Model of Parkinson's Disease in Adult Rats. Int J Mol Sci. 2017 Feb 14;18(2):E406. doi:10.3390/ijms18020406.
- Zhang J, Guo Y, Wang Y, Song L, Zhang R, Du Y. Long-term treadmill exercise attenuates Aβ burdens and astrocyte activation in APP/PS1 mouse model of Alzheimer's disease. Neurosci Lett. 2017; 666(13):70–77. doi:10.1016/j.neulet.2017.12.025.
- Levi O, Michaelson DM. Environmental enrichment stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J Neurochem. 2007 Jan;100(1):202–10.
- Jankowsky JL, Xu G, Fromholt D, Gonzales V, Borchelt DR. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2003 Dec;62(12):1220–7.
- Wang G, He F, Xu Y, Zhang Y. Immunopotentiator thymosin alpha-1 promotes neurogenesis and cognition in the developing mouse via a systemic Th1Bias. Neurosci Bull. 2017 Dec;33(6):675–84.
- Herring A, Blome M, Ambrée O, Sachser N, Paulus W, Keyvani K. Reduction of cerebral oxidative stress following environmental enrichment in mice with alzheimer-like pathology. Brain Pathol. 2010 Jan;20(1):166–75. doi:10.1111/j.1750-3639.2008.00257.x.
- Pan Y, Short JL, Newman SA, Choy KHC, Tiwari D, Yap C, Senyschyn D, Banks WA, Nicolazzo JA. Cognitive benefits of lithium chloride in APP/PS1 mice are associated with enhanced brain clearance of β-amyloid. Brain Behav Immun. 2018 Mar 12:S0889-1591(18)30055-2.
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27(34):9115–29. doi:10.1523/JNEUROSCI.2361-07.2007.
- Fadda F, Cocco S, Stancampiano R, Rossetti ZL. Long-term voluntary ethanol consumption affects neither spatial nor passive avoidance learning, nor hippocampal acetylcholine release in alcohol-preferring rats. Behav Brain Res. 1999 Aug;103(1):71–6.
- Gonzalez B, Abud EM, Abud AM, Poon WW, Gylys KH. Tau spread, apolipoprotein E, inflammation, and more: Rapidly evolving basic science in alzheimer disease. Neurol Clin. 2017 May;35(2):175–90.
- Li W, Li X, Xin X, Kan PC, Yan Y. MicroRNA-613 regulates the expression of brain-derived neurotrophic factor in Alzheimer's disease. Biosci Trends. 2016 Nov 15;10(5):372–7.
- Sato A. The effect of reminiscence music therapy session on changes in depressive symptoms in elderly persons with dementia. J Music Ther. 2000;29(3):170–82.
- Milev RV, Kellar T, Mc Lean M, Mileva V, Luthra V, Thompson S, Peever L. Multisensory stimulation for elderly with dementia: a 24-week single-blind randomized controlled pilot study. Am J Alzheimer's Dis Other Demen. 2008;23(4):372–6. doi:10.1177/1533317508316681.
- Vorhees CV, Williams MT. Morriswater maze:procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58.
- Zhang Y, Zou J, Yang J, Yao Z. 4Abeta1-15-derived monoclonal antibody reduces more abeta burdens and neuroinflammation than homologous vaccinein APP/PS1 Mice. CurrAlzheimerRes. 2015;12(4):384–97.
- Zhang Y, Li Q, Zou J, Zuo Z, Yao Z. Continuous vaccinations of 4Aβ1-15 induces specific fluctuation of inflammatory factors accompany with pathologic alterations alleviation in APP/PS1 mice. Hum Vaccin Immunother. 2015;11(11):2674–81.
- Wang M, Bi W, Fan K. Ameliorating effect of Alpinia oxyphylla-Schisandra chinensis herb pair on cognitive impairment in a mouse model of Alzheimer's disease. Biomed Pharmacother. 2018 Jan;97:128–35.
