?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A case of illegal vaccine sales in Shandong province, China, (hereinafter, the incident), which caused a lack of confidence among vaccination recipients and public panic, was uncovered in March 2016. We conducted a study comprising two cross-sectional surveys: at two months (May 2016) and seven months (October 2016) after the incident. The study aimed to evaluate the impact on immunizations; investigate the variation of the immunization coverage of the National Immunization Program Vaccines (NIPV) and the sales volume growth rate of Category II vaccines; and understand the reasons for non-vaccination and perspectives on immunization. The immunization coverage of NIPV decreased by 5.6 percentage points in the first survey, with a decline of 11.1 in the region of the incident, and decreased by 0.6 in the second survey compared to same period in 2015. The sales volume growth rate of Category II vaccines decreased by 25.8% in the study area and by 48.8% in the region of the incident in April 2016 compared to April 2015. Overall, 15.8% of respondents in the first survey and 7.0% in the second survey did not vaccinate their children according to the NIPV schedule because of the incident (χ2 = 78.463, P < 0.05). The vaccination was likely affected by the incident in varying degrees, especially in the involved region and particularly in relation to Category II vaccines. Overall, 34% of respondents avoided Category II vaccines for their children, indicating that it will take considerable time to eliminate the negative stigma associated with the incident.
Introduction
On March 18, 2016, the news site thepaper.cn reported a case of illegal vaccine sales (hereinafter referred to as the “the incident”) uncovered by the police in East China's Shandong province. The Shandong Police announced that they had arrested a mother and daughter suspected of illegally selling improperly stored vaccines worth more than 570 million Yuan, involving 24 provincial-level regions since 2011. Although produced by licensed manufacturers, the quality of the vaccines was questionable as they were not transported or stored properly. The incident was first reported in late February by the China National Radio News, but it did not attract much attention. Afterwards, thepaper.cn aggressively reported the incident once again and only then did it shock the nation. Moreover, it triggered widespread public concerns over vaccination safety and provoked a public health crisis in China.Citation1 On March 28, the State Council established a special interdisciplinary team to investigate the incident and the regulatory system that failed to prevent the distribution of substandard vaccines. Meanwhile, the World Health Organization (WHO) issued a prompt statement assuring the public that an adverse reaction to the vaccines was unlikely,Citation2 and official test results indicated that such vaccines did not pose a safety risk other than the conventional adverse reactions.Citation3 However, these assurances had little effect on settling the public outrage. This led to a nationwide lack of confidence in vaccinations among the public; some individuals even refused the use of vaccinations entirely.Citation4 On April 13, the interdisciplinary team confirmed that the illegal vaccines had been disposed of; in addition, the State Council announced that more than 200 people had been detained for their involvement in the incident, and 357 government employees had received punishments, such as dismissals and demotions.Citation5 Since then, the public opinion begun to subside.Citation1,Citation6
Vaccines are temperature-sensitive biological products that need to be adequately refrigerated and transported under strictly controlled conditions to ensure vaccine efficacy.Citation7 Since the 1980s, China worked with UNICEF to build a vaccine cold-chain system. The gradual popularization of the system has enabled all regions of China in different climates to provide routine immunization services, avoid the loss of vaccine potency, and consequent invalid vaccination.Citation8 At present, China has achieved nationwide vaccination coverage. In China, vaccines are divided into two categories under the regulation covering distribution and vaccination issued in 2005: Category I vaccines are those that are provided free of charge by the government to the citizens, mainly including the National Immunization Program Vaccines (NIPV); Category II vaccines are the vaccines that need to be self-funded. In contrast to Category I vaccines, which are distributed with stringent cold-chain management by the Centers for Diseases Control and Prevention, the procurement and distribution of Category II vaccines are usually undertaken by enterprises,Citation9 increasing the risk of improper storage and transportation. The incident that started at Shandong province involved only Category II vaccines, which were apparently stored and transported without the proper care to obtain higher profits.Citation1,Citation3,Citation4,Citation6
To evaluate the level of impact of the incident on vaccination and provide scientific evidence for decision-making, we conducted this study in 12 provinces two months and seven months after the incident to measure the vaccination changes and post-incident recovery.
