ABSTRACT
Current available human papillomavirus (HPV) vaccines are based on the major capsid protein L1 virus-like particles (VLPs), which mainly induce type-specific neutralizing antibodies against vaccine types. Continuing to add more types of VLPs in a vaccine raises the complexity and cost of production which remains the principal impediment to achieve broad implementation of HPV vaccines, particularly in developing regions. In this study, we constructed 16L1-31L2 chimeric VLP (cVLP) by displaying HPV31 L2 aa.17-38 on the h4 coil surface region of HPV16 L1, and assessed its immunogenicity in mouse model. We found that the cVLP adjuvanted with alum plus monophosphoryl lipid A could induce cross-neutralizing antibody responses against 16 out of 17 tested HPV pseudoviruses, and the titer against HPV16 was as high as that was induced by HPV16 L1VLP (titer > 105), more importantly, titers over 103 were observed against two HR-HPVs including HPV31 (titer, 2,200) and −59 (titer, 1,013), among which HPV59 was not covered by Gardasil-9, and medium or low titers of cross-neutralizing antibodies against other 13 tested HPV pseudoviruses were also observed. Our data demonstrate that 16L1-31L2 cVLP is a promising candidate for the formulation of broader spectrum HPV vaccines.
Introduction
Persistent infection with high-risk types of human papillomavirus (HPV) is the etiological cause of nearly all cervical cancers as well as a proportion of other anogenital and oropharygeal cancers.Citation1,Citation2 Therefore, these HPV-associated cancers are potentially preventable through global implementation of a vaccine that provides protection against infections by all high-risk HPVs, of which nearly 20 types have been identified from the papillomavirus species of α5 (HPV26, −51, −82), α6 (HPV53, −56, −66), α7 (HPV18, −39, −45, −59, −68, −70), α9 (HPV16, −31, −33, −35, −52, −58, −67), and α11 (HPV73).Citation3
Three available HPV L1VLP-based prophylactic vaccines, bivalent Cervarix (contains HPV16, −18 L1VLPs), quadrivalent Gardasil (contains HPV16, −18, −6, −11 L1VLPs), and nonavalent Gardasil-9 (contains HPV16, −18, −31, −33, −45, −52, −58, −6, −11 L1VLPs), have been shown to mainly induce specific neutralizing antibodies and provide protection against vaccine types.Citation4-8 While Gardasil-9 provides a broad protection against seven types of high-risk HPVs, it does not cover other high-risk HPVs such as HPV59, which is the seventh prevalent HR-HPV in cervical cancers of China.Citation9 Moreover, continuing to add more types of VLPs in a vaccine also raises the complexity and cost of production which remains the principal impediment to achieve a broad implementation of HPV vaccines, particularly in low- and middle-income countries, where nearly 90% of cervical cancer deaths occur.Citation10,Citation11 Thus finding methods which could not only broaden the protection spectrum but also reduce the cost of production of current L1VLP vaccines is highly desired.
Unlike L1VLPs induce mainly specific neutralizing antibodies, immunization of L2 N-terminal peptides, which contain conserved epitopes or peptides of L2 aa.17-36 (RG-1 peptide) from HPV16, −58 or −6, is able to induce cross-neutralizing antibodiesCitation12-17 albeit with a weak immunogenicity. Unfortunately, the cross-neutralizing antibody titers induced by these L2-based monomeric protein vaccines were generally low despite various efforts, such as delivering 3 copies of 16RG-1 peptide to FcγR by either Fc fragment or lipidated single-chain fragment of CD64 mAb,Citation18,Citation19 displaying 3 copies of RG-1 peptide from HPV16, −31, −51 by thioredoxin (Trx),Citation20,Citation21 or presenting concatenate L2 fusion peptide with flagellin.Citation22,Citation23 Moreover, even the induced neutralizing antibody titers, i.e. in FcγR targeting protein sera (titer, 1,040 and 1,280)Citation18,Citation19 and flagellin fusion protein sera (titer, 2,640),Citation22 were over 103, they were observed only against HPV16, while neutralizing antibody titers over 103 were not observed in Trx-31/51 L2 sera.Citation21 Therefore the main challenge of L2-based vaccine is to induce high titers of L2-mediated cross-neutralizing antibodies, as higher titers of neutralizing antibodies usually indicate a longer protective immunity.Citation24-27
To this end, the multimeric proteins of VLPs derived from non-enveloped viruses are ideal L2 peptide vectors for the production of high titer neutralizing antibodies, as the inserted epitopes on these VLPs are regularly and repetitively arranged on the surface regions at high density. Currently, adeno associated virus (AAV) VLP,Citation28 hepatis B virus core protein (HBc) VLPCitation29 and HPV16/18 L1VLPCitation14,Citation30-33 have been used to deliver RG-1 peptides as chimeric VLP vaccines and to induce cross-neutralizing antibodies. Successful examples include for example displaying 16RG-1 peptide (16RG-1 VLP)Citation32 or 58RG-1 peptide (58L2 cVLP)Citation14 in the DE loop, or displaying 16RG-1 peptide in the h4 coil of L1 by use of HPV16 cVLPs in rabbits,Citation34 which can induce as high titers of neutralizing antibodies against HPV16 as above 105 Citation14,Citation32,Citation34 and is as comparably high as that was induced by HPV16 L1VLP.Citation14 However, it should be noted that the reported types of HR-HPVs neutralized with high titers of antibody (over 103) in these cVLPs sera were still limited. For example, titer over 103 was observed only against HPV16 in AAV-16/31RG-1 cVLP (titer, 2,560)Citation28 and only against HPV16 and −18 in HBc-16RG-1 cVLP both in mouse seraCitation29; while in the sera of above two HPV16 cVLPs (16RG-1 VLPCitation32 and 58L2 cVLPCitation14) with HPV16 neutralizing antibody titers of above 105,Citation14,Citation32 the titers of cross-neutralizng antibody induced by the inserted L2 epitopes over 103 were observed against only one HR-HPV type (HPV31, titer, 1,833) and one cutaneous HPV type (HPV3, titer, 1,000)Citation32 in 16RG-1 VLP rabbit sera, and against only HPV58 (titer, 1,080)Citation14 in 58L2 cVLP mouse sera although broad neutralizing antibody responses were all observed.
