ABSTRACT
Background: Community acquired pneumonia (CAP) is a major cause of morbidity and mortality worldwide, and is a leading cause of hospitalization in previously healthy individuals without predisposing risk factors or comorbidities. In this study we determined PCV13 serotype distribution in adults aged ≥50 years with radiographically confirmed CAP in Israel.
Methods: Subjects aged ≥50 years were enrolled from one of three hospitals (Emek Medical Center, Meir Medical Center and Sheba Medical Center) from March 2014 to July 2015. Information was collected on subject demographics, comorbidities, risk factors, and pneumococcal vaccine immunization status. Subjects presented with suspected CAP supported by radiographic evidence, and provided a urine sample and informed consent. Subjects without radiographic confirmation of CAP or who received PPSV23 within 30 days of study enrollment were excluded from the final analysis. Serotype distribution was performed using the urinary antigen detection (UAD) assay and/or microbiological culture.
Results: Overall, 498 subjects with radiographically confirmed CAP were enrolled in the study. Eighty subjects (16.1%) were positive for any S. pneumoniae serotype by ≥1 assay, and 38 (7.6%) were positive for PCV13 serotypes via the UAD. The overall 30-day mortality rate was 1.2%, though S. pneumoniae was not isolated from any case leading to death.
Conclusion: Despite six years of high pneumococcal immunization coverage in children in Israel, we have shown that 7.6% of CAP cases among adults in Israel remain related to PCV13 serotypes; and that the burden of PCV13 may be as high as 47% of observed pneumococcal CAP.
KEYWORDS:
Introduction
Community acquired pneumonia (CAP) is a major cause of morbidity and mortality worldwide,Citation1-4 and is a leading cause of hospitalization in previously healthy individuals without predisposing risk factors or comorbidities.Citation5 CAP can be caused by a variety of agents; however Streptococcus pneumoniae is the most commonly diagnosed pathogen.Citation6 Accordingly, an early study of CAP etiology in Israel showed that 42% of CAP cases among adults admitted to hospitals were due to S. pneumoniae.Citation7
In an effort to reduce the impact of pneumococcal disease, the 7-valent pneumococcal conjugate vaccine (PCV7) was first licensed in Israel in 2007.Citation8 The vaccine was then introduced into the Israeli National Immunization Plan (NIP) in 2009, to be administered at 2, 4 and 12 months of age, with a catch-up campaign in all children <2 years old.Citation8 Subsequently, in late 2010 the 13-valent pneumococcal conjugate vaccine (PCV13) replaced PCV7 in the Israeli NIP, without further catch-up.Citation8 As of June 2013, the proportion of 7–11 month old children who had received ≥2 doses of any PCV13 was 89%.Citation9 The PCV13 vaccine was approved for use in adults with high-risk conditions in Israel in 2013, but was not provided until 2016.Citation10 Current PCV vaccine coverage among adults in Israel is still low, while PPSV23 vaccine is recommended for adults ≥65 years old.
The use of PCV7/13 vaccines has resulted in a significant decline in the overall occurrence of both invasive and non-invasive pneumococcal disease in Israel. Decreases in vaccine-type invasive pneumococcal disease (IPD) have been seen amongst both children and adults.Citation10,Citation11 Vaccination with PCV7/13 has also had an impact on the occurrence of CAP in Israel. Greenberg et al. reported a decrease in annual CAP incidences (per 1,000 inhabitants) in children <5 years old from a mean of 13.8 ± 0.9 in the pre-PCV period to 11.2 ± 2.7 in the PCV7 period and 7.4 in the PCV13 period, representing reductions of 13% and 47%, respectively.Citation9 Less is currently known about the current incidence and serotype distribution of pneumococcal serotypes in adults with CAP in Israel.
