ABSTRACT
The devastating Ebola virus (EBOV) epidemic in West Africa in 2013–2016 accelerated the progress of several vaccines and antivirals through clinical trials, including the replication-competent vesicular stomatitis virus-based vaccine expressing the EBOV glycoprotein (VSV-EBOV). Extensive preclinical testing in animal models demonstrated the prophylactic and post-exposure efficacy of this vaccine, identified the mechanism of protection, and suggested it was safe for human use. Based on these data, VSV-EBOV was extensively tested in phase 1–3 clinical trials in North America, Europe and Africa. Although some side effects of vaccination were observed, these clinical trials showed that the VSV-EBOV was safe and immunogenic in humans. Moreover, the data supported the use of VSV-EBOV as an emergency vaccine in individuals at risk for Ebola virus disease. In this review, we summarize the results of the extensive preclinical and clinical testing of the VSV-EBOV vaccine.
Introduction
Although development of vaccines and antivirals for Ebola virus (EBOV) has been a long-standing goal for many researchers, progress towards a licensed vaccine accelerated in response to the devastating EBOV epidemic in West Africa in 2013–2016. Many vaccine candidates underwent preclinical testing in various animal models, and several entered clinical trials.Citation1 The performance in preclinical tests of the recombinant vesicular stomatitis virus-based vaccine (VSV-EBOV, also known as rVSV-ZEBOV) supported its progress through phase 1–3 clinical trials.
VSV, a member of the Rhabdoviridae family, is a non-segmented, single-stranded, negative-sense RNA virus encoding five genes. The virus can cause transient disease in livestock and can occasionally infect humans who work in close proximity to animals. However, human infections are asymptomatic or at most result in a mild, transient illness.Citation2 VSV was among the first negative-sense RNA viruses to be generated from cloned cDNA, and its small genome and amenability to genetic manipulation make VSV conducive to use as an expression vector for foreign proteins.Citation3,Citation4 In addition to the ability to express foreign antigens, other features of VSV contribute to its suitability as a vaccine platform. The virus can be propagated in a wide variety of mammalian cells, where it grows to high titers, and it elicits strong immune responses in vivo.
Early studies with recombinant VSV vaccines in mice resulted in signs of disease after vaccination, indicating that attenuation of the vaccine vector was necessary.Citation4 Multiple strategies have been developed to achieve attenuation of the VSV vaccine vector, including truncation of the cytoplasmic tail of the VSV glycoprotein (G) to reduce virulence, as well as modification of other structural proteins.Citation5,Citation6 Alternatively, when the antigen expressed is a glycoprotein that facilitates cell entry, removal and replacement of VSV G with a different viral glycoprotein can occur while preserving the immunogenicity of a replication-competent vector.Citation7 This strategy has proven effective for the VSV-EBOV vaccine, in which the EBOV glycoprotein (GP) is inserted into a VSV vector from which the G open reading frame is deleted (VSV-ΔG), resulting in a replication-competent virus particle exhibiting rhabdovirus morphology with EBOV GP expressed on its surface (). Here, we summarize the results of the extensive preclinical testing in animal models (reviewed inCitation8,Citation9) of the VSV-EBOV vaccine, as well as the results of phase 1–3 clinical trials and discuss the potential use of VSV-based vaccines in outbreak situations.
Figure 1. Preclinical testing of the VSV-EBOV vaccine in animal models. Recombinant VSV particles expressing the EBOV GP are produced from a cDNA clone of the VSV genome in which the VSV G is replaced with EBOV GP. The resulting vaccine has been tested in different animal models to assess protective prophylactic efficacy, time to immunity, post-exposure efficacy, cross-protection potential, as well as providing insight into the mechanism of protection.
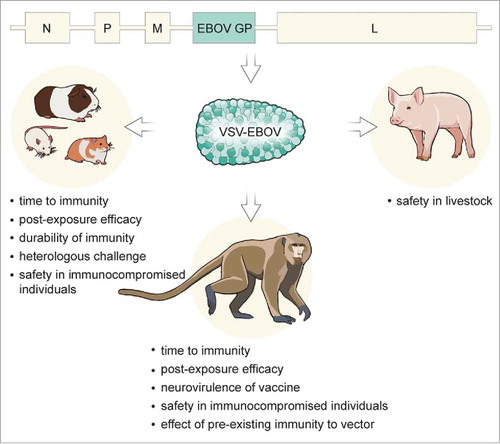
Proof of concept
Following successful mouse studies,Citation7 the efficacy of VSV-EBOV was first demonstrated in cynomolgus macaques in 2005, which are considered the gold standard model for filovirus infection. Intramuscular inoculation with 107 plaque-forming-units (PFU) of VSV-EBOV did not result in signs of disease, but a transient vaccine viremia was detected shortly after vaccination. When challenged intramuscularly with a lethal dose of 1,000 PFU of EBOV 28 days after vaccination, animals were completely protected, with no disease signs and no challenge virus viremia detectable.Citation10 Vaccination with VSV-EBOV also protected cynomolgus macaques from lethal challenge via the aerosol route.Citation11
In the mouse model of EBOV, using mouse-adapted EBOV (MA-EBOV), mice were completely protected from lethal challenge 28 days after intramuscular, intraperitoneal, or intranasal vaccination with VSV-EBOV.Citation12 Cynomolgus macaques vaccinated intranasally or orally with VSV-EBOV were also fully protected from lethal EBOV challenge, suggesting that efficacy of the vaccine is not dependent on the route of administration.Citation13
Rodent models were further used to determine the durability of the immune response to VSV-EBOV. Mice were 100% protected from lethal challenge up to 9 months after vaccination with VSV-EBOV,Citation12,Citation14 and guinea pigs were protected up to 18 months after vaccination,Citation14 suggesting that the antigen-specific immune response is quite durable after vaccination.