HPV31 was detected in 3.5% invasive cervical cancers worldwide (ranked sixth), and it is more prevalent in South America (ranked third), South Europe (ranked third) and Central America (ranked fourth) than other regions.Citation35 But HPV31 neutralizing antibody titers induced by current 16RG-1 based vaccines, such as Trx-16L2,Citation20 16L2-FcγR targeting proteinsCitation19 and HBc-16RG-1 cVLPs,Citation29 were generally very low or undetectable, except for 16RG-1 VLP that induces HPV31 neutralizing antibodies with a titer of ∼1800 in rabbits.Citation32 Contrarily, another report showed that HPV31 neutralizing antibodies and HPV59, −52, −6 neutralizing antibodies were all undetectable in mouse immune sera of two panels of HPV16 cVLP displaying 16RG-1 peptides either in DE loop or h4 coil of HPV16 L1VLP.Citation29 Unfortunately, titers of HPV31 neutralizing antibodies in current 31RG-1 based vaccines, such as Trx-31L2Citation21 and AAV-16/31RG-1 cVLP,Citation28 were all below 103. Our previous work showed that KLH-31RG-1 peptide could induce relatively high levels of neutralizing antibodies against HPV31 and 59 (titers, 3637 and 505).Citation36 To the best of our knowledge, displaying 31RG-1 peptide by use of HPV cVLP has not been reported. Therefore in the present work we constructed 16L1-31L2 cVLP by inserting 31RG-1 peptide in h4 coil region of HPV16 L1VLP, and found that the chimeric VLP could induce cross-neutralizing antibody responses against 16 out of 17 tested HPV PsVs. The titer against HPV16 was as high as that was induced by HPV16 L1VLP (titer > 105, P > 0.05), and more importantly, titers over 103 were observed against two HR-HPVs including HPV31 and −59 (titers, 2,200 and 1,013) in mouse sera. Because the neutralizing antibody titers of three highly prevalent HR-HPV type 16, −31 and −59 in the chimeric VLP mouse sera were observed, we believe that the cVLP may be attractive in formulating low-cost multi-valent HPV VLP vaccines such as replacing 16L1VLP of Cervarix with the cVLP.
Results
Generation of 16L1-31L2 chimeric proteins
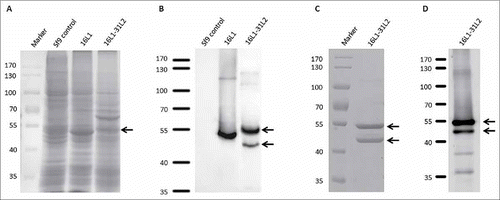
We previously found that HPV31 L2 aa.17-40-KLH peptide induced the production of broad-spectrum neutralizing antibodies.Citation36 So we constructed 16L1-31L2 chimeric protein by displaying HPV31 L2 aa.17-38 on the h4 coil of HPV16 L1 and expressed in baculovirus expression system. The expression of the chimeric protein was confirmed by SDS-PAGE with Coomassie staining () and Western blot analysis with Camvir-1 monoclonal antibody (). The expressed 16L1-31L2 chimeric protein showed a strong band at approximately 55 kDa (, ), which was in accordance with its theoretical molecular weight. In addition, a small band (about 47 kDa) was also observed (). After an ultracentrifugation, the purified protein was further analyzed by SDS-PAGE () and Western blot with polyclonal rabbit serum raised against HPV31 L2 aa.17-40 (). The two bands running at 55 kDa and 47 kDa on SDS-PAGE were observed when 8 μg of chimeric protein was loaded () and were reactive with HPV31 L2 antisera (), indicating that the partially degraded chimeric protein also contained HPV31 L2 peptide.
Figure 1. SDS-PAGE with Coomassie blue staining and Western blot analysis of 16L1-31L2 chimeric protein. Lysate of Sf9 cells expressing 16L1-31L2 or 16L1 protein was analyzed by SDS-PAGE with Coomassie blue staining (A), and Western blot with Camvir-1 MAb (B). Purified 16L1-31L2 chimeric protein was tested by SDS-PAGE with Coomassie blue staining (C) and Western blot with anti-HPV31 L2 polyclonal rabbit serum (D).
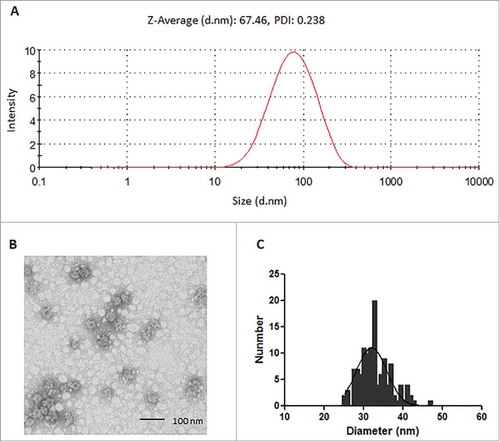
Dynamic light scattering analysis of the purified 16L1-31L2 chimeric proteins showed the uniform hydrodynamic diameter distribution with a mean polydispersity index of 0.238 and an average hydrodynamic diameter of 67.46 nm (). Transmission electron microscopy analysis showed that the chimeric proteins were assembled into VLPs with diameters ranged from 25–48 nm (average diameter, 32 nm) (, ), including a minor portion of regular VLPs and a major portion of small VLPs. These results suggested that the insertion of L2 peptide may have some impact on VLP assembly. It was reported that the insertion of HPV33 RG-1 peptide or HPV58 L2 aa. 55–74 peptide into the h4 coil region of HPV18 L1VLP resulted in smaller sized VLPs (diameter, 20–40 nm), but the HPV18-specific immunogenicity was not affected.Citation30 Thus we speculate that the 16L1-31L2 cVLP may also provide similar immunogenicity as regular sized VLPs.