Understanding the distribution of pneumococcal serotypes present in CAP is important for optimizing pneumococcal disease control in Israel and around the globe. In the past, the lack of a reliable and specific diagnostic assay greatly limited epidemiologic measurement of non-invasive pneumococcal diseases such as CAP. However, the recent development of a serotype specific UAD multiplex assay that allows for the identification of 13 pneumococcal serotypes (serotypes 1, 3, 4, 5, 6A/C, 6B, 7F/A, 9V/A, 14, 18 [A, B, C, F], 19A, 19F, and 23F) in the urine of subjects with CAP allows for a more thorough investigation of serotype distribution. Using this UAD assay we have investigated the proportion of radiographically-confirmed pneumonia caused by pneumococcal serotypes included in the 13-valent pneumococcal conjugate vaccine (PCV13) in adults 50 years and older at three sites in Israel.
Materials and methods
Objective
The study objective was to determine PCV13 serotype distribution in adults aged ≥50 years with radiographically confirmed CAP presenting to 3 hospitals in Israel using the UAD assay and/or microbiological culture.
Design and subjects
This study was approved by institutional review boards and/or independent ethics committees of Emek Medical Center (IRB#0141-13-EMC), Meir Medical Center (IRB#0261-13 MMC) and the Sheba Medical Center (IRB#0789-13). The study was conducted in compliance with the amended Declaration of Helsinki, the International Conference on Harmonisation, Good Clinical Practice Guidelines, and all local regulatory requirements. Written informed consent was obtained from all subjects before enrollment and performance of any study-related procedures.
Subjects aged ≥50 years were enrolled from one of three hospitals: Emek Medical Center, in Afula, Northern Israel, with 550 beds, Meir Medical Center, in Kfar Saba, Hasharon District, with 740 beds and Sheba Medical Center, the largest tertiary hospital in Israel, in Ramat-Gan, Central Israel, with 1400 Acute care beds, from March 2014 to July 2015. Information was collected on subject demographics, comorbidities, risk factors, and pneumococcal vaccine immunization status. Subjects were ≥50 years of age, presented to one of the three hospitals with suspected CAP supported by radiographic evidence (some cases were later designated as healthcare-associated pneumonia ([HCAP]), and provided a urine sample and informed consent. Subjects without radiographic confirmation of CAP or who received PPSV23 within 30 days of study enrollment were excluded from the final analysis.
Streptococcus pneumoniae detection
Each subject underwent three assessments for the detection of S. pneumoniae CAP: 1) microbiology cultures collected per standard of care (blood, pleural fluid, bronchiolar lavage, tracheal aspirate, and respiratory cultures); overall low sensitivity / high specificityCitation12; 2) BinaxNOW® assay (Alere North America, Orlando, FL, USA) for overall detection of S. pneumoniae in the urine (52% to 86% sensitivity, 83% to 97% specificity compared to blood cultureCitation13-17) and 3) the proprietary 13-valent multiplex UAD assay (Pfizer Research, Pearl River, NY, USA) for specific detection of 13 S. pneumoniae serotypes in the urine (97% sensitivity and 100% specificity compared to blood culture).Citation17 UAD results were not used diagnostically. All analyses were descriptive.
Results
Overall, 498 subjects with radiographically confirmed CAP were enrolled in the study (). The mean age of enrollees was 72.6 (median 73, range 50–101), and half of all subjects had previously received PPSV23. The most common at-risk conditions were diabetes mellitus (40.8%), coronary artery disease (33.3%) and chronic obstructive pulmonary disorder (32.1%) (). Most patients received antibiotic treatment (78.9%) before recruitment and 9.6% received prior immunosuppressive therapy. Six deaths were reported; 5 were due to pneumonia with no isolation of S. pneumoniae, and 1 was reported as not directly attributed to the infection. The overall mortality rate measured 30 days after enrollment was 1.2%.
Table 1. Demographics of patients with radiographically confirmed community-acquired pneumonia.
Among the 498 subjects with radiographically confirmed CAP, 80 (16.1%) were positive for any S. pneumoniae serotype by ≥1 assay (/). In total, 38 subjects (7.6%), 48 subjects (9.6%) and 17 subjects (3.7%) were positive for S. pneumoniae via UAD, BinaxNOW and culture, respectively. Some subjects were positive via multiple methods; 2 (0.4%) were positive via all three methods. Positive culture samples were derived from blood (n = 15), sputum (n = 3) and pleural fluid (n = 1).