Experiments in cynomolgus macaques with impaired immune responses due to simian-human immunodeficiency virus infection or depletion of CD4+ or CD8+ T cells showed that CD4+ but not CD8+ T cells were required at the time of vaccination for protection, indicating that VSV-EBOV-mediated protection is mainly a result of the production of EBOV GP-specific antibodies, rather than T cell immunity.Citation15,Citation16
Peri-exposure efficacy
After proving the pre-exposure efficacy of the VSV-EBOV vaccine, follow-up studies were performed to assess the potential of VSV-EBOV as an emergency vaccine, either shortly before or after exposure to EBOV. Mice were fully protected from death and disease when vaccinated as shortly as 24 hours before challenge with MA-EBOV and exhibited only mild disease when treated up to 24 hours post challenge.Citation17 In guinea pigs, partial protection from lethal challenge with guinea pig-adapted EBOV (GPA-EBOV) was observed when animals were vaccinated 24 hours prior to challenge, 1 hour post challenge, or 24 hours post challenge (66%, 83%, and 50% survival, respectively).Citation17 Hamsters vaccinated up to 3 days prior to challenge with MA-EBOV were fully protected and generated high antibody titers without displaying clinical signs of disease. In a post-exposure study, all hamsters in the groups treated immediately or 24 hours after challenge survived, while all hamsters treated 48 hours after challenge succumbed to MA-EBOV infection.Citation18
Rhesus macaques inoculated with VSV-EBOV 20 to 30 minutes after lethal EBOV challenge developed febrile illness, yet 50% of the animals survived, demonstrating partial post-exposure efficacy of VSV-EBOV in nonhuman primates.Citation17 Control animals treated with the VSV-Marburg virus (VSV-MARV) vaccine were not protected from lethal disease. Citation17 Post-exposure efficacy of the VSV-EBOV vaccine against EBOV-Makona, the EBOV strain causing the 2013–2016 West African epidemic, was shown in rhesus macaques, where 50% of animals survived lethal challenge when treated with VSV-EBOV at 1 and/or 24 hours post challenge. In contrast to the post-exposure study, partial survival was also observed in the group of animals treated twice with a control vaccine, the VSV-MARV vaccine, and challenged with EBOV-Makona, again indicating that the mechanism of a short time to protection was most likely based on a non-specific induction of the innate immune system by VSV rather than an antigen-specific response.Citation19
Another study prompted by the 2013–2016 EBOV epidemic examined the time to immunity of VSV-EBOV in cynomolgus macaques using the West African EBOV-Makona strain as challenge virus and found that vaccination as late as 7 days prior to lethal challenge elicited complete protection, while 2 out of 3 animals were protected when vaccinated only 3 days before challenge.Citation20 Based on the absence of antigen-specific antibody responses in the animals vaccinated 3 days before challenge, protection in this group was most likely based on activation of the innate immune response by VSV-EBOV vaccination, providing short-term protection for the duration necessary to develop an EBOV-specific adaptive immune response. This study clearly demonstrated the feasibility of using the VSV-EBOV vaccine in outbreak situations where a short time to protection is essential to aid in reducing transmission of the virus.
Cross-protection
Cross-protection of the VSV-EBOV vaccine within the Zaire ebolavirus species was shown in several studies where animals were vaccinated and subsequently challenged with heterologous strains.Citation10,Citation13 Importantly, it was shown that vaccination of cynomolgus macaques with a VSV-EBOV vaccine expressing the GP from the EBOV-Kikwit strain, isolated during the 1995 EBOV outbreak in Kikwit, Democratic Republic of the Congo,Citation21 that was subsequently used in clinical trials resulted in complete protection from lethal challenge with the EBOV-Makona strain isolated during the West African EBOV epidemic.Citation20 Thus, the currently available VSV-EBOV vaccine will likely be protective in future EBOV outbreaks.