Figure 2. DLS and TEM analysis of 16L1-31L2 chimeric protein. Pure 16L1-31L2 protein was analyzed by DLS (A) and TEM with a magnification of 80,000 × (B). Bar = 100 nm. The diameter distribution of 16L1-31L2 cVLP visualized by TEM was shown by frequency histogram (C).
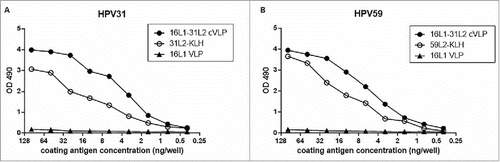
The exposure of HPV31 L2 peptide in 16L1-31L2 cVLP was evaluated by ELISA. HPV31 L2 antisera () and HPV59 L2 antisera () bound strongly to 16L1-31L2 cVLP and HPV31 L2-KLH or HPV59 L2-KLH, but not to HPV16 L1VLP, suggesting that HPV31 L2 peptide in 16L1-31L2 cVLP was well exposed.
Figure 3. Binding activities of 16L1-31L2 cVLP with antisera of HPV31 or −59 L2 peptides. ELISA plates were coated with serial dilutions of 16L1-31L2 cVLP, HPV16 L1VLP, HPV31 L2-KLH and HPV58 L2-KLH (0.39–100 ng/well) respectively. Binding of HPV31 L2 antisera (A) or HPV59 L2 antisera (B) at 1:3000 dilution was detected. Reactivity was determined by measuring the mean optical density (OD) values at 490 nm. The experiment was repeated twice.
The 16L1-31L2 cVLP induced cross-reactive antibodies against L2 peptides from divergent HPV types
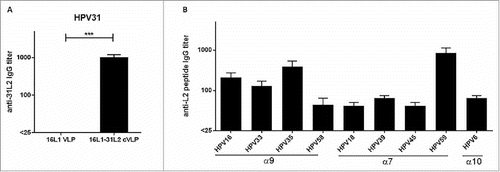
To test the ability of 16L1-31L2 cVLP to induce antibody responses against L2 peptides, sera from mice that were immunized with Alum-MPL adjuvanted 16L1-31L2 cVLPs were analyzed by ELISA (). The 16L1-31L2 cVLP induced robust serum antibodies against HPV31 L2 (mean titer, 1000), while HPV16 L1VLP antisera have no detectable antibodies against HPV31 L2 (). Then we detected the cross-reactive antibodies against L2 peptides from other HPV types. As shown in , cross-reactive antibodies were detected against KLH-L2 fusion proteins from high-risk HPV16, −33, −35, −58 (α9) (titers, 200, 125, 375, 42.5 respectively), HPV18, −39, −45, −59 (α7) (titers, 40, 62.5, 40, 825 repsectively), and low-risk HPV6 (α10) (titer, 62.5), among which titer against HPV59 was the highest. Contrarily, cross-reactive antibodies against the above L2 peptides were undetectable in HPV16 L1VLP antisera (not shown). Therefore, 16L1-31L2 cVLP was able to induce cross-reactive antibodies against L2 peptides from all of 10 detected HPV types.
Figure 4. Cross-reactive antibody titers in sera from mice vaccinated with 16L1-31L2 cVLP. Mice (n = 4) were immunized subcutaneously at weeks 0, 4, 7 and 10 with HPV16 L1VLPs or 16L1-31L2 cVLPs formulated in Alum-MPL, and sera were collected at week 12. KLH-conjugated L2 peptides from HPV6/16/18/31/33/35/39/45/58/59 were coated in ELISA plates, and sera IgG titers against L2 peptides from HPV31 (A) were determined. The sera of 16L1DE-31L2 cVLP were also analyzed for sera IgG against L2 peptides from other 9 HPV types (B). Data are presented as mean titers ± SEM. The statistically significant differences were indicated by: ***, P < 0.001.
The 16L1-31L2 cVLP induced cross-neutralizing antibody responses in mice
To elucidate whether the induced cross-reactive antibodies also have neutralizing activities, the immune sera were further determined by standard pseudovirus-based neutralization assay (PBNA).
Firstly, we detected the neutralizing antibody titers against HPV16. The 16L1-31L2 cVLP induced robust neutralizing antibody responses against HPV16 with a mean titer of 115,200, which is comparable to that was induced by HPV16 L1VLP (P > 0.05, ). This result indicates that the major neutralizing epitopes of HPV16 L1VLP are maintained irrespective of the insertion of HPV31 L2 aa.17-38 peptide. Because 16L1-31L2 cVLPs are smaller than HPV16 L1VLPs (the average diameters are 32 nm and 55 nm, respectively), thus these results demonstrate that the immunogenicity of L1 of the small VLP and regular VLP was comparable with the dose used here.
Figure 5. 16L1-31L2 cVLPs induced broadly cross-neutralizing antibody responses in mice. Mice (n = 4) were vaccinated subcutaneously at weeks 0, 4, 7, 10 with 16L1 VLP or 16L1-31L2 cVLP adjuvanted with Alum-MPL. Sera were collected at week 12 and analyzed for neutralization against HPV16 (A) and HPV31 (B). The sera of 16L1DE-31L2 cVLP were also analyzed for cross-neutralization against other 15 HPV types (C). Data are presented as mean titers ± SEM. The statistically significant differences were indicated by: ns, P > 0.05; **, P < 0.01. The dash line indicated a titer of 1,000.