Figure 1. Identification of S. pneumoniae by multiple diagnostic methods. UAD = urinary antigen detection assay. *n = number of subjects with nonmissing results from ≥1 diagnostic method. Results in this figure are mutually exclusive from group to group. Total number of subjects positive for S. pneumoniae (n = 80).
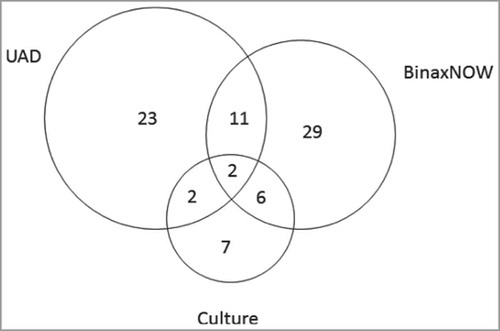
Table 2. Overall identification of S. pneumoniae by diagnostic method.
Of 498 subjects with radiographically confirmed CAP, 51 subjects (10.2%) were positive for an identifiable S. pneumoniae serotype via UAD or culture. Thirty eight subjects (7.6%) were positive for PCV13 serotypes, that is, 47.5% (38 of 80) of all S. pneumoniae isolates identified. Twelve subjects (2.4% of all patients and 15% of the S. pneumoniae isolates) were positive for PCV7-associated serotypes and 26 subjects (5.2%) were positive for 1 of the 6 additional serotypes contained in PCV13 (compared to PCV7). Thirteen subjects (2.6%) were positive for non-PCV13 serotypes, and 42 (8.4% of all patients and 52.5% of all S. pneumoniae isolates) were presumably non-PCV13, i.e. either identified as a non-PCV13 serotype by culture (13 isolates) or detected as S. pneumoniae by Binax but not UAD ( and ). Serotypes 23F (1.4%) and 6B (0.4%) were the most prevalent PCV7-associated serotypes detected, whereas serotype 3 was the most prevalent PCV13 serotype detected (2.4%). One subject had differing UAD and blood serotype results. For this subject, the UAD serotype was identified as 23F, while the blood serotype was identified as 10A. The distribution of PCV7- and PCV13-associated serotypes identified via UAD assay and culture stratified by subject characteristics is shown in .
Figure 2. S. pneumoniae serotype distribution among isolates from S pneumoniae–positive patients tested by UAD assay and culture. PCV7 = 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine; NVT = non–vaccine type; UAD = urinary antigen detection.
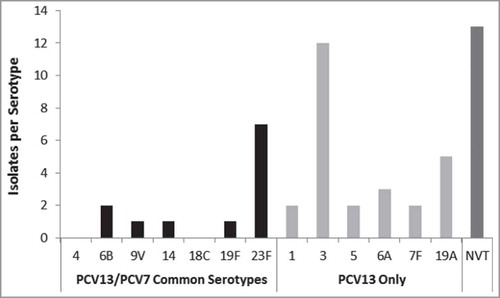
Table 3. PCV7 and PCV13 serotype distribution by patient demographics among isolates from patients with a positive S. pneumoniae isolate.
The agreement in findings (any agreement) between UAD and culture was 90.4% (95% CI 87.3-92.9%); between UAD and BinaxNOW was 88.0% (95% CI 84.8%-90.7%); and between culture and BinaxNOW was 89.8% (95% CI 86.6%-92.4%). Agreement in finding for PCV13 serotypes between UAD and culture was 92.8% (95% CI 90.1%-95.0%).
Discussion
While S. pneumoniae is known to be a common cause of CAP in all age groups, this study sought to determine the serotype distribution of S. pneumoniae in invasive and non-invasive radiographically confirmed CAP in adults 50 years of age or older utilizing standard of care cultures, BinaxNOW, and the UAD assay. In 498 subjects with radiographically confirmed CAP in Israel, we found that 16.1% were positive for any S. pneumoniae serotype by ≥1 assay and 7.6% were positive for PCV13 serotypes. Serotypes 23F (1.4%) and 6B (0.4%) were the most prevalent PCV7-associated serotypes detected, whereas serotype 3 was the most prevalent PCV13 serotype detected (2.4%).