The cross-protective ability of VSV vectors between different Ebolavirus species (Bundibugyo, Taï Forest, Reston, or Sudan ebolavirus) was examined in rodent models. In mice, vaccination with VSV vectors expressing the Taï Forest virus (TAFV), or Reston virus (RESTV) GP were completely protected from MA-EBOV challenge; moreover, 75% of animals receiving a VSV expressing the Sudan virus (SUDV) GP survived. In contrast, there was no cross-species protection in guinea pigs immunized with VSV-based vaccines bearing the GPs of Bundibugyo virus (BDBV), TAFV, RESTV, or SUDV when challenged with guinea pig-adapted EBOV.Citation22 However, vaccination of cynomolgus macaques with VSV-EBOV resulted in partial protection against BDBV infectionCitation23 and vaccination with VSV-EBOV in a prime-boost regimen resulted in complete protection against BDBV.Citation24 Taken together, there is limited interspecies cross-protection afforded by the VSV-EBOV vaccine; efficient cross-species protection will most likely require different approaches such as blending of several monovalent VSV vaccine vectors expressing the glycoproteins of different Ebolavirus species, expression of additional viral proteins from a multivalent VSV-EBOV vector, or expression of different ebolavirus GPs from a multivalent VSV vector.Citation22,Citation25,Citation26
Pre-existing immunity
With the successful development of VSV-based vaccines against highly pathogenic viruses with geographically overlapping endemic areas, such as the VSV-EBOV and a VSV-based vaccine expressing the Lassa virus (LASV) glycoproteins (VSV-LASV),Citation27,Citation28 the effect of pre-existing immunity to the vaccine vector on vaccine efficacy became a concern. To assess the effect of pre-existing immunity to the VSV vector, cynomolgus macaques previously vaccinated with VSV-LASV and challenged with LASV were vaccinated with VSV-EBOV 3 months after LASV challenge, and challenged with a lethal dose of EBOV. Despite high VSV-specific antibody titers at the time of vaccination with VSV-EBOV, the animals produced EBOV GP-specific antibodies and were completely protected from lethal EBOV challenge.Citation29 Thus, the presence of antibodies specific to the VSV vector did not influence protective efficacy of later VSV-based vaccination, indicating that consecutive VSV-based vaccines can be used effectively in the same population.
Vaccine vector safety
The preclinical efficacy data described above made the VSV-EBOV vaccine a very promising candidate vaccine for human use; however, safety concerns regarding the use of a replicating vaccine in humans needed to be addressed. One of these concerns stemmed from the fact that VSV wild type (VSVwt) is a neurotropic virus. Although VSV-EBOV lacks the VSV G, the protein largely responsible for its neurotropism, the neurovirulence of VSV-EBOV was determined in cynomolgus macaques through intrathalamic inoculation. Although inoculation of cynomolgus macaques with recombinant VSVwt via this route results in neurological disease and histologic lesions in the central nervous system, inoculation with VSV-EBOV did not result in the development of neurological disease signs or histologic lesions, indicating a lack of neurovirulence of this vaccine.Citation30 An additional consideration regarding large-scale use of a replication-competent vector was its safety in immunocompromised individuals, a serious concern due to the high prevalence of HIV in EBOV endemic areas in Africa. To address this concern, NOD-SCID mice that are severely immunocompromised due to the lack of functional T and B cells were vaccinated with a high dose of VSV-EBOV; no clinical signs of disease were observed in these animals.Citation12 To further investigate the effect of VSV-EBOV vaccination in immunocompromised individuals, rhesus macaques infected with simian-human immunodeficiency virus were immunized with VSV-EBOV and monitored for signs of disease. None of the animals exhibited evidence of vaccine-associated illness, and four of six animals survived subsequent lethal EBOV challenge, demonstrating safety and partial efficacy in immunocompromised animals.Citation15
In addition to the safety of this vaccine in humans, the ability of VSVwt to cause disease in livestock prompted concerns of a potential spillover of the vaccine from vaccinated individuals to livestock, since VSV is an OIE-listed pathogen. To address this concern, pigs were inoculated with the VSV-EBOV vaccine. Animals did not display signs of disease and virus shedding was observed at a very low level in only one out of six animals, suggesting that exposure of pigs to VSV-EBOV-vaccinated humans is unlikely to cause disease or transmission in pig herds.Citation31 However, further testing in pigs and other livestock species may still be warranted.
Human clinical trials
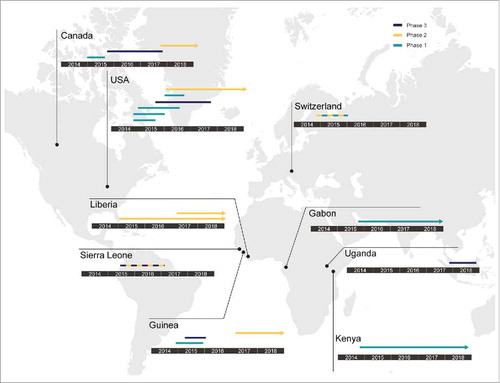
The 2013–2016 epidemic of Ebola virus disease (EVD) in West Africa prompted several phase 1–3 human clinical trials to determine safety and efficacy of antiviral strategies and vaccines. Since October 2014, eighteen human clinical trials of VSV-EBOV have been conducted, are ongoing or planned in North America, Europe, and Africa ().