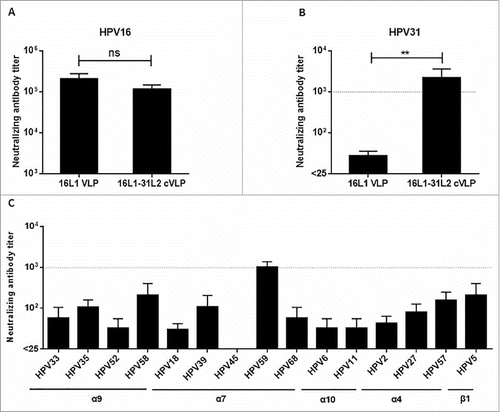
Secondly, we analyzed whether the immune sera could neutralize HPV31. Because it was reported that HPV16 L1VLP induces low level of cross-neutralizing antibodies against HPV31,Citation37 we therefore used HPV16 L1VLP immune sera as a control group. We found that the titer of HPV31 neutralizing antibodies induced by 16L1-31L2 cVLP (titer, 2,200) was significantly higher than that induced by HPV16 L1VLP (titer, 25; P < 0.01, ). Thus we believe that the HPV31 neutralizing antibodies in 16L1-31L2 cVLP immune sera are composed of mainly 31L2-derived neutralizing antibodies.
Next, we selected another 15 types of HPV PsVs to examine the activity of cross-neutralizing antibodies. As shown in , 16L1-31L2 cVLP induced robust titer of HPV59 neutralizing antibodies (titer, 1,013), which is comparable to the titer of HPV31. In addition, medium titers of neutralizing antibodies were detected against HPV58, −5, −57, −35, −39 (titer, 100–206), and low titers of neutralizing antibodies against HPV27, −33, −68, −2, −52, −18, −6, −11 (titer, 25–75) were also observed.
The preliminary data of this study showed that 4 immunizations of low dose (0.1-2 μg) of cVLPs induced only low titers of L2-directed neutralizing antibodies against one non-vaccine type, although comparably high titers of L1-directed neutralizing antibodies as that of HPV16 L1 VLP were induced. Cross-neutralizing antibody responses induced by 3 doses of 10 μg cVLPs was impressive (titer, ∼102), but lower than that of 4 dose immunizations. To guarantee relatively high titers of L2-directed neutralizing antibodies, a relatively high dose of L2-cVLP would be highly recommended in clinical trials. 60 μg of HPV16 L1 VLPs in Gardasil-9 was shown to be well tolerated, thus 60 μg or more cVLPs per dose may be encouraged in clinical trial.
These data demonstrated that the 16L1-31L2 cVLP sera could neutralize both HPV16 with a similarly high titer to that of HPV16 L1VLP sera (titer>105) and HPV31 as well as −59 with robust titer (titer>103) in mice. In our previous study, the 16L1-58L2 cVLP also induced the highest titer of HPV16 neutralizing antibodies and cross-neutralizing antibodies to multiple HPV types in mice, but among which only titer of HPV58 neutralizing antibody was robust (titer>103).Citation14 Considering a higher neutralizing antibody level usually represents a longer protection, it is tempting to speculate that the 16L1-31L2 cVLP may provide a better protective immunity against HPV16, −31 and −59.
Discussion
16RG-1 peptide, i.e. the linear amino acid sequence of HPV16L2 aa.17-36 that is conserved among papillomaviruses, was shown to contain major neutralizing epitopes of 16L2 protein.Citation16,Citation38,Citation39 Therefore, the RG-1 peptides, including HPV16 and other HPV types such as HPV58, −45, −33, −31, −51, or the L2 N-terminal peptides were usually used to construct L2-based vaccines.Citation14,Citation18,Citation19,Citation21,Citation28,Citation30-32,Citation34 The highest titers of L2-mediated neutralizing antibodies induced by these vaccines were reported to range from 1,000-6,400 when different vaccine vectors and various adjuvants including Freud's adjuvant were used.Citation14,Citation18,Citation19,Citation21,Citation28,Citation30-32,Citation34 Contrarily, the highest antibody titers induced by L1VLP are usually ranged from 105–106.Citation14,Citation31,Citation33 The reasonable explanation for this different antibody titers induction is that many conformational epitopes are exposed on the surface of L1VLPs, which could be valuable for the induction of high titer antibodies, whereas epitopes in RG1 peptides are linear sequences, which usually induce low titers of antibodies. We believe that the highest antibody titers induced by RG-1 peptides or other L2 antigens are difficult to be further promoted to a level of over 104. In this study, the highest titer of cross-neutralizing antibodies induced by 16L1-31L2 cVLP reached 2,200, titers over 103 were observed against HPV31 and −59 (titers, 2,200 and 1,013), and medium to low titers of cross-neutralizing antibodies against other 13 HPV types were also observed (titer, 25–206). Moreover, the titers of vector type (HPV16) specific antibodies were as high as that induced by HPV16 L1VLP (titer > 105, P > 0.05) with the same immunization dose (10 μg), indicating that the major conformational epitopes of the L1VLP vector were maintained very well in 16L1-31L2 cVLP. To be noted, reports showed that L1- and L2-directed antibodies neutralize viruses by binding at different sites on the capsids respectively,Citation40 and L2-directed neutralizing antibodies and protective immunity induced by L2-cVLP sustained for at least 82 weeks and 85 weeks in mice.Citation14 Thus extensively measure L2-directed antibodies with a physiologically more relevant assay (more sensitive than standard neutralization assay used in this studyCitation41) and L1-directed neutralizing antibodies, will be helpful for investigations on the protective threshold L1 titer for HPV L1 VLP vaccines and protective threshold L1/L2 titers for HPV L2-cVLP vaccines.
In previous reports on HPV cVLP, RG-1 peptides were usually inserted into the DE loop of L1. In this study, we also inserted 31RG-1 peptide into the DE loop of HPV16 L1VLP, but in the resultant cVLP mouse sera, cross-neutralizing antibody titers over 103 were not detected (Fig. S1). So we think that 31RG-1 epitopes were displayed more properly in the h4 coil than DE loop of HPV16 L1. This was different from 16RG-1 and 58RG-1 peptides, which were displayed more properly in the DE loop rather than in the h4 coil of HPV16 L1.Citation14,Citation33,Citation34 Prediction data by homology modeling showed that secondary structure of 31RG-1 peptide in the DE loop was different from that in the h4 coil regions of HPV16 cVLPs (Fig. S2). We thus speculate that the differences in the amino acid sequences of RG-1 peptides and their flanking regions at both ends, as well as the local secondary structures around the insertion site may all have an influence on the displaying of RG-1 peptides.