The overall prevalence of S. pneumoniae in CAP in the current study was lower than results reported by Said et al in 2013 showing a proportion of CAP attributable to S. pneumoniae of 27%.Citation18 While these numbers are divergent, it's important to consider that studies of CAP etiology are constrained by the sensitivity and specificity of the diagnostic assays being used. As Said and colleagues performed a meta-analysis of many other studies using different methodologies, there is a clear potential for varying results. In addition, no studies from Israel were included in the analysis by Said et al. Recent literature from LeBlanc and colleagues from hospitalized adults in Canada illustrate this. Among 4,769 all-cause CAP cases, they found that a laboratory test alone identified 14.3% as CAP related to S. pneumoniae.Citation19 However, when a battery of four tests was performed (sputum culture, blood culture, a commercial pan-pneumococcal UAD, and a serotype-specific UAD), S. pneumoniae was identified in 23.2% of CAP cases.Citation19 An overall decrease in disease as a result of a herd effect from the vaccination of children cannot be discounted however.
The diagnostic criteria used to identify CAP are also of paramount importance when looking at etiology. Data from a study conducted in Poland illustrate this by showing that among 1,195 adults with CAP, S. pneumoniae was detected in 12.1% of subjects.Citation20 In a subset of 1,166 subjects with radiographically confirmed CAP, S. pneumoniae prevalence fell to 11.2% of subjects.Citation20 Another study by Sherwin and colleagues in the United States demonstrated that 11.0% of radiographically confirmed CAP cases in adults 50 and older were related to PCV13 serotypes (note that PPSV recipients were not excluded from the analysis population).Citation21 This is in contrast to our current observed prevalence of 7.6% PCV13 serotypes in adults with radiographically confirmed CAP.
Our study has several limitations that may, in part, help explain observed differences in prevalence compared to published literature. While hospital and ventilator acquired pneumonia were not to be included in the current study, the diagnosis of CAP is not without difficulty, and in addition, less severe cases of CAP not requiring hospitalization would have been missed. Antibiotic treatment prior to recruitment into the current study may also have had an effect on our results, potentially altering urinary antigen detection. We also did not check for nasopharyngeal carriage in this study; however it has been suggested that a low percentage of adult nasopharyngeal carriers will have positive urinary pneumococcal antigen detection in the absence of pneumococcal disease.Citation22 It is not known to what extent these data can be directly extrapolated to other countries and/or populations. Additionally, the contribution of S. pneumoniae in the etiology of CAP varies according to the published literature and may reflect the inherent variability in study design and the sensitivity and specificity of the laboratory methods for detecting and typing pneumococci in both bacteremic and nonbacteremic CAP. In the current study three methods were employed: 1) microbiology cultures (blood, pleural fluid, bronchiolar lavage, tracheal aspirate, and respiratory culture and serotyping via Quellung reaction); 2) BinaxNOW S. pneumoniae test and 3) Pfizer's urine antigen detection (UAD) assay. The lack of a gold standard test to determine pneumococcal etiology in CAP makes assessment of the performance of the diagnostics challenging.Citation23,Citation24 Although highly specific, the sensitivity of microbiology cultures (blood, pleural fluid, bronchiolar lavage, tracheal aspirate, and respiratory cultures) and subsequent isolation of S. pneumonia is low.Citation12,Citation18,Citation25 Rates of positive blood cultures for adults hospitalized with pneumonia has been reported as low as 3%-8%Citation26-28 and > 50% from those with pneumococcal meningitis.Citation29 The clinically approved method for confirming and serotyping S. pneumoniae IPD cases has been the capsular swelling/Quellung reaction.Citation30,Citation31 This technique has relatively low sensitivity, as it requires viable bacteria in a blood sample.Citation32,Citation33 Additional