ABSTRACT
Respiratory Syncytial Virus (RSV) is the leading cause of pneumonia and bronchiolitis in infants, resulting in significant morbidity and mortality worldwide. In addition, RSV infections occur throughout different ages, thus, maintaining the virus in circulation, and increasing health risk to more susceptible populations such as infants, the elderly, and the immunocompromised. To date, there is no vaccine approved to prevent RSV infection or minimize symptoms of infection. Current clinical trials for vaccines against RSV are being carried out in four very different populations. There are vaccines that target two different pediatric populations, infants 2 to 6 month of age and seropositive children over 6 months of age, as well as women (non-pregnant or pregnant in their third trimester). There are vaccines that target adult and elderly populations. In this review, we will present and discuss RSV vaccine candidates currently in clinical trials. We will describe the preclinical studies instrumental for their advancement, with the goal of introducing new preclinical models that may more accurately predict the outcome of clinical vaccine studies.
Introduction
RSV is the most significant cause of serious lower respiratory tract infection in infants and young children worldwide.Citation83,Citation90 Globally, RSV infection leads to ∼3.2 million hospitalizations and ∼59,000 deaths yearly.Citation84 Almost half of these hospitalizations and death occur in children younger than 6 months old.Citation84 In addition, severe RSV disease in children is highly associated with prematurity, bronchopulmonary dysplasia, or congenital heart disease.Citation20 Infection in early infancy has been reported to result in allergic and/or asthmatic symptoms later in life.Citation27
To date, there is no approved vaccine to prevent RSV infection. A clinical trial of a formalin-inactivated RSV vaccine (FI-RSV) in the mid-1960's led during the following RSV season to a 80% increase in hospitalizations of vaccinated infants and children, and two deaths,.Citation17,Citation29,Citation45,Citation47 Since that time, development of RSV subunit vaccines has been hampered by lingering concerns over “vaccine enhanced disease.” Almost three decades after the failed FI-RSV trial, infants at high risk for severe RSV disease were treated prophylactically with RSV immune globulin (RespiGam™) that reduced RSV-mediated hospitalizations by 41%.Citation75 Subsequently, a monoclonal antibody, Synagis™ (palivizumab), directed against Region II (RII)Citation55 of the RSV Fusion (F) protein, replaced RespiGam™ and reduced RSV-related hospitalizations by >55%.Citation69 However, due to its high cost and lack of therapeutic efficacy, only the USA routinely administers Synagis™ prophylactically to high-risk infants.
RSV is relatively stable antigenically and most adults are seropositiveCitation85; however, re-infections are common, even within the same infection season. Thus, it is not surprising that RSV is also a cause of morbidity and mortality in the elderly.Citation26,Citation39 In the elderly, infections can be quite severe and have been associated with a senescent immune system.Citation26,Citation39 Annually, ∼11,000 older adults die in the USA of illness related to RSV infection.Citation11 This suggests that immunity from infection is neither sufficient nor long-lasting.Citation37,Citation42 Multiple vaccination trials of several different subunit RSV vaccine candidates in adults; however, have all resulted in disappointing outcomesCitation2,Citation3 suggesting the presence of an immune-inhibitory mechanism that influences the outcome of RSV vaccination in individuals who have been previously infected and reinfected. Finally, RSV infection is also underappreciated in transplant and immunodeficient patients.Citation10,Citation40
Choosing the appropriate target population for an RSV Vaccine
Due to the failure of the RSV vaccine clinical trial in the 1960's, clinical trials in the very young, RSV-naïve population have employed live-attenuated RSV vaccines, which are considered safe (). Subunit or vectored vaccines are first tested in “RSV-experienced” individuals since it is generally accepted that pre-existent infection protects from developing vaccine-enhanced disease.Citation2,Citation3 In addition, in RSV-experienced individuals live attenuated vaccines are considered to be less efficacious due to their more restricted replication. Thus, boosting pre-existent immunity might require targeting specific memory immune responses using purified specific or vector-expressed antigens.
Table 1. Summary of Current RSV Vaccines in Clinical Trials.
There is an ongoing debate over which target population(s) would benefit most from an RSV vaccine.Citation2,Citation44,Citation58 Most newborns are protected against RSV for ∼3 months by circulating maternal antibodies.Citation18 The reduction in the incidence of RSV acute lower respiratory infection (ALRI) during the first months of life has been correlated with higher levels of RSV-specific maternal antibodiesCitation34,Citation38 and is supported by the efficacy of Synagis adminsitration to premature infants. However, the peak incidence of RSV disease is observed between the ages of 2–8 months,Citation38,Citation68,Citation86 the time when maternal passively-transferred immunity is waning, rendering this population highly vulnerable. This situation suggests that increasing levels of maternal antibodies against RSV could potentially benefit infants during this critical window of susceptibility to severe RSV infection. Coupled with an immature immune system, as well as safety concerns and regulatory issues, the reality of an RSV vaccine for infants may only become achievable through development of a live attenuated RSV vaccine that results in a balance between immunogenicity and reactogenicity, a goal still sought by many groups.Citation1,Citation43,Citation46
In a meta-analysisCitation63 and in a recent study analyzing RSV in resource-constrained populations,Citation71 it was noted that almost all RSV-associated mortality in developed countries occurred in children <1 year, whereas RSV deaths in developing countries also occurred in children during both the first and the second year of life. One possible interpretation is that in developing countries, many seropositive young children are at risk of fatal RSV-disease. It is also important to note that transmission of RSV among household members is high.Citation71 Thus, interventions that reduce this RSV transmission could have a major beneficial impact in the population. It is believed that a vaccine that protects both young children and adults could, directly or indirectly, reduce the impact of RSV infection in the greatly endangered infant and elderly populations. Vaccination could boost maternal anti-RSV antibodies that would result in enhanced and prolonged passive immunity to RSV in infants.Citation44 A recent study predicted that if vaccination during pregnancy extends maternal protection by just an additional 4 months, a ∼30% decrease in the rate of infection in infants would be realized.Citation71 Enhancing immunity to RSV in household members by vaccination of seropositive young children and adults is expected to decrease virus circulation and the risk of infection of more vulnerable populations. Recent studies conducted in Kenya and Finland showed that for the majority of the families of children hospitalized with RSV, the source of the infant's RSV infection was either an older sibling or a parent.Citation41,Citation60 In fact, it has been estimated that the re-infection rate in children infected as infants is between 74–83% in their second year and 45–65% in their third year of life.Citation37
Immunological hurdles in RSV vaccination
Undoubtedly, infants between 3 to 8 months are at the peak of vulnerability to RSV infection, disease, and mortality.Citation38,Citation68,Citation86 This situation correlates with the fact that during this timeframe maternal antibodies reach their nadir, reducing their protective effect. Increasing the protection of infants at this critical age is the ultimate goal of many RSV vaccine projects. This goal could be achieved through (1) direct vaccination of infants or (2) vaccination of mothers prior to delivery to enhance maternal transfer of antibodies against RSV to the newborn. Studies using the cotton rat, the experimental “gold standard” model for RSV studies, are ongoing and provide evidence of inhibitory checkpoints that vaccines must overcome to achieve efficacy in infants.