Figure 2. Concluded, ongoing and planned human clinical trials of VSV-EBOV. Since 2014, VSV-EBOV has been evaluated globally in phase 1, 2 and 3 clinical trials. The countries where these clinical trials were conducted, and their phase (1-3) are shown. Lines indicate completed clinical trials; arrows indicate clinical trials still ongoing at the time of this writing.
The first two phase 1, placebo-controlled, double-blind, dose-escalation trials of the VSV-EBOV vaccine were conducted in the USA (NCT02269423, NCT02280408).Citation32 In these trials, volunteers received a dose of 3 × 106, 2 × 107, or 1 × 108 PFU of the vaccine or placebo, administered either as a single dose or as two identical doses 28 days apart. ELISA results showed that all vaccinated volunteers had undergone seroconversion to EBOV GP by day 28. The groups that received a dose of 2 × 107 or 1 × 108 PFU had higher geometric mean titers against EBOV GP than the group that received a dose of 3 × 106 PFU. Furthermore, there was no significant difference in the geometric mean titer between the group that received a dose of 2 × 107 PFU and the group that received a dose of 1 × 108 PFU. Systemic adverse events such as injection-site pain, headache, fatigue, myalgia, fever, chills, and arthralgia were observed, which is consistent with other live virus vaccines. These symptoms were generally mild and transient, and resolved by day 4 post vaccination. In the two-dose regimen, the second dose was less reactogenic than the initial dose.Citation33 Serum sample analysis by Khurana et al. resulted in the identification of novel GP epitopes and, using a pseudo-particle assay in vitro, showed that IgM responses after vaccination are neutralizing, but a second dose administered 28 days after the first one did not significantly increase the antibody titer or repertoire.Citation33
Three open-label, dose-escalation phase 1 trials and one randomized, double-blind, controlled phase 1/2 trial to assess the safety, side-effect profile, and immunogenicity of VSV-EBOV were simultaneously performed in Europe and Africa. All participants were injected with vaccine doses ranging from 3 × 105 to 5 × 107 PFU or placebo (NCT02283099, NCT02296983).Citation34,Citation35 The results from these three trials demonstrated good antibody responses in all vaccinees and neutralizing antibodies were elicited by day 28 in 107 out of 126 vaccinees (85%).Citation36,Citation37 However, during one trial in Geneva, 11 out of 51 (22%) participants without any previous history of joint disease had an onset of arthralgia in the second week after vaccination. Of them, 8 participants had received 1 × 107 PFU and 3 had received 5 × 107 PFU. Moreover, a mild maculopapular rash mainly on the limbs developed in 3 participants who had arthritis. These data suggested that lower vaccine doses might be better tolerated but still immunogenic. The Geneva trial thus resumed at the dose of 3 × 105 PFU (low-dose vaccinees) and compared safety and immunogenicity results with 1 × 107 or 5 × 107 PFU (high-dose vaccinees). Although similar seroconversion rates were observed, the EBOV GP-specific and neutralizing antibodies of low-dose vaccinees were significantly lower than that of high-dose vaccinees. Furthermore, 13 low-dose vaccinees still developed arthritis. Therefore, this study did not support a strategy of dose reduction to prevent vaccine-induced arthritis.Citation38 Samples collected during this study were analyzed by Rechtien et al., using a systems vaccinology approach and showed that an early innate immune signature, specifically NK cell activation and IP-10 levels, correlates with antigen-specific antibody responses.Citation39 In-depth analysis of samples from one trial corroborated this finding of increased IP-10 levels after vaccination and found a general activation of T cells with a significant induction of Th1 cytokines in response to vaccination.Citation35 A different study found a correlation between the antibody response to vaccination and an increase in the circulation of a specific subset of CD4+ T cells, cTfh17 cells.Citation40
In addition to the safety data, immunogenicity data that were available in January 2015 were pooled from some North American phase 1 trials and assessed for dose selection. As a result, 2 × 107 PFU was selected as the dose for phase 2 and 3 trials in West Africa. A randomized, placebo-controlled phase 2 trial of the vaccine was performed in Liberia during the epidemic (NCT02344407). A total of 1,500 adults underwent randomization and were followed for 12 months.Citation41 One month after vaccination, the vaccinees had elicited high antibody responses that were largely maintained throughout the 12 months.