potential drawbacks of this method are its reliance on technical experience and the subjective interpretation of assay results.Citation31 The other two diagnostics used in this study are urine antigen detection assays. The BinaxNOW S. pneumoniae diagnostic tests for the presence of pneumococcal C-polysaccharide (C-PS) antigens in the urine (and in the cerebrospinal fluid of patients with meningitis) using an immunochromatographic membrane test kit. The sensitivity of this assay is documented by the manufacturer as 86% for bacteremic pneumococcal pneumonia.Citation16 However, the sensitivity of this method has been called into question by users in the field.Citation34,Citation35 Lower sensitivity and specificity (between 52% to 82% and 83% to 97%, respectively) have been reported in studies involving adult populations with CAP.Citation13-15,Citation17 While the BinaxNOW® assay detects C-PS antigen, which is present in all S. pneumoniae strains; it does not distinguish among different pneumococcal serotypes. To this end a proprietary Luminex platform-based multiplex UAD assay that allows for the identification of 13 pneumococcal serotypes (serotypes 1, 3, 4, 5, 6A/C, 6B, 7F/A, 9V/A, 14, 18 [A, B, C, F], 19A, 19F, and 23F) in the urine of subjects with CAP was used for a more thorough investigation of serotype distribution.Citation17 The overall validated sensitivity and specificity of this assay is reported to be 97.1% and 100%, respectively.Citation17 While any of these individual assays could have underestimated the burden of CAP in this study, it's likely that the combination of these assays provides a better assessment of the role of S. pneumoniae in both bacteremic and non-bacteremic CAP.
Despite six years of high pneumococcal immunization coverage in children in Israel, we have shown that 7.6% of CAP cases among adults in Israel remain related to PCV13 serotypes; and that the burden of PCV13 may be as high as 47% of observed pneumococcal CAP.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Prior presentation
These data have not been previously presented.
Acknowledgments
We thank the subjects who participated in this study, study investigators, nurses, coordinators, the clinical testing laboratory staff, and the clinical research associates and scientists at Pfizer. All authors had full access to all study data. This study was sponsored by Pfizer Inc. Medical writing support was provided by Scott Vuocolo PhD, (Pfizer Collegeville, PA).
Additional information
Funding
References
- Angoulvant F, Levy C, Grimprel E, Varon E, Lorrot M, Biscardi S, Minodier P, Dommergues MA, Hees L, Gillet Y, et al. Early impact of 13-Valent Pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clin Infect Dis. 2014;58(7):918–24. doi:10.1093/cid/ciu006. PMID:24532543.
- Chan CHS, Cohen M, Pang J. A Prospective Study of Community-Acquired Pneumonia in Hong Kong. Chest. 1992;101(2):442–6. doi:10.1378/chest.101.2.442. PMID:1735270.
- File TM, Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgraduate Med. 2010;122(2):130–41. doi:10.3810/pgm.2010.03.2130.
- Quan TP, Fawcett NJ, Wrightson JM, Finney J, Wyllie D, Jeffery K, Jones N, Shine B, Clarke L, Crook D, et al. Increasing burden of community-acquired pneumonia leading to hospitalisation, 1998–2014. Thorax. 2016;71(6):535–42. doi:10.1136/thoraxjnl-2015-207688. PMID:26888780.
- Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68(11):1057–65. doi:10.1136/thoraxjnl-2013-204282. PMID:24130229.
- File TM, Jr. Streptococcus pneumoniae and community-acquired pneumonia: a cause for concern. Am J Med. 2004;117 Suppl 3A:39S–50S. PMID:15360096.
- Lieberman D, Schlaeffer F, Boldur I, Lieberman D, Horowitz S, Friedman MG, Leiononen M, Horovitz O, Manor E, Porath A. Multiple pathogens in adult patients admitted with community-acquired pneumonia: a one year prospective study of 346 consecutive patients. Thorax. 1996;51(2):179–84. doi:10.1136/thx.51.2.179. PMID:8711652.
- Ben-Shimol S, Greenberg D, Givon-Lavi N, Elias N, Glikman D, Rubinstein U, Dagan R, Israeli Bacteremia and Meningitis Active Surveillance Group. Rapid reduction in invasive pneumococcal disease after introduction of PCV7 into the National Immunization Plan in Israel. Vaccine. 2012;30(46):6600–7. doi:10.1016/j.vaccine.2012.08.012. PMID:22939907.