Paradoxically, these two vaccine populations are strongly interrelated by immunological parameters that work against efficacy of vaccination. On the one hand, vaccination of infants is inhibited by the presence of circulating maternal anti-RSV antibodies,Citation22,Citation61 albeit the impact of low levels of maternal antibodies on the efficacy of infant vaccination has not yet been addressed adequately. In addition, in maternal vaccination, the goal is to enhance maternal antibodies before delivery to achieve maximum efficacy and length of protection in newborns. However, maternal vaccination, as is the case for vaccination of adults or the elderly, is also cumbersome since boosting preexistent immunity to RSV may be counteracted by immunosuppressive mechanisms,Citation14,Citation61 depending on the characteristics of the vaccine used.
Clinical trials underway and their preclinical testing history
A recount of current RSV vaccines in clinical trials is listed on . Updates on the status or RSV vaccines are posted regularly on the PATH Vaccine Resource Library (https://vaccinesources.org/rsv.php).
RSV protein antigens
Vaccine candidates have most commonly used the RSV fusion (F) protein of the virus as the antigen due to its capacity to generate most of the RSV neutralizing antibodies and its high level of conservation across RSV strains.Citation20 The F protein of RSV exists in two different conformations.Citation57 The post-fusion F protein (post-F), exposes epitopes that bind the protective monoclonal antibody palivizumab, has the most stable conformation, and has been used extensively in the past and current vaccine studies. The pre-fusion F protein (Pre-F) conformation, in addition to binding to palivizumab, also exposes at least four additional newly recognized epitopes that are the targets for neutralization by the majority of human high affinity neutralizing antibodies.Citation65 Pre-F is now being included in the newest RSV F protein vaccine trials.Citation53,Citation77
Novavax (Gaithersburg, MD, USA) has modified and cloned the RSV F protein (post-F conformation) into a baculovirus vector for expression in insect Sf9 cells. The recombinant protein was glycosylated and cleaved into covalently-linked F2 and F1 polypeptides that form homotrimers. The expressed and purified F protein trimers assemble into protein nanoparticles with the F protein forming rosettes.Citation87 In preclinical studies using naïve cotton rats, Smith et al. demonstrated strong induction of anti-RSV neutralizing antibodies (NA, >8 Log2) when animals were vaccinated with adjuvanted vaccine (120 micrograms aluminum phosphate) and at the highest concentrations of F protein preparation (30 micrograms). Under these conditions, full protection of the lung and the nose of challenged cotton rats was achievedCitation74,Citation87 without triggering vaccine-enhanced disease.Citation74 In a final preclinical study, pregnant, RSV-naïve guinea pigs were vaccinated with the F nanoparticles with or without adjuvant (alum). The vaccine induced high titers of anti-RSV antibodies that were efficiently transferred to newborn animals. However, no challenge results for the litters were reported.Citation31
An F nanoparticle vaccine has been tested in at least 11 different clinical trials that started in 2010 and in a wide variety of RSV-seropositive populations including the elderly, adults, pregnant and non-pregnant females, and toddlers (,Citation4,Citation28,Citation32,Citation33). This vaccine formulation is the most advanced RSV vaccine clinical candidate, currently undergoing Phase 3 efficacy testing for maternal vaccination (NCT 02624947) and has enrolled over 8000 mothers worldwide. The vaccine strongly induced production of anti-F and palivizumab-competing antibodies (PCA). Pre-existent NA against RSV in a healthy human population was enhanced moderately (reaching a maximum of ∼2.7 to 3.5 Log2 increase against RSV A in women vaccinated once or twice with 60 to 90 micrograms of adjuvanted vaccine).Citation32 Although the increase in NA titers reported in this study was encouraging, further investigation of the correlation between NA and reduction in viral spread or RSV symptoms is needed. In a more recent study using a similar population of healthy women, a comparison of one or two doses of the vaccine to evaluate immunogenicity was carried out.Citation4 A smaller increase in NA titers was evidenced in this study. Similarly, a study in adults of both sexes also resulted in less than a two-fold increase in NA.Citation28
GlaxoSmithKline (GSK; Brentford, UK) has developed RSV fusion proteins stabilized in the pre-F (DS-Cav1) or in the post-F fusion conformationCitation56 that were produced in Chinese hamster ovary (CHO) cells. These vaccines were tested for their ability to boost pre-existent NA responses in cattle.Citation88 This model has the advantage that most cattle are infected with bovine RSV early in life, and thus, the effect of vaccination can be measured by determining enhancement of the NA response to conserved F protein epitopes (i.e., most of the antigenic sites in the RSV F protein are conserved between human RSV and bovine RSV)Citation21 and neutralization capacity generated. A single immunization with non-adjuvanted pre-F protein, but not with post-F vaccine, strongly boosted RSV NA responses (∼3 Log2) to RSV A and B. However, this study has some limitations. The authors cannot rule out the possibility that the animals were previously vaccinated against bovine RSV, a practice common in the USA. In addition, a direct maternal immunization efficacy study is not feasible in this model due to the inability of antibodies to be transported across the placenta. In a Phase 1 human studyCitation51 adult males were vaccinated once using doses of 10, 30, and 60 micrograms of the pre-F protein in the absence or presence of adjuvant (aluminum hydroxide, 500 μg).Citation6 The boosted antibody response was strong at 30 days post-vaccination with a reported increase in NA titers between 3- and 4.9-fold. In addition, ∼85% of the subjects responded to vaccination. Further studies with the highest dose, without or with adjuvant, are underway in women of childbearing age (). Initial Phase 2 studies in non-pregnant women of childbearing age resulted in the selection of a dose of 60 micrograms of the pre-F protein without adjuvant for follow-up clinical studies.Citation5
MedImmune (Gaithersburg, MD, USA) developed a soluble form of the post-F protein of the RSV A2 F sequence that lacks the transmembrane domain and is purified from supernatants of stably transfected Chinese Hamster Ovary (CHO) cells. The antigen was formulated with the adjuvant glucopyranosyl lipid A (GLA),Citation50 a synthetic analog of monophosphoryl lipid A (a Toll-like receptor-4 [TLR-4] agonist) in a squalene-based oil-in-water stable emulsion (SE). In a series of preclinical studies using mice, cotton rats, and Sprague Dawley rats,Citation49 vaccination and boosting induced strong NA responses in all models, complete protection of the upper and lower respiratory tract (in cotton rats), a balanced Th1/Th2 response (in mice and rats), and induction of F protein-specific CD4 and CD8 T cell responses. Subsequent Phase 1 and 2a clinical trials in adultsCitation23,Citation24 showed that the vaccine was safe and the dose of soluble F with adjuvant was selected for the Phase 2b study. Unfortunately, this vaccine formulation failed to meet its efficacy endpoint in Phase 2bCitation25 and new formulations of soluble F protein are currently being explored pre-clinicallyCitation82
Scientists at the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH; Bethesda, MD) used a stabilized formulation of the DS-Cav1 protein (pre-F) to test in mice, non-human primates, and calves for immunogenicity.Citation56,Citation78 Although several adjuvants were tested in these studies, alum was one of the strongest, and they have initiated Phase 1 trials in adults with this formulation (NCT03049488).