In 2015, three phase 3 clinical trials were conducted. The first was an open-label, cluster-randomized ring vaccination phase 3 trial in Guinea (PACTR201503001057193).Citation42,Citation43 In this trial, contacts and contacts of contacts of individuals with EVD were grouped into clusters and these clusters were randomized to receive a single dose of the VSV-EBOV vaccine (2 × 107 PFU) immediately or with a 21-day delay. In total, 4,539 contacts and contacts of contacts were assigned to the immediate vaccination group, and 4,557 participants were assigned to the delayed vaccination group. No cases of EVD occurred 10 or more days after randomization among randomly assigned contacts and contacts of contacts vaccinated in immediate clusters. However, 16 cases were determined in those receiving the vaccine with the 21-day delay. These results show that the VSV-EBOV vaccine is indeed fast-acting and efficacious against EVD in humans. In this study, around 50% of the vaccinees reported at least one adverse event in the first 14 days after vaccination, but these were typically mild (headache, fatigue, and muscle pain). Eighty serious adverse events were identified, of which two were judged to be related to vaccination (one febrile reaction and one anaphylaxis) and one possibly related (influenza-like illness). All three individuals recovered without sequelae.
The second trial was an open-label, individually randomized controlled phase 2/3 trial conducted in Sierra Leone (NCT02378753 and PACTR201502001027220).Citation44 Vaccination was completed in December 2015, and more than 8,000 participants were enrolled and vaccinated. A total of 64 participants had illnesses that were investigated as suspected EVD, of whom 60 provided specimens for testing, but none were confirmed as EVD. No serious adverse events, including arthritis, related to vaccination were reported, and the data are generally consistent with data found in the phase 1 trials of the vaccine.
The last study was a randomized, double-blind, multicenter phase 3 clinical trial in the USA, Spain, and Canada (NCT02503202).Citation45 The trial was designed to assess the safety and immunogenicity of 3 consistency lots (2 × 107 PFU) and a high-dose lot (1 × 108 PFU) of VSV-EBOV vaccine. The vaccine was generally well-tolerated with some adverse events, and no vaccine-related severe adverse events or deaths were reported. The safety of the VSV-EBOV vaccine in immunocompromised individuals, first investigated in preclinical testing,Citation15 was confirmed by the inclusion of human immunodeficiency virus (HIV) positive participants in one clinical trial. No serious adverse events were reported in these volunteers within a month of vaccination, although a lower portion of HIV-positive individuals generated an antibody response to the vaccine than HIV-negative participants.Citation41 No effect of sex on immunogenicity of the vaccine was found; a study including children ages 6 and over showed that VSV-EBOV is safe and immunogenic in children.Citation41,Citation46 The findings of these clinical trials support the use of VSV-EBOV as an emergency vaccine in individuals at risk for EVD such as contacts and contacts of contacts of confirmed EVD cases.
Conclusion
The data summarized above show that VSV-EBOV is a safe and efficacious vaccine for use in humans. While this vaccine is still not licensed, several clinical trials are ongoing () to provide further data to expand the efficacy and safety profile of the VSV-EBOV vaccine. Although several other EBOV vaccine platforms have been developed and progressed to clinical trials, the unique advantage of the VSV-EBOV is the rapid protection after vaccination and its potential post-exposure efficacy. The post-exposure treatment properties of the VSV-EBOV vaccine have been used on several occasions in laboratory and healthcare workers exposed to and potentially infected with EBOV.Citation47,Citation48 The use of the VSV-EBOV vaccine in thousands of people during phase 2/3 clinical trials also showed that it is feasible to manufacture large-scale vaccine batches for deployment in outbreak situations.
Importantly, the VSV vaccine platform is amenable to adaptation to other emerging and re-emerging pathogens of concern, including other filoviruses, paramyxoviruses, and arenaviruses.Citation27,Citation49,Citation50 In addition, a dual VSV-based vector expressing the GPs of viruses from two different virus families was shown to protect against both viruses in an animal model with similar short time to protection and post-exposure efficacy as VSV-EBOV.Citation18,Citation51 Now that the safety, efficacy, and feasibility of the VSV platform have been proven, it is time to finally license this platform, as has been recently done in Russia. In addition, moving VSV-based vaccines for other pathogenic viruses with outbreak potential, such as MARV, LASV, and Nipah virus, forward to clinical trials should be a priority.
Disclosure of potential conflicts of interest
H. F. claims intellectual property regarding the vesicular stomatitis virus-based filovirus vaccine. The authors declare no other conflicts of interests.
Acknowledgments
The authors would like to thank Ryan Kissinger and Jackson Emanuel (NIAID) for assistance with this manuscript.
Additional information
Funding
References
- Mire CE, Geisbert TW, Feldmann H, Marzi A. Ebola virus vaccines – reality or fiction? Expert Rev Vaccines. 2016;15:1421–30. doi:10.1080/14760584.2016.1178068. PMID:27078187.
- Lichty B, Power A, Stojdl D, Bell J. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–6. doi:10.1016/j.molmed.2004.03.003. PMID:15121047.
- Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci USA. 1995;92:4477–81. doi:10.1073/pnas.92.10.4477. PMID:7753828.
- Roberts A, Kretzschmar E, Perkins A, Forman J, Price R, Buonocore L, Kawaoka Y, Rose JK. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72:4704–11. PMID:9573234.
- Cooper D, Wright KJ, Calderon PC, Guo M, Nasar F, Johnson JE, et al. Attenuation of recombinant vesicular stomatitis virus-human immunodeficiency virus type 1 vaccine vectors by gene translocations and g gene truncation reduces neurovirulence and enhances immunogenicity in mice. J Virol. 2008;82:207–19. doi:10.1128/JVI.01515-07. PMID:17942549.
- Fang X, Zhang S, Sun X, Li J, Sun T. Evaluation of attenuated VSVs with mutated M or/and G proteins as vaccine vectors. Vaccine. 2012;30:1313–21. doi:10.1016/j.vaccine.2011.12.085. PMID:22222871.
- Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, Volchkov V, Klenk HD, Feldmann H, Ströher U. Properties of replication-competent Vesicular Stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78:5458–65. doi:10.1128/JVI.78.10.5458-5465.2004. PMID:15113924.
- Yamaoka S, Banadyga L, Bray M, Ebihara H. Small animal models for studying filovirus pathogenesis. In: Mühlberger E, Hensley LL, Towner JS, eds. Marburg- and Ebolaviruses: From ecosystems to molecules. Cham: Springer International Publishing, 2017:195–227.
- Shurtleff AC, Bavari S. Animal models for ebolavirus countermeasures discovery: what defines a useful model? Expert Opin Drug Discov. 2015;10:685–702. doi:10.1517/17460441.2015.1035252. PMID:26004783.
- Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–90. doi:10.1038/nm1258. PMID:15937495.
- Geisbert TW, Daddario-Dicaprio KM, Geisbert JB, Reed DS, Feldmann F, Grolla A, Ströher U, Fritz EA, Hensley LE, Jones SM, et al. Vesicular stomatitis virus-based vaccines protect nonhuman primates against aerosol challenge with Ebola and Marburg viruses. Vaccine. 2008;26:6894–900. doi:10.1016/j.vaccine.2008.09.082. PMID:18930776.
- Jones SM, Stroher U, Fernando L, Qiu X, Alimonti J, Melito P, Bray M, Klenk HD, Feldmann H. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J Infect Dis. 2007;196 Suppl 2:S404–12. doi:10.1086/520591. PMID:17940977.
- Qiu X, Fernando L, Alimonti JB, Melito PL, Feldmann F, Dick D, Ströher U, Feldmann H, Jones SM. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One. 2009;4:e5547. doi:10.1371/journal.pone.0005547. PMID:19440245.
- Wong G, Audet J, Fernando L, Fausther-Bovendo H, Alimonti JB, Kobinger GP, Qiu X. Immunization with vesicular stomatitis virus vaccine expressing the Ebola glycoprotein provides sustained long-term protection in rodents. Vaccine. 2014;32:5722–9. doi:10.1016/j.vaccine.2014.08.028. PMID:25173474.
- Geisbert TW, Daddario-Dicaprio KM, Lewis MG, Geisbert JB, Grolla A, Leung A, Paragas J, Matthias L, Smith MA, Jones SM, et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Pathog. 2008;4:e1000225. doi:10.1371/journal.ppat.1000225. PMID:19043556.
- Marzi A, Engelmann F, Feldmann F, Haberthur K, Shupert WL, Brining D, Scott DP, Geisbert TW, Kawaoka Y, Katze MG, et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc Natl Acad Sci U S A. 2013;110:1893–8. doi:10.1073/pnas.1209591110. PMID:23319647.
- Feldmann H, Jones SM, Daddario-DiCaprio KM, Geisbert JB, Stroher U, Grolla A, Bray M, Fritz EA, Fernando L, Feldmann F, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathogens. 2007;3:e2. doi:10.1371/journal.ppat.0030002. PMID:17238284.
- Tsuda Y, Safronetz D, Brown K, LaCasse R, Marzi A, Ebihara H, Feldmann H. Protective efficacy of a bivalent recombinant vesicular stomatitis virus vaccine in the Syrian hamster model of lethal Ebola virus infection. J Infect Dis. 2011;204 Suppl 3:S1090–7. doi:10.1093/infdis/jir379. PMID:21987746.
- Marzi A, Hanley PW, Haddock E, Martellaro C, Kobinger G, Feldmann H. Efficacy of vesicular stomatitis virus-ebola virus postexposure treatment in rhesus macaques infected with ebola virus makona. J Infect Dis. 2016;214:S360–S6. doi:10.1093/infdis/jiw218. PMID:27496978.