Results
Immunization coverage of NIPV
shows the differences in the immunization coverage of NIPV in April and September 2016 compared to April and September 2015 in the different regions. In the first survey, the immunization coverage of NIPV was 86.4% in April 2016 for the survey area, which decreased by an average of 5.6 percentage points compared with that in April 2015. Additionally, there was a decrease of 11.1 and 1.3 percentage points in Regions I and II, respectively. In the second survey, the immunization coverage of NIPV for the study area was 93.3% in September 2016, which represented an decrease of 0.6 percentage points compared with that in September 2015, with an increase of −2.0 and 0.4 percentage points in Regions I and II, respectively.
Table 1. Comparison of the change of vaccination rates of NIPV in the survey area in the two surveys.
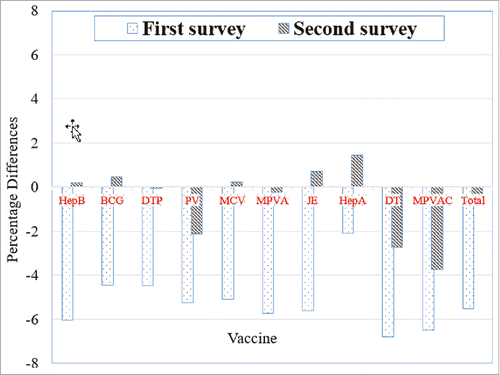
illustrates the differences in immunization coverage of 10 common NIPV in the April and September 2015 and 2016 for the study area. In the first survey, the immunization coverage in April 2016 decreased by varying degrees, and seven types of NIPV declined by more than 5 percentage points compared with April 2015. In the second survey, the immunization coverage of five types of NIPV in September 2016 had already increased in varying degrees, along with a decrease of 2–4 percentage points in the application of polio vaccines, diphtheria–tetanus vaccine and Group A and C meningococcal vaccine, compared with that in September 2015.
Figure 1. Differences in the immunization coverage of the 10 types of NIPV in the study area in the two surveys. Notes: 1. NIPV include Hepatitis B vaccine (HepB), Bacillus Calmette Guerin vaccine (BCG), diphtheria-tetanus-pertussis vaccine (DTP), polio vaccine (PV), measles-containing vaccine (MCV), epidemic cerebrospinal meningitis group A polysaccharide vaccine (MPVA), Japanese Encephalitis vaccine (JE), Hepatitis A vaccine (HepA), Diphtheria–Tetanus (DT) vaccine, and epidemic cerebrospinal meningitis group A and C polysaccharide vaccine (MPVAC). 2. The first survey compared changes in the NIPV vaccination rates between April 2016 and April 2015. 3. The second survey compared changes in the NIPV vaccination rates between September 2016 and September 2015. 4. Bar labeled “Total” represents the aggregate of the 10 NIPV reviewed in the study regions.
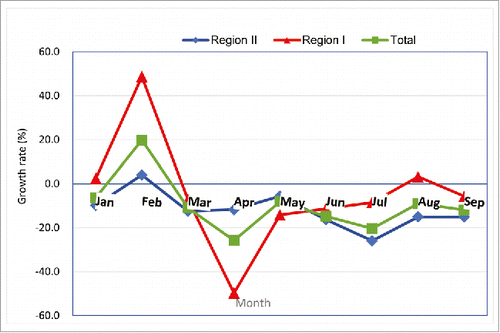
Sales volume growth rate of Category II vaccines
shows the sales volume growth rate of Category II vaccines from January to September 2016 compared to that of same period in 2015 for the different regions. In the study area, the sales volume growth rate in February 2016 was high (19.8%), followed by a slight decrease in March, the largest decrease (by 25.8%) in April, and a further decrease by approximately 10% in September 2016. In Region I, the largest decrease (by 48.8%) in the sales volume growth rate was in April 2016, after which it increased moderately between May and August. The sales volume growth rate was 5.7% lower in September 2016 than in September 2015. In Region II, the sales volume growth rate reached the greatest decrease (by 25.9%) in July after a decrease of 11.8% in April 2016. The sales volume growth rate was more than 15% lower in September 2016 compared with that in September 2015.