Bavarian Nordic (Kvistgaard, Denmark) recently announced their initial 6 month follow-up data from the Phase 2 trial of Modified Vaccinia Ankara (MVA)-BN® RSV, a universal vaccine candidate designed to induce protective immune responses against both RSV A and B subtypes. The company reported that the vector has been tested in many clinical trials and is safe in adult and immune compromised populations. For the RSV vaccine, MVA-BN® expressed 5 proteins of RSV: the F, the G protein from RSV A and B type, the N, and the M2 proteins. The randomized, placebo-controlled trial evaluated the safety and immunogenicity of the recombinant vaccine in 421 healthy adults aged 55 and older. The subjects were enrolled into four active arms of the study, which examined the effects of both a high (5 × 108) and a low (1 × 108) dose, administered as either one or two vaccinations (day 0, 28), compared to a placebo arm. At the 6 month follow up, a persistent antibody response against multiple RSV targets could still be observed. No information is published about preclinical studies for this vaccine.
VIB (Ghent, Belgium), University of Ghent developed a prototype vaccine based on the ectodomain of the small hydrophobic (SH) protein surface antigen of RSV conjugated to keyhole limpet hemocyanin (KHL), a carrier protein that increases SH immunogenicity.Citation81 Although the ectodomain domain of the SH protein is known to be poorly immunogenic and did not generate NA in vaccinated animals, a significant reduction in viral replication in the lungs of mice and cotton rats was shown. This efficacy was dependent on T cell receptor binding of anti-SH antibodies and probably to the action of SH-specific T cells. ImmunoVaccine Inc. (Nova Scotia, Canada) formulated the SH antigen with their adjuvant vaccine platform, DepoVaxTM, or with alum (). The Phase 1 trial consisted of testing their vaccine in adults using two doses of each vaccine. The vaccine had an acceptable safety profile and induced antigen-specific antibody responses.Citation52
Mucosis (now under VirtuVax, Odijk, The Netherlands) developed a mucosal vaccine based on the purified pre-F conformation attached non-covalently to bacteria-like particles. This vaccine was tested in preclinical studies in mice and cotton rats by administering the vaccine intranasally in three immunization courses.Citation76 The vaccine conferred full and partial protection of the lung in the mouse and in the cotton rat model, respectively. The vaccine underwent Phase I clinical trials (). As reported by the company, Phase 1 studies require follow up clinical trials to determine optimal dose.
A Bacillus Calmette-Guérin (BCG) vaccine expressing the nucleoprotein (N) of hRSV as a good inducer of T cell responses, was formulated under current good manufacturing practices by the Universidad Católica de Chile, and has been tested in the mouse model of RSV infection. This vaccine showed protection of the lung, reduction of lung cell infiltration, and production of a strong CD4 and CD8 T cell response after only one vaccination.Citation13,Citation16 Currently, this formulation is entering a Phase 1 clinical trial () targeting adult males who have previously received BCG vaccination against Mycobacterium tuberculosis (NCT03213405). However, since the BCG vaccine has never been licensed in the USA, the use of this modified RSV vaccine could be restricted in this country.
Adenovirus vectored RSV vaccines
Four groups, Janssen (Beerse, Belgium), GSK, ReiThera (Rome, Italy) with the Oxford Vaccine Group (Oxford, UK), and Vaxart (San Francisco, CA, USA), have developed adenovirus-vectored vaccines against RSV, and all are currently undergoing Phase 1 or Phase 2 clinical trials ().
Janssen's adenovirus vectored vaccines consist of low-serovalent adenoviral vectors Ad26 and Ad35, expressing the RSV fusion protein in the pre-F configuration. These vaccines have been tested in vaccination or vaccination and boosting (using alternate vectors) preclinical studies using mice and cotton rats using the intramuscular route of immunization.Citation93 Both a single vaccination or vaccination and boosting generated high levels of NA and protection against RSV A in cotton rats. Vaccination and heterologous vector boosting also generated full protection against RSV B challenge. A first clinical study was performed using previous design of prime and heterologous subtype boosting (NCT02561871). However, most recent clinical trials have focused only in vaccination with Ad26 subtype (reference NCT002926430, NCT330625, and NCT03334695).
GSK's adenovirus RSV vaccine is based on a chimpanzee adenovirus subtype 155 expressing the RSV F, N, and M2-1 proteins. In unpublished preclinical studies (https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM559666.pdf.), it was demonstrated that the vaccine reduced viral replication, disease, and lung pathology in all three models of infection: mouse, cotton rat, and calf. In addition, mouse studies have demonstrated that there is a balanced Th1/Th2 response to the vaccine. Subsequently, the vaccine was tested in two consecutive clinical trials using vaccination and boosting strategies. A Phase 1 study was performed in individuals 18–45 years of age (NCT02491463), whereas a Phase 2 trial targeting RSV seropositive infants 12 to 23 months of age (NCT02927873) is underway and no results have been presented yet.
Vaxart is one of the leaders in oral-mucosal vaccines. Their vaccine consists of an oral-delivery tablet that contains a non-replicating E1-, E3-deleted Adenovirus subtype 5 (Ad5) vector encoding the RSV F protein, adjuvanted with double stranded RNA. Clinical trials are under way (NCT02830932). Although a vaccine using the same platform was tested for influenza in mouse, ferrets, and in humansCitation48,Citation79,Citation80 showing promising results, up to date no preclinical studies using the RSV vaccine have been reported.