Most of reports on HPV cVLPs used rabbit model to assess the neutralizing antibody responsesCitation14,Citation30,Citation31,Citation33,Citation42 because rabbits usually produce high titers of antibody responses, whereas titers of antibodies produce by mice were relatively low.Citation14,Citation22,Citation28,Citation33 For example, BPV L1-16L2 cVLPCitation33 and 16L1-58L2 cVLPCitation14 induced higher titers of cross-neutralizing antibodies in rabbits than in mice. For 16L1-58L2 cVLP vaccine, the titers of HPV6, −31, −57, −11 were all above 103 (2,600, 2,025, 1,100 and 1,000, respectively) in rabbit sera, but they were only 165, 25, 160 and 90, respectively, in mouse sera.Citation14 In this study, we used mouse model for immunogenicity analysis, and found that the titers of HPV58, −5, −57, −35, −39 induced by 16L1-31L2 cVLP were as high as 100–206. We thus believe that when the cVLP in this work was used in rabbits, theoretically higher titers of antibodies against HPV58, −5, −57, −35, −39 will be expected, as 16L1-58L2 cVLP induced higher titers of neutralizing antibodies in rabbits.
Passive transfer of L1VLP and L2 immune sera was shown to provide naïve animals against challenges with papillomaviruses from native virionsCitation43,Citation44 or PsVs,Citation42,Citation45-47 and neutralizing antibody titers were analyzed to evaluate the immunity induced by HPV vaccines.Citation25,Citation48,Citation49 Dynamic analysis of neutralizing antibodies (including cross-neutralizing antibodies) that are induced by HPV L1VLP or L2 in animal modelsCitation14 as well as clinical trialsCitation27,Citation50 demonstrated that the neutralizing antibodies with higher titers usually take a longer time to decline, which therefore is predicted to provide a longer protective immunity. In this study, robust neutralizing antibody responses of three highly prevalent HR-HPVs were observed in 16L1-31L2 cVLP mouse sera and the L1VLP-directed antibody titers of HPV16 were as high as over 105. More importantly, the L2-directed antibody titers of HPV31 and −59 were over 103. Whereas the L2-directed neutralizing antibody titers above 103 that were induced by current HPV cVLPs were either not observed or against only one HR-HPV type (HPV31 or −58).Citation14,Citation33 So we believe that the 16L1-31L2 cVLP may have some superiority in updating HPV L1VLP vaccines, for example, replace the HPV16 L1VLP in bivalent L1VLP vaccines with 16L1-31L2 cVLP will be helpful to broaden the protection spectrum meanwhile reduce the complexity. Immune interference was not observed in animal model studies on co-immunization of HPV18 cVLPs that were produced in baculovirus system and bivalent L1VLP vaccine, as the titers of both L2-directed cross-neutralizing antibodies and L1VLP-type specific neutralizing antibodies were not reduced,Citation30 which is consistent with our unpublished data of co-administration of 16L1-58L2 cVLP and 18L1 VLP.
In conclusion, our study demonstrates that the 16L1-31L2 cVLP is a promising HPV16 cVLP for the development of a low-cost HPV vaccine with broad spectrum.
Materials and methods
Construction, expression and purification of 16L1-31L2 chimeric protein
Based on our previous work, HPV16 L1VLPs were expressed at high level in baculovirus expression system with an optimized HPV16 L1 gene (accession number: GU556964).Citation51 In this study, HPV31 L2 aa.17-38 gene sequence was genetically engineered into a h4-βJ coil of the above HPV16 L1 between nt.1290 and nt.1297 (aa.430 and aa.433) by PCR strategy. The 16L1-31L2 construct was cloned into a pFastBac1 vector and expressed in the Bac-to-Bac baculovirus expression system according to the manufacturer's instructions (Invitrogen).
The expressed 16L1-31L2 chimeric proteins were purified by CsCl ultracentrifugation as described previously.Citation14,Citation51 Briefly, the cell lysate was purified by ultracentrifugation on 27% CsCl (w/w)-PBS density gradients for 20 h, then the interested fractions were collected and dialyzed against 0.5 M NaCl-PBS at 4°C for 2–3 h. After an ultra-centrifugation on 5% sucrose (w/w)/60% CsCl (w/w)-0.5 M NaCl-PBS for 2.5 h, the collected fraction was then loaded on 27% CsCl (w/w)-0.5 M NaCl-PBS density gradients and centrifuged for 20 h. VLPs were dialyzed against PBS at 4°C for 3 days. Cell lysates or purified proteins were analyzed by SDS-PAGE with Coomassie blue staining or Western blot with Camvir-1 (MILLIPORE, MAB885) or polyclonal rabbit serum raised against HPV31 L2 aa.17-40.
Dynamic light scattering (DLS) and transmission electron microscopy (TEM)
DLS measurements were performed on a Malvern Zetasizer Nano ZS (Malvern). Samples were equilibrated to 25 °C before analysis. The hydrodynamic size was recorded as Z-average hydrodynamic diameter. The average data from three independent measurements of the same sample were reported.
For TEM analysis, purified proteins were adsorbed on a carbon-coated grid for 1 minute, rinsed with distilled water, negatively stained with 1% uranyl acetate for 3 minutes and examined with a TEM-1400 electron microscope operating at 80 kV with a magnification of 80,000 ×. The particle size distribution histogram was generated from diameters of 150 VLPs measured randomly.