Household surveys
Characteristics of children
We interviewed 2,255 and 2,189 guardians of children in the first and second surveys who did not receive the NIPV vaccination according to the vaccination schedule after the incident, respectively. The response rate was 100% and 95.4% for the first and second surveys, respectively. Over 51% of the respondents lived in urban areas and >85% were the children's parents. The majority of the respondents have at least junior high school education level. A total of 86.8% of the respondents gave a definite yes when asked if they had heard of the incident. The main information source regarding the incident was the Internet, accounting for 49.6% of the respondents.
Reasons for avoiding the immunizations and willingness to receive NIPV vaccination
There were statistically significant differences among the variables between the two surveys in the different regions ().
Table 2. Reasons for avoiding immunizations with NIPV and perceptions of guardians on vaccination after the incident in the different survey areas.
Awareness rates and reasons for avoiding the immunizations
In the first and second survey, the awareness rates were 87.9% and 78.9% for the survey area (χ2 = 65.882, ρ < 0.05), 89.8% and 82.8% for Region I (χ2 = 23.934, ρ < 0.05), and 85.9% and 74.9% for Region II (χ2 = 41.782, ρ < 0.05), respectively. The percentage of respondents who did not take their children for NIPV vaccination as scheduled because of the incident was 15.8% and 7.0% for the survey area (χ2 = 78.463, ρ < 0.05), 19.0% and 11.6% for Region I (χ2 = 19.379, ρ < 0.05), and 12.2% and 2.3% for Region II (χ2 = 82.159, ρ < 0.05), respectively.
Perspectives on vaccine safety and effectiveness
In the first and second survey, the proportions of respondents with negative views on vaccine safety were 22.7% and 20.7% for the survey area (χ2 = 53.327, ρ < 0.05), 15.8% and 23.0% for Region I (χ2 = 98.994, ρ < 0.05), and 30.5% and 18.2% for Region II (χ2 = 43.860, ρ < 0.05), respectively. The proportions of respondents with a negative perspective on vaccine effectiveness were 19.6% and 14.7% for the survey area (χ2 = 41.847, ρ < 0.05), 12.4% and 15.8% for Region I (χ2 = 38.855, ρ < 0.05), and 27.8% and 13.5% (χ2 = 66.116, ρ < 0.05) for Region II, respectively.
Subsequent decision of vaccination
In the first and second survey, the percentage of respondents that were not negatively influenced by the incident were 47.6% and 57.4% for the survey area (χ2 = 130.241, ρ < 0.05), 45.9% and 58.4% for Region I (χ2 = 60.187, ρ < 0.05), and 49.5% and 56.4% for Region II (χ2 = 78.712, ρ < 0.05), respectively. The proportion of respondents who chose not to vaccinate their children with Category II vaccines increased from 27.6% to 36.1% for Region II while there was a slight decrease by 0.2% in Region I.
Discussion
Although the efficacy and safety of vaccines have continued to improve, negative information about vaccines still disrupts public confidence in vaccinations globally.Citation10 The present study referred to an incident in which an illegal dealer sold Category II vaccines, that were stored and transported inappropriately without using cold-chain equipment; therefore, the efficacy and safety of the vaccinations could not be guaranteed. The media intensively reported the incident, causing public panic and distrust of vaccinations.Citation1,Citation3,Citation4,Citation11 Thus, we conducted a study to assess the impact of the incident on vaccinations through two surveys applied in 12 provinces in China. The first survey aimed to investigate the impact of the incident on vaccination in May 2016 and the second survey aimed to assess the post-incident recovery in October 2016.