ReiThera has used a combination vectored vaccine based on chimpanzee Adenovirus 3 and Modified Vaccinia Ankara, both encoding the F, N, and M2-1 proteins of RSV. They have tested the vaccine in mice and cotton rats for immunogenicity, protection, and safety after RSV challenge and for immunogenicity in non-human primates (macaques).Citation70 In mice, the vaccination afforded complete protection irrespective of the route of vaccination (intranasally or intramuscularly). In cotton rats the vaccine induced stronger protection when applied intranasally, and lasted at least 3 months after vaccination. In the macaque model, cellular responses to the vaccine required boosting. In a bovine model, vaccination and boosting of bRSV seronegative cows with the virus-vectored vaccines induced high titers of NA and protection against a heterologous bRSV challenge.Citation89 The vaccine was further tested in a human Phase 1 trial where it was well-tolerated and enhanced RSV immunity in the adult population.Citation35,Citation36
Animal studies
Preclinical studies for testing RSV vaccine candidates have used several laboratory rodent models, most commonly mouse and cotton rat, although rats, guinea pigs, ferrets, lambs, calves, and non-human primates have been also utilized. These studies are necessary to determine the immunological potency of the antigen, as well as testing basic safety parameters, including the potential development of vaccine enhanced disease upon viral challenge. Most of these vaccines have achieved high levels of NA and protection in naïve animals. A population that has been exposed to RSV and is therefore “immune experienced,” has been found to be much more challenging. Most newborns and infants have detectable levels of NA or total antibodies against RSV. In newborns and infants, these antibodies are acquired through passive transfer from the mother, and these antibodies persist in the newborn's circulation no longer than six months after birth.Citation15 By the age of 2 years, almost all children have been infected with RSV, indicating that most of them have developed some type of RSV immune response. It is believed that in most cases of RSV disease that occur during this age period there is an overlap of maternal- and infant-generated anti-RSV antibodies.
Although maternally transferred immunity is not fully protective, antibodies with different specificities against the various viral proteins are circulating early in life and will most likely interfere differentially with subsequent vaccinations. With the exception of limited preclinical studies using lambs, calves (susceptible to bovine and lamb RSV), and cotton rats (susceptible to human strains of RSV), the majority of the RSV vaccine preclinical studies have been carried out in RSV-naïve animals or in young animals in the absence of maternal antibodies.
Inhibitory effect of pre-existing maternal antibodies
It was recognized early that pre-existing antibodies against RSV have a modulatory effect on the development of immunity and protection by RSV infection or vaccination of the offspring.Citation61 It has been suggested that the presence of maternal antibodies against RSV in babies exert a suppressive effect that inhibits development of the antibody responses during RSV infection of the infants. This phenomena was recently evidenced in a study where RSV acute and convalescent serum samples were compared in infants <2 months old (high maternal antibodies) and in infants >2 month to <24 months old (low maternal antibodies).Citation91 Infants infected with RSV between 0 and 2 months of age showed no significant increase in NA upon infection. However, those between 2 and 24 months showed a clear increase. In addition, a highly significant negative correlation between the antibody levels against the F protein antigen in the acute infection and the fold-change in antibody titers one month post-infection demonstrates that age was not the only factor involved in the low response. Overall, these data exemplify the inhibitory environment that must be overcome by a vaccine delivered to neonates.
The kinetics of decay of the RSV-specific maternal antibodies was previously estimated. The half-life of maternal antibodies seems to vary in different populations, ranging between 20 to 80 days.Citation12,Citation59,Citation67 Most of the RSV neutralizing activity is undetectable in infants who are 4–6 month of age.Citation66,Citation67 The mechanism by which the level of antibodies (neutralizing or not) influences repression of RSV immunity is still not clear.
The negative effect of the passively transferred RSV antibodies in developing RSV immunity has been tested in animal models of RSV infection. Cotton rats that received hyperimmune RSV antiserum showed suppressed antibody responses to vaccination with RSV glycoproteins, but not to unrelated antigens.Citation62 Furthermore, it was demonstrated that cotton rats born from RSV-infected mothers responded poorly to RSV vaccination.Citation8,Citation72 The extent to which the response to the vaccine was blunted was directly proportional to the titers of circulating maternal antibodies.Citation73
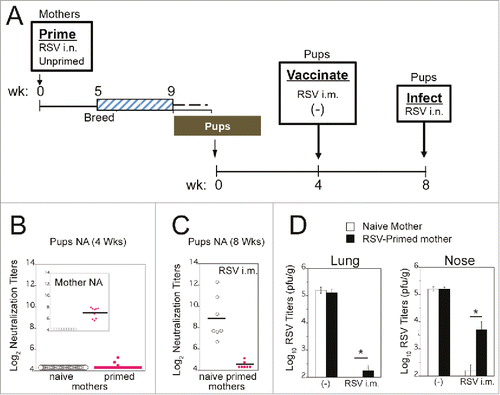
In a recent study, female cotton rats, either naïve or previously infected with RSV, were set in breeding pairs 5 weeks after infection (). Four weeks after birth, the pups showed low to undetectable levels of maternal antibodies remaining in circulation () compared to their mothers. This is due to the rapid decay of maternal antibodies in the cotton rat pups, in which the IgG half-life is ∼7 days. Most importantly, even in the presence of this miniscule amount of NA, a significant inhibitory effect on vaccination in animals born to RSV-infected mothers was detected. This was evidenced by the poor induction of a NA response to the vaccine (, compare the response of vaccinated pups born from naïve vs. primed mothers), and the reduced protection against RSV imparted by vaccination in pups born from infected mothers (). These studies strongly support the notion that vaccination of naïve animals most likely overestimates vaccine efficacy. Moreover, this over-estimation may alter the predicted outcome of clinical trials in which most of the recruitment occurs in RSV seropositive individuals, or in infants, with detectable levels of maternal antibodies, that can result in “vaccine blunting.”