Enzyme linked immunosorbent assay (ELISA)
The binding of 16L1-31L2 cVLP to HPV L2-KLH immune sera was analyzed by ELISA as described before.Citation19 Briefly, ELISA plates were coated with serial dilutions of 16L1-31L2 cVLPs, HPV16 L1VLPs, HPV31 L2 aa.17-40-KLH and HPV58 L2 aa.15-37-KLH (0.39–100 ng/well) at 4°C overnight, and then blocked with 5% bovine serum albumin (BSA) at room temperature for 2 h. Immune sera of HPV31 L2-KLH and HPV59 L2-KLH (diluted at 1:3000) were respectively added to the plates and incubated at room temperature for 2 h, followed by 3 washes with PBS. HRP-conjugated goat anti-rabbit IgG (1:3000) was added and incubated at 37°C for 1 h. Enzyme activity was revealed with substrate O-phenylenediamine (Sigma-Aldrich, Missouri, USA) and the reaction was stopped by 2M H2SO4. The absorbance was measured at 490 nm.
The sera IgG titers against different types of HPV L2 peptides were also analyzed by ELISA. Briefly, plates were respectively coated with HPV6 L2 aa.16-39-KLH, HPV16 L2 aa.17-36-KLH, HPV18 L2 aa.16-39-KLH, HPV31 L2 aa.17-40-KLH, HPV33 L2 aa.16-39-KLH, HPV35 L2 aa.16-38-KLH, HPV39 L2 aa.16-38-KLH, HPV45 L2 aa.16-39-KLH, HPV58 L2 aa.15-37-KLH or HPV59 L2 aa. 18-40-KLH (100 ng/well) at 4°C overnight. After blocking, plates were incubated with serially diluted immune sera at RT for 2 h, and then incubated with HRP-conjugated goat anti-mouse IgG (1:3000) at 37 °C for 1 h. Enzyme activity was detected as described above. The binding titer was determined as the reciprocal of the highest serum dilution with an OD490 greater than 0.1 and 2-fold higher than control sera at the same dilution.
Animal immunizations
BALB/c mice were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences, and kept in the animal facility of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. All animal studies were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Science, and all protocols were reviewed and approved by the Institutional Animal Care and Use Committee.
Female BALB/c mice (n = 4) were vaccinated subcutaneously at weeks 0, 4, 7 and 10 with 10 μg of 16L1-31L2 cVLPs formulated with Alum-MPL, respectively. HPV16 L1VLPs adjuvanted with Alum-MPL was used as a control. At week 12, sera were collected and subjected to ELISA analysis and pseudovirus-based neutralization assay.
HPV pseudovirus (PsV) preparation
PsVs of HPV2, −5, −6, −11, −16, −18, −27, −31, −33, −35, −39, −45, −52, −57, −58, −59, −68 with reporter plasmid pfwB which encoding green fluorescence protein (GFP) were produced in 293TT cells as previously described.Citation51-53
Standard PsV-based neutralization assay (PBNA)
Standard PBNA was performed as described.Citation51-53 Briefly, 293TT cells were seeded in 96-well plate at 1.5 × 104 cells/well and incubated overnight at 37 °C. The mixture of serially diluted antisera and PsVs were transferred to pre-plated 293TT cells and cultured for 72 h. The 293TT cells were harvested by trypsin digestion, and then detected by FACS. The endpoint titer was calculated as the reciprocal of the highest serum dilution with percent infection inhibition higher than 50%.
Data analysis
Statistical significance was determined by two-tailed, unpaired t-test using Graphpad Prism 5.0. P values < 0.05 were considered to be statistically significant.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
KHVI_A_1464355_Supplementary_Materials.docx
Download MS Word (444.9 KB)Acknowledgments
We would like to thank Prof. John T. Schiller, Dr. Susana Pang, Dr. Christopher B. Buck (National Cancer Institute, Maryland) and Prof. Martin Müller (German Cancer Research Center) for their generously offering 293TT cells, pfwB plasmid, p2SHELL plasmid, p5SHELL plasmid, p6SHELL plasmid, p11L1w plasmid, p11L2w plasmid, p16SHELL plasmid, p18SHELL plasmid, p31SHELL plasmid, p45SHELL plasmid, p68SHELL plasmid; Prof. Tadahito Kanda (National Institute of Infectious Diseases, Tokyo) for his kindly providing p58SHELL plasmid and p52SHELL plasmid; Prof. Lutz Gissmann (German Cancer Research Center) for his kindly providing HPV27L1 plasmid, HPV27L2 plasmid, HPV57L1 plasmid, HPV57L2 plasmid; and Dr. Simon Beddows and Dr. Phil Luton (Health Protection Agency, UK) for their offering p35SHELL plasmid, p39SHELL plasmid and p59SHELL plasmid.
Additional information
Funding
References
- de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol [Internet]. 2012 [cited 2016 Aug 21];13:607–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22575588 doi:10.1016/S1470-2045(12)70137-7.
- Cubie HA. Diseases associated with human papillomavirus infection. Virology [Internet]. 2013;445:21–34. Available from: doi:10.1016/j.virol.2013.06.007.
- Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer? J Pathol. 2014;234:431–5. doi:10.1002/path.4424. PMID:25124771
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007;369:2161–70. doi:10.1016/S0140-6736(07)60946-5. PMID:17602732
- Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine [Internet]. 2012;30:F123–38. Available from: doi:10.1016/j.vaccine.2012.04.108.
- Garland SM, Kjaer SK, Muñoz N, Block SL, Brown DR, Dinubile MJ, Lindsay BR, Kuter BJ, Perez G, Dominiak-Felden G, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: A systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;63:519–27. doi:10.1093/cid/ciw354. PMID:27230391
- Romanowski B, Schwarz TF, Ferguson L, Peters K, Dionne M, Behre U, Schulze K, Hillemanns P, Suryakiran P, Thomas F, et al. Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: Five-year clinical data and modeling predictions from a randomized study. Hum Vaccin Immunother [Internet]. 2016;12:20–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26176261 doi:10.1080/21645515.2015.1065363.
- Vesikari T, Brodszki N, van Damme P, Diez-Domingo J, Icardi G, Petersen LK, Tran C, Thomas S, Luxembourg A, Baudin M. A randomized, double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) Versus Gardasil® in 9–15-year-old girls. Pediatr Infect Dis J [Internet]. 2015;34:992–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26090572 doi:10.1097/INF.0000000000000773.