The first survey showed that although NIPV were not involved in the incident, the immunization coverage for NIPV in the study area decreased in varying degrees compared with that in the corresponding month of the previous year, being this decrease more pronounced in Region I compared with Region II. Furthermore, the sales volume growth rate of Category II vaccines in April 2016 decreased by 25.8% in the study area and by 49.8% in Region I compared with that in the corresponding month of the previous year. The findings indicated that vaccination was very likely affected by the incident in the survey area, and that Category II vaccines were more seriously affected in the different regions. Chen Wei et al.Citation12 pointed out that the immunization coverage of NIPV in Tianjin City, China in March 2016 dropped 1.47% to 8.92% compared with that in March 2015, whereas varicella vaccination (Category II vaccine in China) decreased >40% compared with that in March 2015. Chen Baolin et al.Citation13 in Urumqi City and WangCitation14 in Henan province also found that the number of Category II vaccinations had decreased >50% in the same periods while NIPV vaccination suffered moderately negative effects. These findings were consistent with ours. However, Luo et al.Citation15 found that the immunization coverage of NIPV in Hubei province of China significantly dropped >20%. These findings indicate that NIPV vaccination was subject to a greater negative impact in some areas than in others. After the government took the necessary steps to contain the incident and carried out positive publicity and health education, the immunization coverage of NIPV in September 2016 only decreased by an average of 0.6% compared with that in September 2015. This indicated that the negative influence on NIPV vaccination might be weakening or may have even gradually disappeared. We also found a slight decrease in the vaccination of a few NIPV in September, likely because of the temporary shortage due to the adjustment of the immunization strategy for poliomyelitis eradication in response to the WHO resolution starting on May 1, 2016,Citation16,Citation17 and/or delays on the biological product approval by the government.Citation18 Additionally, the study shows that the use of Category II vaccines has gradually improved by 10% to 15% between August and September 2016, which seems to indicate that the concerns raised by the incident had eased down after the temporary shortage of Category II vaccines due to the revision of the “Chinese regulations for vaccine storage and transportation.”Citation18
According to the analysis of public opinion, the incident was classified into four stages: incubation, outbreak, investigation and disposal, and extinction periods. The public concern raised by the incident gradually subsided after the state council released the final settlement of the incident on April 13, 2016.Citation1,Citation19 In the present study, we also found that the largest decrease in sales volume of Category II vaccines occurred one month after the incident (April 2016), but that it then rebounded after May 2016. Accordingly, this study showed that there was a substantial impact on vaccination as vaccinations decreased after the incident; however, the public seemed to remain observant and did not rush to make a decision while the situation was unclear. Thus, a fast response to the incident might be helpful to mitigate the negative impact in such situations.
The incident also seemed to indicate that vaccination became a victim of its own success, meaning that the growing effectiveness of vaccines made the public pay close attention to the risks of vaccination rather than to the burden of the disease the vaccine was designed to prevent. This has led to a loss of confidence in vaccinations among the public, which has become a global issue.Citation20 As the Internet has changed the way people access information and communicate, it has also deepened the trend of vaccine distrust.Citation21 Nearly 50% of the public information of the incident came from the Internet, and the emotional aspect of the incident worsened the public panic and mistrust.Citation1 According to the first survey in this study, the proportion of respondents not willing to have their children vaccinated for NIPV according to the vaccination schedule because of the incident reached 15.8% in the study area and was even as high as 19% in Region I. As the incident developed, public attention and awareness decreased. More than 20% of the respondents had not heard of the incident, and the proportion of children who were not vaccinated according to the vaccination schedule because of the incident dropped sharply to 7% by the second survey. However, the proportion of children who lack of motivation to receive NIPV increased from 2.1% to 4.2% in the study area, which seems to indicate that the impact of the incident did not completely disappear and that long-term efforts must be made. Moreover, although the incident involved only Category II vaccines made by legal manufacturers without safety issues identified by repeated testing by the government and the WHO,Citation2,Citation3 the proportion of respondents with definitive negative views still reached 20.7% on vaccine safety and 14.7% on vaccine effectiveness in the study areas during the second survey. These facts indicated that the public trust in vaccination is not always based on scientific evidence but often results from many factors related to psychology, social science, and politics.Citation10 Although the proportion of parents who were hesitant about their children receiving vaccinations decreased from 22.3% to 8.6% in the second survey, 34% of respondents still avoided Category II vaccines for their children. This further suggests that raising the public confidence regarding the safety of Category II vaccines and eliminating the impact of the incident is a long-term task.
The study has several limitations; thus, the results must be cautiously interpreted when extrapolating to a larger population. First, this was a cross-sectional study without a control group, the results can be suggestive but are too weak to establish any causal relationship among the related factors.Citation22 A further inferential study is needed to identify such relationships. Second, it may overestimate the impact because of the sample of health centers with definite clues and the developed provinces with convenient transportation and communication may not be the representative of the entire country. Finally, the structural temporary shortage of Category II vaccines after the adjustment of procurement and distribution since April 23, 2016,Citation23 and the nationwide shortage of polio vaccine because of insufficient production after adjustment of the immunization strategy on May 1, 201624 affected the vaccination to some extent.