- Greenberg D, Givon-Lavi N, Ben-Shimol S, Ziv JB, Dagan R. Impact of PCV7/PCV13 introduction on community-acquired alveolar pneumonia in children <5 years. Vaccine. 2015;33(36):4623–9. doi:10.1016/j.vaccine.2015.06.062. PMID:26116251.
- Regev-Yochay G, Katzir M, Strahilevitz J, Rahav G, Finn T, Miron D, Maor Y, Chazan B, Schindler Y, Dagan R, et al. The herd effects of infant PCV7/PCV13 sequential implementation on adult invasive pneumococcal disease, six years post implementation; a nationwide study in Israel. Vaccine. 2017;35(18):2449–56. doi:10.1016/j.vaccine.2017.03.031. PMID:28342668.
- Ben-Shimol S, Givon-Lavi N, Grisaru-Soen G, Megged O, Greenberg D, Dagan R. Comparative incidence dynamics and serotypes of meningitis, bacteremic pneumonia and other-IPD in young children in the PCV era: Insights from Israeli surveillance studies. Vaccine. 2017;(17):30707–7. doi:10.1016/j.vaccine.2017.05.059.
- Saha S, Darmstadt G, Naheed A, Arifeen S, Islam M, Fatima K, Breiman R, Sack D, Hamer D. Improving the sensitivity of blood culture for Streptococcus pneumoniae. J Trop Pediatr. 2011;57(3):192–6. doi:10.1093/tropej/fmq070. PMID:20736384.
- Stralin K, Kaltoft MS, Konradsen HB, Olcen P, Holmberg H. Comparison of two urinary antigen tests for establishment of pneumococcal etiology of adult community-acquired pneumonia. J Clin Microbiol. 2004;42(8):3620–5. doi:10.1128/JCM.42.8.3620-3625.2004. PMID:15297507.
- Murdoch DR, Laing RT, Mills GD, Karalus NC, Town GI, Mirrett S, Reller LB. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol. 2001;39(10):3495–8. doi:10.1128/JCM.39.10.3495-3498.2001. PMID:11574562.
- Ishida T, Hashimoto T, Arita M, Tojo Y, Tachibana H, Jinnai M. A 3-year prospective study of a urinary antigen-detection test for Streptococcus pneumoniae in community-acquired pneumonia: utility and clinical impact on the reported etiology. J Infect Chemother. 2004;10(6):359–63. doi:10.1007/s10156-004-0351-1. PMID:15614462.
- BinaxNow® [package insert]. Scarborough, ME: Alere Scarborough, Inc.; 2012.
- Pride MW, Huijts SM, Wu K, Souza V, Passador S, Tinder C, Song E, Elfassy A, McNeil L, Menton R, et al. Validation of an immunodiagnostic assay for detection of 13 Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Vaccine Immunol. 2012;19(8):1131–41. doi:10.1128/CVI.00064-12. PMID:22675155.
- Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O'Brien KL, AGEDD Adult Pneumococcal Burden Study Team, Andreo F, Beovic B, Blanco S, Boersma WG, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PloS One. 2013;8(4):e60273. doi:10.1371/journal.pone.0060273. PMID:23565216.
- LeBlanc JJ, ElSherif M, Ye L, MacKinnon-Cameron D, Li L, Ambrose A, Hatchette TF, Lang AL, Gillis H, Martin I, et al. Burden of vaccine-preventable pneumococcal disease in hospitalized adults: A Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) network study. Vaccine. 2017;35(29):3647–54. doi:10.1016/j.vaccine.2017.05.049. PMID:28554501.
- Harat R, Alexander R, Gray S, Gutterman EM, Pluta J, Pride M, Shite S, Fijolek J, Kozub J. Prospective, population-based surveillance of the burden of Streptococcus pneumoniae in community-acquired pneumonia in older adults, Chrzanow County, Poland, 2010 to 2012. Pneumonol Alergol Pol. 2016;84(2):95–103. doi:10.5603/PiAP.2016.0007. PMID:27238167.