- Meta-analysis performed by IARC's Infections and Cancer Epidemiology Group [Internet]. ICO HPV Inf. Cent. [cited 2017 Aug 30]; Available from: http://www.hpvcentre.net/parser.php?xml=M1_Cervical cancer by histology_HPV type distribution&iso=XWX_CHN&title=M1. HPV prevalence estimates – Invasive cervical cancer – HPV type distribution (Country/Regions)
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin [Internet]. 2015;65:87–108. Available from: doi:10.3322/caac.21262.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J cancer [Internet]. 2015;136:E359–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25220842 doi:10.1002/ijc.29210.
- Pastrana D V, Gambhira R, Buck CB, Pang YYS, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RBS. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–72. doi:10.1016/j.virol.2005.04.011. PMID:15885736
- Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RBS. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009;101:782–92. doi:10.1093/jnci/djp106. PMID:19470949
- Chen X, Liu H, Wang Z, Wang S, Zhang T, Hu M, Qiao L, Xu X. Human papillomavirus 16L1-58L2 chimeric virus-like particles elicit durable neutralizing antibody responses against a broad-spectrum of human papillomavirus types. Oncotarget [Internet]. 2017;8:63333–44. Available from: http://www.oncotarget.com/fulltext/19327
- Roden RBS, Yutzy WH, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing Epitopes. Virology [Internet]. 2000;270:254–7. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0042682200902721 doi:10.1006/viro.2000.0272.
- Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RBS. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol [Internet]. 2007;81:13927–31. Available from: doi:10.1128/JVI.00936-07.
- Kawana Y, Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Human papillomavirus type 16 minor capsid protein l2 N-terminal region containing a common neutralization epitope binds to the cell surface and enters the cytoplasm. J Virol [Internet]. 2001;75:2331–6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=114816&tool=pmcentrez&rendertype=abstract doi:10.1128/JVI.75.5.2331-2336.2001.
- Chen X, Liu H, Zhang T, Liu Y, Xie X, Wang Z, Xu X. A vaccine of L2 epitope repeats fused with a modified IgG1 Fc induced cross-neutralizing antibodies and protective immunity against divergent human papillomavirus types. PLoS One [Internet]. 2014;9:e95448. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24802101 doi:10.1371/journal.pone.0095448.
- Zhang T, Liu H, Chen X, Wang Z, Wang S, Qu C, Zhang J, Xu X. Lipidated L2 epitope repeats fused with a single-chain antibody fragment targeting human FcγRI elicited cross-neutralizing antibodies against a broad spectrum of human papillomavirus types. Vaccine [Internet]. 2016;34:5531–9. Available from: doi:10.1016/j.vaccine.2016.10.009.
- Rubio I, Bolchi A, Moretto N, Canali E, Gissmann L, Tommasino M, M??ller M, Ottonello S. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20-38) peptide displayed on bacterial thioredoxin. Vaccine 2009;27:1949–56. doi:10.1016/j.vaccine.2009.01.102. PMID:19368776
- Seitz H, Canali E, Ribeiro-Müller L, Pàlfi A, Bolchi A, Tommasino M, Ottonello S, Müller M. A three component mix of thioredoxin-L2 antigens elicits broadly neutralizing responses against oncogenic human papillomaviruses. Vaccine 2014;32:2610–7. doi:10.1016/j.vaccine.2014.03.033. PMID:24662712
- Kalnin K, Tibbitts T, Yan Y, Stegalkina S, Shen L, Costa V, Sabharwal R, Anderson SF, Day PM, Christensen N, et al. Low doses of flagellin-L2 multimer vaccines protect against challenge with diverse papillomavirus genotypes. Vaccine 2014;32:3540–7. doi:10.1016/j.vaccine.2014.04.032. PMID:24780250
- Kalnin K, Chivukula S, Tibbitts T, Yan Y, Stegalkina S, Shen L, Cieszynski J, Costa V, Sabharwal R, Anderson SF, et al. Incorporation of RG1 epitope concatemers into a self-adjuvanting Flagellin-L2 vaccine broaden durable protection against cutaneous challenge with diverse human papillomavirus genotypes. Vaccine [Internet]. 2017;35:4942–51. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0264410X17310125 doi:10.1016/j.vaccine.2017.07.086.
- Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, Salmerón J, McNeil S, Stapleton JT, Bouchard C, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis [Internet]. 2016 [cited 2016 Jul 14];0:1048–56. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1473309916301207
- GlaxoSmithKline Vaccine HPV-007 Study Group, Romanowski B, de Borba PC, Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier RM, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet (London, England) [Internet]. 2009;374:1975–85. Available from: doi:10.1016/S0140-6736(09)61567-1.
- De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, Sanchez N, Schuind A. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine [Internet]. 2010;28:6247–55. Available from: doi:10.1016/j.vaccine.2010.07.007.
- Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, Lalezari J, David M-P, Lin L, Struyf F, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: end-of-study analysis of a Phase III randomized trial. Hum Vaccin Immunother [Internet]. 2014;10:3435–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25483701 doi:10.4161/hv.36121.
- Nieto K, Weghofer M, Sehr P, Ritter M, Sedlmeier S, Karanam B, Seitz H, Müller M, Kellner M, Hörer M, et al. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS One [Internet]. 2012;7:e39741. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22761884 doi:10.1371/journal.pone.0039741.
- Wang D, Li Z, Xiao J, Wang J, Zhang L, Liu Y, Fan F, Xin L, Wei M, Kong Z, et al. Identification of Broad-Genotype HPV L2 Neutralization Site for Pan-HPV Vaccine Development by a Cross-Neutralizing Antibody. PLoS One [Internet]. 2015;10:e0123944. Available from: doi:10.1371/journal.pone.0123944.
- Boxus M, Fochesato M, Miseur A, Mertens E, Dendouga N, Brendle S, Balogh KK, Christensen ND, Giannini SL. Broad cross-protection is induced in preclinical models by a human papillomavirus vaccine composed of L1/L2 chimeric virus-like particles. J Virol [Internet]. 2016;90:6314–25. Available from: doi:10.1128/JVI.00449-16.