Although the present results need to be interpreted with caution, this study showed that vaccinations were most likely negatively affected by the incident to varying degrees, particularly in the region involved in the incident and particularly regarding the use of Category II vaccines. In the second survey, a considerable proportion of respondents mentioned a lack of interest in vaccinating their children with Category II vaccines, which indicates that long-term measures are necessary to eliminate such negative effects.
Materials and methods
Study design and sampling methods
In this study, we conducted two cross-sectional surveys: at two months (in May 2016) and at seven months (in October 2016) after the incident. We measured the variation of the immunization coverage of NIPV and sales volume growth rates of Category II vaccines to evaluate the impact of the incident on vaccinations. We employed the stratified sampling method to select 12 provinces as the study areas according to willingness to cooperate, geographical distribution and the definite clues of the incident provided by the police, which are published on the website of Shandong Food and Drug Administration,Citation25 including six provinces with definite clues that were involved in the incident (Region I) and six provinces that were not involved in the incident (Region II). The eastern, middle and western parts of China respectively selected two provinces for each region. In Region I, we sampled the townships from the list of those townships involved in the incident provided by the police. In Region II, two districts in the most developed metropolises of Beijing, Shanghai, and Tianjin, and one district and one county with convenient transportation and advanced communications in the rest of three provinces, were selected at the provincial level. Each county/district randomly sampled three townships. In total, each province investigated at least two counties and six townships. Finally, 76 townships or community health centers were surveyed twice in the different periods of study. Aside from performing the survey at health centers, we also conducted household interviews to find at least 30 children aged less than 3 years who had not received immunization for NIPV according to the vaccination schedule after the incident in each township to determine the reason for the lack of immunization and the perspectives of their guardians on vaccination. In total, there were at least 180 children interviewed in each province for each survey.
Data collection
Unified questionnaires were used to collect the data, and investigators received training for application of the questionnaires before performing the fieldwork. The NIPV vaccination data in April and September 2015 and 2016, and the sales volume data of Category II vaccines from January to September 2015 and 2016 were respectively collected for each township in the two surveys based on the immunization registry and vaccine delivery information kept at the health facilities. Afterwards, investigators carried out household surveys to interview the guardians of children 3 years of age or less who did not comply with their NIPV vaccination according to the vaccination schedule in order to determine the reason for the lack of immunization and their level of confidence in the vaccination program applied in the surveyed towns.
Data analysis
The difference of the immunization coverage of NIPV in April and September 2016 was compared with that during the corresponding period of the previous year by cumulatively calculating the impact of the NIPV vaccination by townships for each regions. Furthermore, we calculated the monthly sales volume growth rates of Category II vaccines from January to September 2016 based on the following formula:
The chi-squared test was used to compare the differences in sample rates between different survey rounds and regions with statistical significance. We used EPIDATA 3.0 (The EpiData Association, Odense, Denmark) to build the database, collect the data, and run double entry and logic checks. Descriptive analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA) and Excel.
Ethical issues
This work was performed by the Chinese Center for Disease Control and Prevention. We obtained verbal informed consent from all the participants before the household investigation. We did not collect any private information of the participants during the interviews; therefore, this study did not require ethics approval.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the staff of the provincial, prefectural and county Centers for Diseases Control and Prevention and township health centers for conducting the survey.
Additional information
Funding
References
- Jie L. Public opinion public attitudes and response analysis. China Public Heal Manag. 2017;33:316–9.
- World Health Organization. Incorrect Storage or Expired Vaccines rarely Cause Toxic Reactions. 2016. http://news.xinhuanet.com/world/2016-03/22/c_1118409442.htm. Accessed 20 Oct 2017.
- Joint Investigation Team of the State Council. The Investigation Progress of the Shandong Jinan Illegal Vaccine Sales Event. 2016. http://news.xinhuanet.com/2016-04/13/c_1118613998.htm. Accessed 20 Oct 2017.
- Wei-dong L, Qi L. The Causes and Countermeasures of Public Panic Caused by Sudden Public Events Taking“Vaccine event”in Shandong as an Example. J Yichun Univ. 2016;38:27–30.
- The Xinhua News Agency. China Punishes 357 Officials over Vaccine Scandal. 2016. http://www.xinhuanet.com/english/2016-04/14/c_135276050.htm. Accessed 9 Mar 2018.