- Sherwin RL, Gray S, Alexander R, McGovern PC, Graepel J, Pride MW, Purdy J, Paradiso P, File TM, Jr. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged >/ = 50 years with community-acquired pneumonia. J Infect Dis. 2013;208(11):1813–20. doi:10.1093/infdis/jit506. PMID:24092845.
- Palmu AA, Kaijalainen T, Saukkoriipi A, Leinonen M, Kilpi TM. Nasopharyngeal carriage of Streptococcus pneumoniae and pneumococcal urine antigen test in healthy elderly subjects. Scandinavian J Infect Dis. 2012;44(6):433–8. doi:10.3109/00365548.2011.652162.
- Wunderink RG, Self WH, Anderson EJ, Balk R, Fakhran S, Courtney DM, Qi C, Williams DJ, Zhu Y, Whitney CG, et al. Pneumococcal Community-Acquired Pneumonia Detected by Serotype-Specific Urinary Antigen Detection Assays. Clin Infect Dis. 2018;66(10):1504–10. doi:10.1093/cid/cix1066. PMID:29342250.
- Klugman KP, Madhi SA, Albrich WC. Novel Approaches to the Identification of Streptococcus pneumoniae as the Cause of Community-Acquired Pneumonia. Clin Infect Dis. 2008;47(Supplement_3):S202–6. doi:10.1086/591405. PMID:18986290.
- Musher DM, Alexandraki I, Graviss EA, Yanbeiy N, Eid A, Inderias LA, Phan HM, Solomon E. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine (Baltimore). 2000;79(4):210–21. doi:10.1097/00005792-200007000-00002. PMID:10941350.
- Werno AM, Murdoch DR. Medical microbiology: laboratory diagnosis of invasive pneumococcal disease. Clin Infect Dis. 2008;46(6):926–32. doi:10.1086/528798. PMID:18260752.
- Ruiz M, Ewig S, Marcos MA, Martinez JA, Arancibia F, Mensa J, Torres A. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Critical Care Med. 1999;160(2):397–405. doi:10.1164/ajrccm.160.2.9808045.
- Bandettini R, Melioli G. Laboratory diagnosis of Streptococcus pneumoniae infections: past and future. J Prev Med Hyg. 2012;53(2):85–88. PMID:23240165.
- Kirkpatrick B, Reeves DS, MacGowan AP. A review of the clinical presentation, laboratory features, antimicrobial therapy and outcome of 77 episodes of pneumococcal meningitis occurring in children and adults. J Infect. 1994;29(2):171–82. doi:10.1016/S0163-4453(94)90698-X. PMID:7806880.
- O'Brien KL, Nohynek H, World Health Organization Pneumococcal Vaccine Trials Carriage Working G. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatric Infect Dis J. 2003;22(2):e1–11. doi:10.1097/01.inf.0000049347.42983.77.
- Austrian R. The quellung reaction, a neglected microbiologic technique. Mt Sinai J Med. 1976;43(6):699–709. PMID:13297.
- Musher DM. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin Infect Dis. 1992;14(4):801–7. doi:10.1093/clinids/14.4.801. PMID:1576274.
- Arai S, Konda T, Wad A, Matsunaga Y, Okabe N, Watanabe H, Inouye S. Use of antiserum-coated latex particles for serotyping Streptococcus pneumoniae. Microbiol Immunol. 2001;45(2):159–62. doi:10.1111/j.1348-0421.2001.tb01284.x. PMID:11293482.
- Smith MD, Derrington P, Evans R, Creek M, Morris R, Dance DA, Cartwright K. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41(7):2810–3. doi:10.1128/JCM.41.7.2810-2813.2003. PMID:12843005.
- Leeming JP, Cartwright K, Morris R, Martin SA, Smith MD, South-West Pneumococcus Study G. Diagnosis of invasive pneumococcal infection by serotype-specific urinary antigen detection. J Clin Microbiol. 2005;43(10):4972–6. doi:10.1128/JCM.43.10.4972-4976.2005. PMID:16207950.