- Marzi A, Robertson S, Haddock E, Feldmann F, Hanley P, Scott D, Strong JE, Kobinger G, Best SM, Feldmann H. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science. 2015;349:739–42. doi:10.1126/science.aab3920. PMID:26249231.
- Musong M, Muyembe T, Kibasa. Outbreak of Ebola Viral Hemorrhagic Fever – Zaire, 1995. MMWR Suppl. 1995;44:381–2.
- Marzi A, Ebihara H, Callison J, Groseth A, Williams KJ, Geisbert TW, Feldmann H. Vesicular stomatitis virus-based Ebola vaccines with improved cross-protective efficacy. J Infect Dis. 2011;204 Suppl 3:S1066–74. doi:10.1093/infdis/jir348. PMID:21987743.
- Falzarano D, Feldmann F, Grolla A, Leung A, Ebihara H, Strong JE, Marzi A, Takada A, Jones S, Gren J, et al. Single immunization with a monovalent vesicular stomatitis virus-based vaccine protects nonhuman primates against heterologous challenge with Bundibugyo ebolavirus. J Infect Dis. 2011;204 Suppl 3:S1082–9. doi:10.1093/infdis/jir350. PMID:21987745.
- Mire CE, Geisbert JB, Marzi A, Agans KN, Feldmann H, Geisbert TW. Vesicular stomatitis virus-based vaccines protect nonhuman primates against Bundibugyo ebolavirus. PLoS Negl Trop Dis. 2013;7:e2600. doi:10.1371/journal.pntd.0002600. PMID:24367715.
- Geisbert TW, Geisbert JB, Leung A, Daddario-DiCaprio KM, Hensley LE, Grolla A, Feldmann H. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J Virol 2009;83:7296–304. doi:10.1128/JVI.00561-09. PMID:19386702.
- Mire CE, Geisbert JB, Versteeg KM, Mamaeva N, Agans KN, Geisbert TW, Connor JH. A single-vector, single-injection Trivalent Filovirus vaccine: Proof of concept study in outbred guinea pigs. J Infect Dis. 2015;212 Suppl 2:S384–8. doi:10.1093/infdis/jiv126. PMID:25957964.
- Geisbert TW, Jones S, Fritz EA, Shurtleff AC, Geisbert JB, Liebscher R, Grolla A, Ströher U, Fernando L, Daddario KM, et al. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2005;2:e183. doi:10.1371/journal.pmed.0020183. PMID:15971954.
- Safronetz D, Mire C, Rosenke K, Feldmann F, Haddock E, Geisbert T, Feldmann H. A recombinant vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa viruses. PLoS Negl Trop Dis. 2015;9:e0003736. doi:10.1371/journal.pntd.0003736. PMID:25884628.
- Marzi A, Feldmann F, Geisbert TW, Feldmann H, Safronetz D. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses. Emerg Infect Dis. 2015;21:305–7. doi:10.3201/eid2102.141649. PMID:25625358.
- Mire CE, Miller AD, Carville A, Westmoreland SV, Geisbert JB, Mansfield KG, Feldmann H, Hensley LE, Geisbert TW. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl Trop Dis. 2012;6:e1567. doi:10.1371/journal.pntd.0001567. PMID:22448291.
- de Wit E, Marzi A, Bushmaker T, Brining D, Scott D, Richt JA, Geisbert TW, Feldmann H. Safety of recombinant VSV-Ebola virus vaccine vector in pigs. Emerg Infect Dis. 2015;21:702–4. doi:10.3201/eid2104.142012. PMID:25811738.
- Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Hu Z, Muñoz P, Moon JE, Ruck RC, Bennett JW, et al. A recombinant vesicular stomatitis virus ebola vaccine. N Eng J Med. 2017;376:330–41. doi:10.1056/NEJMoa1414216..
- Khurana S, Fuentes S, Coyle EM, Ravichandran S, Davey RT, Jr, Beigel JH. Human antibody repertoire after VSV-Ebola vaccination identifies novel targets and virus-neutralizing IgM antibodies. Nat Med. 2016;22:1439–47. doi:10.1038/nm.4201. PMID:27798615.
- ElSherif MS, Brown C, MacKinnon-Cameron D, Li L, Racine T, Alimonti J, Rudge TL, Sabourin C, Silvera P, Hooper JW, et al. Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. CMAJ. 2017;189:E819–e27. doi:10.1503/cmaj.170074. PMID:28630358.
- Dahlke C, Kasonta R, Lunemann S, Krahling V, Zinser ME, Biedenkopf N, Fehling SK, Ly ML, Rechtien A, Stubbe HC, et al. Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization. EBioMedicine. 2017;19:107–18. doi:10.1016/j.ebiom.2017.03.045. PMID:28434944.
- Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, Yerly S, Dayer JA, Kraehling V, Kasonta R, et al. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe. N Eng J Med. 2016;374:1647–60. doi:10.1056/NEJMoa1502924..