- Min XIAO, Yingying LI. Discussion on the Supervision Problems and Suggestions in the Case of Shandong Vaccine. China Pharm. 2016.;27:2161–3.
- Kumru OS, Joshi SB, Smith DE, Middaugh CR, Prusik T, Volkin DB. Biologicals Vaccine Instability in the Cold Chain: Mechanisms, Analysis and Formulation Strategies. Biologicals. 2014;42:237–259. Accessed 20 Oct 2017. doi:10.1016/j.biologicals.2014.05.007. PMID:24996452
- Ministry of Foreign Trade and Economic Cooperation of China. The coopertion between China EPI and Unicef goes well. Int Trade 1995.;5:40–5.
- The State Council of China. Regulations on the management of vaccine circulation and vaccination. 2005..
- Larson HJ, Cooper LZ, Eskola J, Katz SL, Ratzan S. Addressing the vaccine confidence gap. Lancet. 2011.;378:526–35. doi:10.1016/S0140-6736(11)60678-8. PMID:21664679.
- Rui-hua Z, Da-ren Z, Si-zhang H, Al E. Reflection on Shandong vaccine from the perspective of the self-media. Chinese J Disaster Med. 2016.;4:422–4.
- Wei CHEN, Zhi-gang G. Influence of media report of illegal vaccine sales in Shandong province on attitude and behavior of parents about vaccination of their children in Tianjin city:a quick survey in 2016. Chinese J Public Heal. 2016.;32:881–4.
- Bao-lin C, Pei-sheng W, Xia L. Analysis of the impact of Shandong vaccine events on the partial vaccination in Urumqi City. Chinese J Pest Control. 2017.;33:578–9.
- Chang- W, Yan W, Ming- LU. Analysis of “Shandong Vaccine Event” on parental attitude and practice to vaccination in 2 counties of Henan Province. Chinese J Heal Educ. 2017.;33:255–8.
- Yu-e L, Jin-ze L, Ning L, Al E. Influence of the Illegal Vaccine Trade Event on the Immunization Program in Hubei Province. Chinese J vaccinces Immun. 2016.;22:420–3.
- World Health Assembly. Poliomyelitis. 2016.. http://apps.who.int/gb/ebwha/pdf_files/WHA68/A68_21Add1-en.pdf. Accessed 28 Sep 2016.
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, April 2016—conclusions and recommendations. Wkly Epidemiol Rec. 2016.;91:266–72. PMID:27236869.
- People's Dly. Online. Behind the Shortage of Category II Vaccine.2016.. http://health.people.com.cn/n1/2016/0715/c398004-28557047.html. Accessed 15 Jul 2016.
- Lin YU. Evolution of Internet public opinions about illegal vaccine sales in Shandong province. Chinese J public Heal Manag. 2017.;33:320–2.
- Cooper LZ, Larson HJ, Katz SL. Protecting Public Trust in Immunization. Pediatrics. 2008.;122:149–53. Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2008-0987. Accessed 28 Sep 2017. doi:10.1542/peds.2008-0987. PMID:18595998.
- Bean SJ. Emerging and continuing trends in vaccine opposition website content. Vaccine. 2011.;29:1874–80. doi:10.1016/j.vaccine.2011.01.003. PMID:21238571.
- Levin KA. Study design III: Cross-sectional studies. Evid Based Dent. 2006.;7:24–5. doi:10.1038/sj.ebd.6400375. PMID:16557257.
- People's Daily Online. The Shortage of A Few of Category II Vaccines was Occurring in Jiangsu Province. 2016..http://js.people.com.cn/n2/2016/0709/c360306-28637529.html. Accessed 9 Jul 2016.
- National Health and Family Planning Commission. China Responds to WHO Resolutions to Implement A New Polio Vaccine Immunization Strategy. http://www.nhfpc.gov.cn/jkj/s3582/20104/8c760a934d5b4d41a81752915c58d304.shtml. Accessed 29 Apr 2016.
- Shandong Food and Drug Administration. A Notice on the Clues about Shandong Illegal Vaccine Sales Incident. 2016.. http://www.sdfda.gov.cn/art/2016/3/19/art_3543_133258.html. Accessed 19 Mar 2016.