Figure 1. RSV vaccination of animals with passively transferred maternal RSV immunity. (A) Diagram of the experiment. Female cotton rats were primed by infection with RSV A/Long (105 pfu i.n.) or left unprimed. All females were set in breeding pairs 5 weeks after RSV infection, and on week 9, females began delivering pups. At 4 weeks of age, pups were vaccinated with live RSV i.m., or left unvaccinated (-). Four weeks later, serum was collected from each pup and animals were challenged with RSV i.n. Four days after infection, pups were euthanized for determination of lung and nose viral titers. (B) NA titers of 4-week old pups born to unprimed or primed mothers. Insert shows mothers' NA titers in sera collected before delivery, indicating that all primed mothers produced NA. (C) NA titers in sera obtained from pups born to naïve or RSV-primed mothers after i.m. vaccination with live RSV (samples collected at week 8 prior to RSV challenge). (D) Lung and nose viral titers measured in samples obtained from pups born to naïve or RSV-primed mothers after vaccination of pups with PBS (-), or live RSV i.m. All animals were challenged i.n. with RSV/A Long (105 pfu) and euthanized 4 days later. ANOVA followed by Student-Newman-Keuls post hoc test. *p<0.01. Data taken from reference.Citation8
Inadequacy of current and past pre-clinical RSV vaccine models
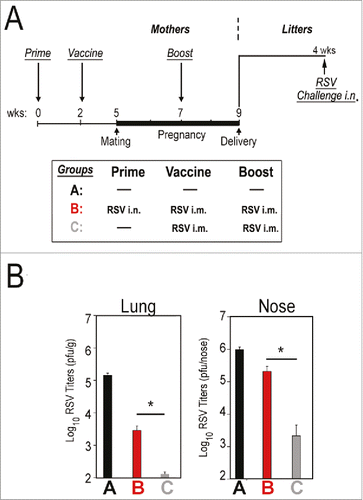
Antibodies against RSV are found universally in sera of adult humans. Our initial studies using maternal immunization uncovered a fact that was not obvious. The pre-existent RSV immunity has a specific inhibitory effect on RSV vaccination that will thus influence the result of maternal vaccination and the underlying protection that is passively transferred to the infants. When naïve cotton rat dams were vaccinated with live RSV i.m., the protection of the pups was more efficient than when the same vaccine was given to previously RSV-infected, vaccinated dams (, compare groups B and C). This phenomenon needs to be taken into account since most of the preclinical studies to test/validate prototype vaccines for maternal immunization have used naïve dams for vaccination.Citation9,Citation30,Citation31 In our study, the vaccine-induced protection of the litter was evidenced only later (4 weeks after birth), when waning of maternal immunity is more evident, in contrast to testing it on or close to the day of delivery, when most of the animals are fully protected independent of the vaccination status. Overall, this data strongly supports the idea that we must use a different set of conditions for testing prototype RSV vaccines in order to find a successful vaccine for vaccination during pregnancy.
Figure 2. Efficiency of protection against RSV in juvenile cotton rats born from vaccinated, unprimed or primed mothers. (A) Female cotton rats were separated into three groups. One group of females remained unprimed and unvaccinated (Group A) as a control. Another group was primed by infection with RSV i.n. and then vaccinated with live RSV i.m. two weeks after priming and boosted during pregnancy (Group B). The last group of females remained unprimed, but was vaccinated and boosted (Group C). Litters from mothers in each group were challenged 4 weeks after birth and euthanized on day 4 post-challenge. (B) Quantification of lung and nose viral titers in samples of RSV-challenged, 4-week old juvenile cotton rats. Bars represent the mean ± SE. The inhibitory effect on vaccination in seropositive mothers was evaluated by comparing litters born from mothers in Group B (primed) and Group C (unprimed). N = 13-25 pups per group. ANOVA followed by Student-Newman-Keuls post hoc test. *p < 0.01. Data taken from reference.Citation7
Maternal vaccination against RSV can also protect mothers
Transfer of IgG and IgA was previously studied by Malek et al..Citation54 While the levels of maternal IgG slowly, but continuously, decrease during pregnancy, levels of IgG and IgA exponentially increase in the fetal circulation, rising from ∼10% of the maternal level to significantly higher levels at the end of the gestation period. We have recently shown in the cotton rat model that the decrease in RSV antibodies during the perinatal period is significant. Dams previously immunized with live RSV i.m. or infected i.n. exhibited a strong decline in serum NA titers against RSV in samples obtained within a week of delivery.Citation7 This decrease in protective anti-RSV antibodies late in pregnancy or soon after delivery could signal a window of time during which severe RSV infection could develop in the mother. In fact, recent studies have suggested a certain degree of immunosuppression for RSV during late pregnancy.Citation64 Also, several cases of maternal RSV infection and hospitalization have been reported, and highlight the poorly recognized effects of RSV infection on late-term or new mothers.Citation19,Citation92 Experiments are currently underway to determine the impact that the decrease in protective antibodies in cotton rat dams could have upon RSV challenge.
A final note
More than a dozen strong programs for developing a vaccine against RSV are now in place and there is a good chance that 50 years after the unfortunate FI-RSV trials, a RSV vaccine will be approved for protection in one of the many populations affected by RSV. Regardless of which population is chosen, approval of an RSV vaccine will be the first step toward protection of the most endangered groups. The high cost of clinical trials, the immunological heterogeneity encountered in the human population for RSV, and the difficulties of defining surrogates of efficacy, will still challenge these programs. Preclinical studies that define and target these hurdles will help to better model RSV vaccine efficacy in humans and to accelerate RSV vaccine development.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Anderson AJ, Snelling TL, Moore HC, Blyth CC. Advances in Vaccines to Prevent Viral Respiratory Illnesses in Children. Paediatr Drugs. 2017;19(6):523–31. doi:10.1007/s40272-017-0257-x. PMID:28808938.
- Anderson LJ. Respiratory syncytial virus vaccine development. Semin Immunol. 2013;25(2):160–71. doi:10.1016/j.smim.2013.04.011. PMID:23778071.
- Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31 Suppl 2, B209–215. doi:10.1016/j.vaccine.2012.11.106.
- August A, Glenn GM, Kpamegan E, Hickman SP, Jani D, Lu H, Fries LF. A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine. 2017;35(30):3749–59. doi:10.1016/j.vaccine.2017.05.045. PMID:28579233.
- Beran J, Lickliter JD, Schwarz TF, Johnson C, Chu L, Domachowske JB, Dieussaert I. Safety and immunogenicity of 3 formulations of an investigational respiratory syncytial virus vaccine in non-pregnant women: results from two phase II trials. J Infect Dis. 2018;217(10):1616–25. doi:10.1093/infdis/jiy065. PMID:29401325.
- Blais N, Gagne M, Hamuro Y, Rheault P, Boyer M, Steff AM, Martin D. Characterization of Pre-F-GCN4t, a Modified Human Respiratory Syncytial Virus Fusion Protein Stabilized in a Noncleaved Prefusion Conformation. J Virol. 2017;91(13). doi:10.1128/JVI.02437-16. PMID:28404847.
- Blanco JCG, Pletneva LM, Otoa RO, Patel MC, Vogel SN, Boukhvalova MS. Preclinical assessment of safety of maternal vaccination against respiratory syncytial virus (RSV) in cotton rats. Vaccine. 2017;35(32):3951–8. doi:10.1016/j.vaccine.2017.06.009. PMID:28624306.
- Blanco JC, Pletneva LM, Oue RO, Patel MC, Boukhvalova MS. Maternal transfer of RSV immunity in cotton rats vaccinated during pregnancy. Vaccine. 2015;33(41):5371–9. doi:10.1016/j.vaccine.2015.08.071. PMID:26335771.
- Blodorn K, Hagglund S, Fix J, Dubuquoy C, Makabi-Panzu B, Thom M, Valarcher JF. Vaccine safety and efficacy evaluation of a recombinant bovine respiratory syncytial virus (BRSV) with deletion of the SH gene and subunit vaccines based on recombinant human RSV proteins: N-nanorings, P and M2-1, in calves with maternal antibodies. PLoS ONE. 2014;9(6), e100392. doi:10.1371/journal.pone.0100392. PMID:24945377.
- Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102(3A):27–30; discussion 42-23. doi:10.1016/S0002-9343(97)00007-7. PMID:10868139.
- Branche AR, Falsey AR. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs Aging. 2015;32(4):261–9. doi:10.1007/s40266-015-0258-9. PMID:25851217.
- Brandenburg AH, Groen J, van Steensel-Moll HA, Claas EC, Rothbarth PH, Neijens HJ, Osterhaus AD. Respiratory syncytial virus specific serum antibodies in infants under six months of age: limited serological response upon infection. J Med Virol. 1997;52(1):97–104. doi:10.1002/(SICI)1096-9071(199705)52:1%3c97::AID-JMV16%3e3.0.CO;2-Y. PMID:9131465.
- Bueno SM, Gonzalez PA, Cautivo KM, Mora JE, Leiva ED, Tobar HE, Kalergis AM. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci U S A. 2008;105(52):20822–7. doi:10.1073/pnas.0806244105. PMID:19075247.
- Buynak EB, Weibel RE, Carlson AJ, McLean AA, Hilleman MR. Further inestigation of live respiratory virus vaccine administered parenterally (40433). Proc Soc Exp Biol Med. 1979;160:272–7. doi:10.3181/00379727-160-40433.
- Capella C, Chaiwatpongsakorn S, Gorrell E, Risch ZA, Ye F, Mertz SE, Peeples ME. Prefusion F, Postfusion F, G Antibodies, and Disease Severity in Infants and Young Children With Acute Respiratory Syncytial Virus Infection. J Infect Dis. 2017;216(11):1398–406. doi:10.1093/infdis/jix489. PMID:29029312.
- Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus Calmette-Guerin promotes virus clearance and protects from infection. J Immunol. 2010;185(12):7633–45. doi:10.4049/jimmunol.0903452. PMID:21084664.
- Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89(4):449–63. doi:10.1093/oxfordjournals.aje.a120957. PMID:4305200.
- Chu HY, Englund JA. Maternal immunization. Clin Infect Dis. 2014;59(4):560–8. doi:10.1093/cid/ciu327. PMID:24799324.
- Chu HY, Katz J, Tielsch J, Khatry SK, Shrestha L, LeClerq SC, Englund JA. Clinical Presentation and Birth Outcomes Associated with Respiratory Syncytial Virus Infection in Pregnancy. PLoS ONE. 2016;11(3), e0152015. doi:10.1371/journal.pone.0152015. PMID:27031702.
- Collins PL, Crowe JE. Respiratory syncytial virus and metapneumovirus. (5th ed. Vol. 2). Philadelphia: Lippincott-Williams & Wilkins; 2007
- Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, Lanzavecchia A. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501(7467):439–43. doi:10.1038/nature12442. PMID:23955151.
- Crowe JE, Jr., Williams JV. Immunology of viral respiratory tract infection in infancy. Paediatr Respir Rev. 2003;4(2):112–9. doi:10.1016/S1526-0542(03)00033-2. PMID:12758048.
- Falloon J, Ji F, Curtis C, Bart S, Sheldon E, Krieger D, Esser MT. A phase 1a, first-in-human, randomized study of a respiratory syncytial virus F protein vaccine with and without a toll-like receptor-4 agonist and stable emulsion adjuvant. Vaccine. 2016;34(25):2847–54. doi:10.1016/j.vaccine.2016.04.002. PMID:27102821.
- Falloon J, Talbot HK, Curtis C, Ervin J, Krieger D, Dubovsky F, Esser MT. Dose Selection for an Adjuvanted Respiratory Syncytial Virus F Protein Vaccine for Older Adults Based on Humoral and Cellular Immune Responses. Clin Vaccine Immunol. 2017;24(9):e00157–17. doi:10.1128/CVI.00157-17. PMID:28679495
- Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, Falsey AR. An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults. J Infect Dis. 2017;216(11):1362–70. doi:10.1093/infdis/jix503. PMID:29029260.
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in elderly adults. Drugs Aging. 2005;22(7):577–87. doi:10.2165/00002512-200522070-00004. PMID:16038573.
- Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191(1):34–44. doi:10.1164/rccm.201405-0901PP. PMID:25369458.
- Fries L, Shinde V, Stoddard JJ, Thomas DN, Kpamegan E, Lu H, Glenn GM. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun Ageing. 2017;14:8. doi:10.1186/s12979-017-0090-7. PMID:28413427.
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89(4):435–48. doi:10.1093/oxfordjournals.aje.a120956. PMID:4305199.
- Garg R, Latimer L, Wang Y, Simko E, Gerdts V, Potter A, van Drunen Littel-van den Hurk S. Maternal immunization with respiratory syncytial virus fusion protein formulated with a novel combination adjuvant provides protection from RSV in newborn lambs. Vaccine. 2016;34(2):261–9. doi:10.1016/j.vaccine.2015.11.029. PMID:26616551.
- Glenn GM, Fries LF, Smith G, Kpamegan E, Lu H, Guebre-Xabier M, Flyer D. Modeling maternal fetal RSV F vaccine induced antibody transfer in guinea pigs. Vaccine. 2015;33(47):6488–92. doi:10.1016/j.vaccine.2015.08.039. PMID:26319066.
- Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, Piedra PA. A Randomized, Blinded, Controlled, Dose-Ranging Study of a Respiratory Syncytial Virus Recombinant Fusion (F) Nanoparticle Vaccine in Healthy Women of Childbearing Age. J Infect Dis. 2016;213(3):411–22. doi:10.1093/infdis/jiv406. PMID:26259809.
- Glenn GM, Smith G, Fries L, Raghunandan R, Lu H, Zhou B, Piedra PA. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2013;31(3):524–32. doi:10.1016/j.vaccine.2012.11.009. PMID:23153449.
- Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98(5):708–15. doi:10.1016/S0022-3476(81)80829-3. PMID:7229749.
- Green CA, Scarselli E, Sande CJ, Thompson AJ, de Lara CM, Taylor KS, Pollard AJ. Chimpanzee adenovirus- and MVA-vectored respiratory syncytial virus vaccine is safe and immunogenic in adults. Sci Transl Med. 2015;7(300):300ra126. doi:10.1126/scitranslmed.aac5745. PMID:26268313.
- Green CA, Scarselli E, Voysey M, Capone S, Vitelli A, Nicosia A, Pollard AJ. Safety and immunogenicity of novel respiratory syncytial virus (RSV) vaccines based on the RSV viral proteins F, N and M2-1 encoded by simian adenovirus (PanAd3-RSV) and MVA (MVA-RSV); protocol for an open-label, dose-escalation, single-centre, phase 1 clinical trial in healthy adults. BMJ Open. 2015;5(10), e008748. doi:10.1136/bmjopen-2015-008748. PMID:26510727.
- Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–8. doi:10.1093/infdis/163.4.693. PMID:2010624.
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi:10.1056/NEJMoa0804877. PMID:19196675.
- Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179(1):25–30. doi:10.1086/314567. PMID:9841818.
- Harrington RD, Hooton TM, Hackman RC, Storch GA, Osborne B, Gleaves CA, Meyers JD. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;165(6):987–93. doi:10.1093/infdis/165.6.987. PMID:1583345.
- Heikkinen T, Valkonen H, Waris M, Ruuskanen O. Transmission of respiratory syncytial virus infection within families. Open Forum Infect Dis. 2015;2(1), ofu118. doi:10.1093/ofid/ofu118. PMID:25884006.
- Henderson FW, Collier AM, Clyde WA, Jr., Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300(10):530–4. doi:10.1056/NEJM197903083001004. PMID:763253.
- Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development – A global agenda. Vaccine. 2016;34(26):2870–5. doi:10.1016/j.vaccine.2016.03.109. PMID:27105562.
- Kaaijk P, Luytjes W, Rots NY. Vaccination against RSV: is maternal vaccination a good alternative to other approaches? Hum Vaccin Immunother. 2013;9(6):1263–7. doi:10.4161/hv.24096. PMID:23442726.
- Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89(4):405–21. doi:10.1093/oxfordjournals.aje.a120954. PMID:4305197.
- Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:259–84. doi:10.1007/978-3-642-38919-1_13.
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. doi:10.1093/oxfordjournals.aje.a120955. PMID:4305198.
- Kim L, Martinez CJ, Hodgson KA, Trager GR, Brandl JR, Sandefer EP, Doll WJ, Liebowitz D, Tucker SN. Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule. Scientific Reports 2016;6:37295 DOI: doi:10.1038/srep37295. PMID:27881837.
- Lambert SL, Aslam S, Stillman E, MacPhail M, Nelson C, Ro B, Tang RS. A novel respiratory syncytial virus (RSV) F subunit vaccine adjuvanted with GLA-SE elicits robust protective TH1-type humoral and cellular immunity in rodent models. PLoS ONE. 2015;10(3), e0119509. doi:10.1371/journal.pone.0119509. PMID:25793508.
- Lambert SL, Yang CF, Liu Z, Sweetwood R, Zhao J, Cheng L, Woo J. Molecular and cellular response profiles induced by the TLR4 agonist-based adjuvant Glucopyranosyl Lipid A. PLoS ONE. 2012;7(12), e51618. doi:10.1371/journal.pone.0051618. PMID:23284726.
- Langley JM, Aggarwal N, Toma A, Halperin SA, McNeil SA, Fissette L, Dieussaert I. A Randomized, Controlled, Observer-Blinded Phase 1 Study of the Safety and Immunogenicity of a Respiratory Syncytial Virus Vaccine With or Without Alum Adjuvant. J Infect Dis. 2017;215(1):24–33. doi:10.1093/infdis/jiw453. PMID:27694633.
- Langley J, MacDonald L, Weir G, Mackinnon-Cameron D, Math M, Ye L, Halperin SA. A Phase I Randomized, observer-blind controlled, dose escalation trial of the safety and tolerability of two intramuscular doses of DPX-RSV(A), a respiratory syncytial virus (RSV) vaccine containing RSV SH antigen and a noveladjuvant DepoVax, or SH antigen co-administered with alum hydorxide or placebo to healthy adults >50-64 years of age. Open Forum of Infectious Disease. 2016;3(Suppl-1):1270.
- Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, Palomo C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc Natl Acad Sci U S A. 2012;109(8):3089–94. doi:10.1073/pnas.1115941109. PMID:22323598.
- Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36(5):248–55. doi:10.1111/j.1600-0897.1996.tb00172.x. PMID:8955500.
- McLellan JS. Neutralizing epitopes on the respiratory syncytial virus fusion glycoprotein. Curr Opin Virol. 2015;11:70–75. doi:10.1016/j.coviro.2015.03.002.
- McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Kwong PD. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–8. doi:10.1126/science.1243283. PMID:24179220.
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Graham BS. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–7. doi:10.1126/science.1234914. PMID:23618766.
- Munoz FM. Respiratory syncytial virus in infants: is maternal vaccination a realistic strategy? Curr Opin Infect Dis. 2015;28(3):221–4. doi:10.1097/QCO.0000000000000161. PMID:25918956.
- Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21(24):3465–7. doi:10.1016/S0264-410X(03)00352-9. PMID:12850361.
- Munywoki PK, Koech DC, Agoti CN, Lewa C, Cane PA, Medley GF, Nokes DJ. The source of respiratory syncytial virus infection in infants: a household cohort study in rural Kenya. J Infect Dis. 2014;209(11):1685–92. doi:10.1093/infdis/jit828. PMID:24367040.
- Murphy BR, Alling DW, Snyder MH, Walsh EE, Prince GA, Chanock RM, Hemming VG, Rodriguez WJ, Kim HW, Graham BS, et al.. Effect of age and preexisting antibody on serum antibody response of infants and children to the F and G glycoproteins during respiratory syncytial virus infection. J Clin Microbiol. 1986;24(5):894–8. PMID:3771779.
- Murphy BR, Olmsted RA, Collins PL, Chanock RM, Prince GA. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J Virol. 1988;62(10):3907–10. PMID:3047432.
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, Huda T. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)-associated acute lower respiratory infections in children. Lancet. 2010;375(9725):1545–55. doi:10.1016/S0140-6736(10)60206-1. PMID:20399493.
- Nandapalan N, Taylor CE, Greenwell J, Scott M, Scott R, Hey EN, Toms GL. Seasonal variations in maternal serum and mammary immunity to RS virus. J Med Virol. 1986;20(1):79–87. doi:10.1002/jmv.1890200110. PMID:3760842.
- Ngwuta JO, Chen M, Modjarrad K, Joyce MG, Kanekiyo M, Kumar A, Graham BS. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med. 2015;7(309):309ra162. doi:10.1126/scitranslmed.aac4241. PMID:26468324.
- Nyiro JU, Kombe IK, Sande CJ, Kipkoech J, Kiyuka PK, Onyango CO, Nokes DJ. Defining the vaccination window for respiratory syncytial virus (RSV) using age-seroprevalence data for children in Kilifi, Kenya. PLoS ONE. 2017;12(5):e0177803. doi:10.1371/journal.pone.0177803. PMID:28531224.
- Ochola R, Sande C, Fegan G, Scott PD, Medley GF, Cane PA, Nokes DJ. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS ONE. 2009;4(12):e8088. doi:10.1371/journal.pone.0008088. PMID:19956576.