- Huttner A, Combescure C, Grillet S, Haks MC, Quinten E, Modoux C, Agnandji ST, Brosnahan J, Dayer JA, Harandi AM, et al. A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa. Sci Transl Med. 2017;9:eaaj1701. doi:10.1126/scitranslmed.aaj1701. PMID:28404856.
- Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, Eickmann M, Finckh A, Goncalves AR, Hooper JW, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2015;15:1156–66. doi:10.1016/S1473-3099(15)00154-1. PMID:26248510.
- Rechtien A, Richert L, Lorenzo H, Martrus G, Hejblum B, Dahlke C, Kasonta R, Zinser M, Stubbe H, Matschl U, et al. Systems vaccinology identifies an early innate immune signature as a correlate of antibody responses to the ebola vaccine rVSV-ZEBOV. Cell Reports. 2017;20:2251–61. doi:10.1016/j.celrep.2017.08.023. PMID:28854372.
- Farooq F, Beck K, Paolino KM, Phillips R, Waters NC, Regules JA, Bergmann-Leitner ES. Circulating follicular T helper cells and cytokine profile in humans following vaccination with the rVSV-ZEBOV Ebola vaccine. Scientific Reports. 2016;6:27944. doi:10.1038/srep27944. PMID:27323685.
- Kennedy SB, Bolay F, Kieh M, Grandits G, Badio M, Ballou R, Eckes R, Feinberg M, Follmann D, Grund B, et al. Phase 2 Placebo-controlled trial of two vaccines to prevent ebola in Liberia. N Eng J Med. 2017;377:1438–47. doi:10.1056/NEJMoa1614067..
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, Carroll MW, Dean NE, Diatta I, Doumbia M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet (London, England). 2017;389:505–18. doi:10.1016/S0140-6736(16)32621-6. PMID:28017403.
- Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet (London, England). 2015;386:857–66. doi:10.1016/S0140-6736(15)61117-5. PMID:26248676.
- Widdowson M, Schrag S, Carter R, Carr W, Legardy-Williams J, Gibson L, Lisk DR, Jalloh MI, Bash-Taqi DA, Kargbo SA, et al. Implementing an Ebola Vaccine Study — Sierra Leone. MMWR Suppl. 2016;65:98–106. doi:10.15585/mmwr.su6503a14. PMID:27387395.
- Halperin SA, Arribas JR, Rupp R, Andrews CP, Chu L, Das R, Simon JK, Onorato MT, Liu K, Martin J, et al. Six-month safety data of recombinant vesicular stomatitis virus-zaire ebola virus envelope glycoprotein vaccine in a Phase 3 double-blind, placebo-controlled randomized study in healthy adults. J Infect Dis. 2017;215:1789–98. doi:10.1093/infdis/jix189. PMID:28549145.
- Agnandji ST, Fernandes JF, Bache EB, Obiang Mba RM, Brosnahan JS, Kabwende L, Pitzinger P, Staarink P, Massinga-Loembe M, Krähling V, et al. Safety and immunogenicity of rVSVDeltaG-ZEBOV-GP Ebola vaccine in adults and children in Lambarene, Gabon: A phase I randomised trial. PLoS Med. 2017;14:e1002402. doi:10.1371/journal.pmed.1002402. PMID:28985239.
- Gunther S, Feldmann H, Geisbert TW, Hensley LE, Rollin PE, Nichol ST, Ströher U, Artsob H, Peters CJ, Ksiazek TG, et al. Management of accidental exposure to Ebola virus in the biosafety level 4 laboratory, Hamburg, Germany. J Infect Dis. 2011;204 Suppl 3:S785–90. doi:10.1093/infdis/jir298. PMID:21987751.
- Wong KK, Davey RT, Jr, Hewlett AL, Kraft CS, Mehta AK, Mulligan MJ, Beck A, Dorman W, Kratochvil CJ, Lai L, et al. Use of Postexposure prophylaxis after occupational exposure to Zaire ebolavirus. Clin Infect Dis. 2016;63:376–9. doi:10.1093/cid/ciw256. PMID:27118786.
- DeBuysscher BL, Scott D, Marzi A, Prescott J, Feldmann H. Single-dose live-attenuated Nipah virus vaccines confer complete protection by eliciting antibodies directed against surface glycoproteins. Vaccine. 2014;32:2637–44. doi:10.1016/j.vaccine.2014.02.087. PMID:24631094.
- Reynolds P, Marzi A. Ebola and Marburg virus vaccines. Virus Genes. 2017;53:501–15. doi:10.1007/s11262-017-1455-x. PMID:28447193.
- Brown KS, Safronetz D, Marzi A, Ebihara H, Feldmann H. Vesicular stomatitis virus-based vaccine protects hamsters against lethal challenge with Andes virus. J Virol. 2011;85:12781–91. doi:10.1128/JVI.00794-11. PMID:21917979.