- Huber B, Schellenbacher C, Jindra C, Fink D, Shafti-Keramat S, Kirnbauer R. A chimeric 18L1-45RG1 virus-like particle vaccine cross-protects against oncogenic alpha-7 human papillomavirus types. PLoS One 2015;10:1–18. doi:10.1371/journal.pone.0120152.
- Schellenbacher C, Kwak K, Fink D, Shafti-Keramat S, Huber B, Jindra C, Faust H, Dillner J, Roden RBS, Kirnbauer R. Efficacy of RG1-VLP vaccination against infections with genital and cutaneous human papillomaviruses. J Invest Dermatol [Internet]. 2013;133:2706–13. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3826974&tool=pmcentrez&rendertype=abstract doi:10.1038/jid.2013.253.
- Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol [Internet]. 2009;83:10085–95. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2748020&tool=pmcentrez&rendertype=abstract doi:10.1128/JVI.01088-09.
- Kondo K, Ochi H, Matsumoto T, Yoshikawa H, Kanda T. Modification of human papillomavirus‐like particle vaccine by insertion of the cross‐reactive L2‐epitopes. J Med Virol. 2008;80:841–6. doi:10.1002/jmv.21124. PMID:18360909
- Bruni L, Barrionuevo-Rosas L, Albero G, Aldea M, Serrano B, Valencia S, Brotons M, Mena M, Cosano R, Muñoz J, Bosch FX, de Sanjosé S CX. Human papillomavirus and related diseases in the world. ICO Inf Cent HPV Cancer [Internet]. 2016; Available from: www.hpvcentre.com
- Zhang T, Chen X, Liu H, Zhou Y, Wang Z, Xu X. Conservative epitope of human papillomavirus type 31 L2 can induce broad-spectrum neutralizing antibodies. Basic Clin Med. 2017;37:1552–6.
- Combita AL, Touze A, Bousarghin L, Christensen ND, Coursaget P. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J Virol [Internet]. 2002;76:6480–6. Available from:http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12050360 doi:10.1128/JVI.76.13.6480-6486.2002.
- Conway MJ, Cruz L, Alam S, Christensen ND, Meyers C. Cross-neutralization potential of native human papillomavirus N-terminal L2 epitopes. PLoS One [Internet]. 2011 [cited 2016 Sep 26];6:e16405. Available from: doi:10.1371/journal.pone.0016405.
- Rubio I, Seitz H, Canali E, Sehr P, Bolchi A, Tommasino M, Ottonello S, Müller M. The N-terminal region of the human papillomavirus L2 protein contains overlapping binding sites for neutralizing, cross-neutralizing and non-neutralizing antibodies. Virology [Internet]. 2011 [cited 2016 Sep 26];409:348–59. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0042682210006641 doi:10.1016/j.virol.2010.10.017.
- Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, Schiller JT. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe [Internet]. 2010;8:260–70. Available from: doi:10.1016/j.chom.2010.08.003.
- Day PM, Pang YYS, Kines RC, Thompson CD, Lowy DR, Schiller JT. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol [Internet]. 2012 [cited 2016 Sep 26];19:1075–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22593236 doi:10.1128/CVI.00139-12.
- Huber B, Schellenbacher C, Shafti-Keramat S, Jindra C, Christensen N, Kirnbauer R. Chimeric L2-based virus-like particle (VLP) vaccines targeting cutaneous human papillomaviruses (HPV). PLoS One [Internet]. 2017 [cited 2017 Feb 8];12:e0169533. Available from: doi:10.1371/journal.pone.0169533.
- Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol [Internet]. 1995 [cited 2017 Sep 8];69:3959–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7745754
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci [Internet]. 1995 [cited 2017 Sep 8];92:11553–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8524802 doi:10.1073/pnas.92.25.11553.
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med [Internet]. 2007 [cited 2017 Sep 8];13:857–61. Available from: doi:10.1038/nm1598.
- Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol [Internet]. 2011;85:13253–9. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd = Retrieve&db=PubMed&dopt=Citation&list_uids=21976653 doi:10.1128/JVI.06093-11.
- Seitz H, Ribeiro-Muller L, Canali E, Bolchi A, Tommasino M, Ottonello S, Muller M. Robust in vitro and in vivo neutralization against multiple high-risk HPV types induced by a thermostable thioredoxin-L2 vaccine. Cancer Prev Res [Internet]. 2015;8:932–41. Available from: doi:10.1158/1940-6207.CAPR-15-0164.
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David M-PM, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin [Internet]. 2008;4:425–34. Available from:http://www.ncbi.nlm.nih.gov/pubmed/18948732 doi:10.4161/hv.4.6.6912.
- Villa LL, Costa RLR, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–8. doi:10.1016/S1470-2045(05)70101-7. PMID:15863374
- Einstein MH, Levin MJ, Chatterjee A, Chakhtoura N, Takacs P, Catteau G, Dessy FJ, Moris P, Lin L, Struyf F, et al. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: follow-up through Month 48 in a Phase III randomized study. Hum Vaccin Immunother [Internet]. 2014;10:3455–65. Available from: doi:10.4161/hv.36117.
- Zhang T, Xu Y, Qiao L, Wang Y, Wu X, Fan D, Peng Q, Xu X. Trivalent Human Papillomavirus (HPV) VLP vaccine covering HPV type 58 can elicit high level of humoral immunity but also induce immune interference among component types. Vaccine [Internet]. 2010;28:3479–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20211219 doi:10.1016/j.vaccine.2010.02.057.
- Buck CB, Pastrana D V, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med [Internet]. 2005 [cited 2016 Aug 15];119:445–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16350417
- Pastrana DV, Buck CB, Pang Y-YS, Thompson CD, Castle PE, FitzGerald PC, Krüger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology [Internet]. 2004 [cited 2016 Aug 15];321:205–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15051381 doi:10.1016/j.virol.2003.12.027.