- Ohuma EO, Okiro EA, Ochola R, Sande CJ, Cane PA, Medley GF, Nokes DJ. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am J Epidemiol. 2012;176(9):794–802. doi:10.1093/aje/kws257. PMID:23059788.
- The Impact-RSV Study Group. Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics. 1998;102(3):531–7. doi:10.1542/peds.102.3.531.
- Pierantoni A, Esposito ML, Ammendola V, Napolitano F, Grazioli F, Abbate A, Vitelli A. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol Ther Methods Clin Dev. 2015;2:15018. doi:10.1038/mtm.2015.18. PMID:26015988.
- Poletti P, Merler S, Ajelli M, Manfredi P, Munywoki PK, Nokes D, Melegaro A. Evaluating vaccination strategies for reducing infant respiratory syncytial virus infection in low-income settings. BMC Med. 2015;13(1):49. doi:10.1186/s12916-015-0283-x. PMID:25857701.
- Prince GA, Horswood RL, Camargo E, Suffin SC, Chanock RM. Parenteral immunization with live respiratory syncytial virus is blocked in seropositive cotton rats. Infect Immun. 1982;37(3):1074–8. PMID:6752019.
- Prince GA, Potash L, Horswood RL, Camargo E, Suffin SC, Johnson RA, Chanock RM. Intramuscular inoculation of live respiratory syncytial virus induces immunity in cotton rats. Infect Immun. 1979;23(3):723–8. PMID:572342.
- Raghunandan R, Lu H, Zhou B, Xabier MG, Massare MJ, Flyer DC, Glenn GM. An insect cell derived respiratory syncytial virus (RSV) F nanoparticle vaccine induces antigenic site II antibodies and protects against RSV challenge in cotton rats by active and passive immunization. Vaccine. 2014;32(48):6485–92. doi:10.1016/j.vaccine.2014.09.030. PMID:25269094.
- The PREVENT Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99(1):93–99. doi:10.1542/peds.99.1.93. PMID:8989345.
- Rigter A, Widjaja I, Versantvoort H, Coenjaerts FE, van Roosmalen M, Leenhouts K, de Haan CA. A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles. PLoS ONE. 2013;8(8):e71072. doi:10.1371/journal.pone.0071072. PMID:23951084.
- Ruckwardt TJ, Morabito KM, Graham BS. Determinants of early life immune responses to RSV infection. Curr Opin Virol. 2016;16:151–7. doi:10.1016/j.coviro.2016.01.003. PMID:26986236.
- Sastry M, Zhang B, Chen M, Joyce MG, Kong WP, Chuang GY, Kwong PD. Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS ONE. 2017;12(10), e0186854. doi:10.1371/journal.pone.0186854. PMID:29073183.
- Scallan CD, Lindbloom JD, Tucker SN. Oral Modeling of an Adenovirus-Based Quadrivalent Influenza Vaccine in Ferrets and Mice. Infect Dis Ther. 2016;5(2):165–83. doi:10.1007/s40121-016-0108-z. PMID:27071663.
- Scallan CD, Tingley DW, Lindbloom JD, Toomey JS, Tucker SN. An adenovirus-based vaccine with a double-stranded RNA adjuvant protects mice and ferrets against H5N1 avian influenza in oral delivery models. Clin Vaccine Immunol. 2013;20(1):85–94. doi:10.1128/CVI.00552-12. PMID:23155123.
- Schepens B, Sedeyn K, Vande Ginste L, De Baets S, Schotsaert M, Roose K, Saelens X. Protection and mechanism of action of a novel human respiratory syncytial virus vaccine candidate based on the extracellular domain of small hydrophobic protein. EMBO Mol Med. 2014;6(11):1436–54. doi:10.15252/emmm.201404005. PMID:25298406.
- Schneider-Ohrum K, Cayatte C, Bennett AS, Rajani GM, McTamney P, Nacel K, McCarthy MP. Immunization with Low Doses of Recombinant Postfusion or Prefusion Respiratory Syncytial Virus F Primes for Vaccine-Enhanced Disease in the Cotton Rat Model Independently of the Presence of a Th1-Biasing (GLA-SE) or Th2-Biasing (Alum) Adjuvant. J Virol. 2017;91(8):e02180–16. doi:10.1128/JVI.02180-16. PMID:28659488
- Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect Dis. 2001;183(1);16–22. doi:10.1086/317655. PMID:11076709.
- Shi T, McAllister DA, O'Brien KL, Simoes EAF, Madhi SA, Gessner BD, Network RSV. Global Epidemiology. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–58. doi:10.1016/S0140-6736(17)30938-8. PMID:28689664.
- Siber GR, Leszcynski J, Pena-Cruz V, Ferren-Gardner C, Anderson R, Hemming VG, Walsh EE, Burns J, McIntosh K, Gonin R, et al. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992;165(3):456–63. doi:10.1093/infdis/165.3.456. PMID:1538152.
- Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(5 Suppl), S118–126. doi:10.1067/S0022-3476(03)00511-0. PMID:14615710.
- Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M, Glenn G. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS ONE. 2012;7(11), e50852. doi:10.1371/journal.pone.0050852. PMID:23226404.
- Steff AM, Monroe J, Friedrich K, Chandramouli S, Nguyen TL, Tian S, Carfi A. Pre-fusion RSV F strongly boosts pre-fusion specific neutralizing responses in cattle pre-exposed to bovine RSV. Nat Commun. 2017;8(1):1085. doi:10.1038/s41467-017-01092-4. PMID:29057917.
- Taylor G, Thom M, Capone S, Pierantoni A, Guzman E, Herbert R, Vitelli A. Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci Transl Med. 2015;7(300):300ra127. doi:10.1126/scitranslmed.aac5757. PMID:26268314.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. doi:10.1001/jama.289.2.179. PMID:12517228.
- Trento A, Rodriguez-Fernandez R, Gonzalez-Sanchez MI, Gonzalez-Martinez F, Mas V, Vazquez M, Melero JA. The Complexity of Antibody Responses Elicited against the Respiratory Syncytial Virus Glycoproteins in Hospitalized Children Younger than 2 Years. Front Microbiol. 2017;8:2301. doi:10.3389/fmicb.2017.02301. PMID:29213258.
- Wheeler SM, Dotters-Katz S, Heine RP, Grotegut CA, Swamy GK. Maternal Effects of Respiratory Syncytial Virus Infection during Pregnancy. Emerg Infect Dis. 2015;21(11):1951–5. doi:10.3201/eid2111.150497. PMID:26485575.
- Widjojoatmodjo MN, Bogaert L, Meek B, Zahn R, Vellinga J, Custers J, Schuitemaker H. Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35 expressing the respiratory syncytial virus (RSV) fusion protein induce protective immunity against RSV infection in cotton rats. Vaccine. 2015;33(41):5406–14. doi:10.1016/j.vaccine.2015.08.056. PMID:26319741.
