?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Objective To delineate seroepidemiology of VZV in children aged 1–14 years in Hangzhou, to evaluate immunological response of VarV via 2 dose regimen immunization of VarV, for improving immunization strategy of VarV. Methods From 2014–2016, a multi-stage stratified random sampling method was employed to select participants included via physical examination for children in the Community Health Centre in Hangzhou. Results were compared among 11 various age groups: 1-,2-,3-,4-,5-,6-,7-,8-,9-,10-,11–14 years. Demographic data and vaccination history of all subjects derived from Zhejiang Information System for Immunization Program. Then, the second dose of the VarV was conducted on children aged 4–6 years who had immunization history of one dose of VarV. ELISA was used to detect VZV IgG in serum samples. Results 895 subjects with available information were included. The rate of VZV IgG seropositivity was 65.59% and the geometric mean concentration (GMC) for VZV IgG was 5.14 ± 1.89 mIU/ml. The GMC in urban subjects were higher than rural ones. Both the rate of VZV IgG seropositivity and the GMC in children aged 4–6 years groups were statistically lower than participants younger than 4 years and aged 7–14 years (1-,2-,3-,7-,8-,9-,10-,11–14 years). 627 subjects had immunization history of VarV. Both the rate of VZV IgG seropositivity and the GMC in subjects had immunization history of VarV was higher than who had no immunization history.90 subjects were included after the 2nd dose immunization of VarV. Both the rate of VZV IgG seropositivity and the GMC were significantly increased after the immunization of the 2nd dose of VarV. Conclusions The GMC for VZV IgG in children aged 4–6 years were lower than participants groups (1-,2-,3-,7-,8-,9-,10-,11–14 years).2 doses regimen immunization of VarV are effective for increasing both the rate of VZV IgG seropositivity and the GMC in these subjects.
Introduction
Varicella (also known as chickenpox) is a worldwide acute respiratory infectious disease transmitted through respiratory tract (such as inhalation of aerosols from vesicular fluid of skin lesions) and also by daily direct contact mainly. Varicella is highly contagious disease, the highest incidence is among children in kindergartens and primary schools. At present, the disease burden caused by varicella in China is mainly manifested in the incidence of varicella and the cost of direct medical services (including outpatient treatment and hospitalization fees) and other indirect cost (including the escort fees, the cost of personnel, financial and material resources during the disposition of the outbreak). According to a retrospective sampling survey on the incidence of varicella among children conducted by Chinese Center For Disease Control And Prevention, there were 4,469,900 cases of varicella among children younger than 14 years in 2007. If medical cost of every varicella outpatient case is 77.86 USD in 2007, the outpatient cost of these three provinces (Shandong, Gansu and Hunan) was 0.36 billion USD. The average hospitalization cost of every varicella case was 634.91 USD and the hospitalization rate at 0.54%, then the hospitalization economic burden was 16.24 million USD.Citation1,Citation2 And varicella is also a special risk for immunocompromised patients, and pregnant women are at risk of life-threatening maternal pneumonia or congenital diseases of the newborn. In addition, once the varicella leads to the outbreaks, it will need serious social and economic burden.
Vaccination is the most effective and reliable method to prevent varicella. In March 1995, the vaccine was licensed for use in the United States.Citation3 With the rise in vaccination rates of VarV, the incidence, mortality and health care costs associated with varicella declined dramatically.Citation4 In China, routine immunization of children was recommended in 1998, with single dose of VarV vaccinated at 12 months old. Surveys found that the number of immunized school-age children in Beijing and Shanghai has exceeded 60% and 70%. But since 2003, the incidence of varicella has risen and there have been outbreaks in schools in the vaccinated population. In addition, immunity induced by the vaccine will decrease over time, which also can increase the risk of a breakthrough varicella.Citation5 It is suggested that the vaccination strategy of single dose VarV could not provide enough immunity to prevent the outbreaks of varicella.Citation6-7
In order to prevent varicella transmission and outbreak, 2 doses of VarV immunization are preferred.Citation8 The Advisory Committee on Immunization Practices (ACIP) in United States revised the VarV vaccination guidelines in 2006. Then, a routine 2-dose schedule (at ages 12–15 months and 4–6 years) was recommended and has been in place since 2006.Citation6 Meanwhile, In Germany and Greece, 2 doses of the VarV have been included in the national immunization program (NIP).Citation9 At present, the vaccine-schedule of VarV of multiple areas in China is still more than 12 months old and single dose procedure, but the Beijing CDC has introduced the routine 2 doses of inoculation procedure in 2012. In addition, the vaccination strategy of 2 doses of VarV were also recommended in Heilongjiang, Shandong, and Fujian province, regional coverage from the northern part of China to the southern part. In Hangzhou City, 2 doses of the VarV have been recommended since 2014.
The objectives of this present study were to delineate seroepidemiology of VZV in children aged 1–14 in Hangzhou, China and to evaluate immunological response of VarV via 2 dose regimen immunization, for improving immunization strategy of VarV.
Results
Population seroepidemiology of VZV IgG
Socio-demographic information and Vaccination
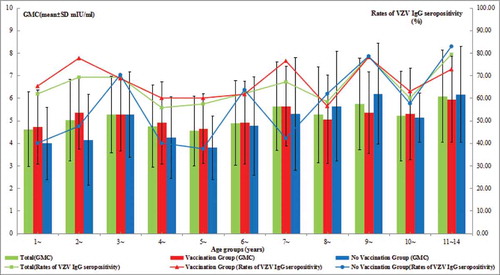
895 subjects with available information were included in the present study. There were 505 male and 390 female, average age was 5.35 ± 3.73 years. 627(70.06%) person have immunization history of one dose of VarV and average inoculation age was 1.36 ± 0.80 years. The vaccination rate of VarV was different in various regions (χ2 = 27.688, P < 0.001), it's 61.31% in urban while 77.48% in rural. The vaccination rate of VarV was 68.91% in male and 71.54% in female, there was no significant difference between the gender. The vaccination rate of VarV among 11 different age groups were statistically significance (χ2 = 98.478, Pfor trend < 0.001), which was declined with age older ().
Table 1. Seroepidemiology of VZV IgG in children among various groups via univariate analysis.
Seroepidemiology of VZV IgG in children among various groups via univariate analysis
The rate of VZV IgG seropositivity was 65.59% and the GMC for VZV IgG was 5.14 ± 1.89 mIU/ml. In univariate analysis, overall, significantly higher rates of VZV IgG seropositivity and the GMC for VZV IgG were found in female compared with men, urban compared with rural, subjects with one dose of VarV compared with who without inoculation of VarV, participants age groups (1-,2-,3-,7-,8-,9-,10-,11–14 years) compared with aged 4–6 years groups () (all P-values <0.05) ().
627 subjects had immunization history of 1-dose of VarV. The rate of VZV IgG seropositivity of them was 67.62% and the GMC for VZV IgG was 5.13 ± 1.75 mIU/ml, while the rate of VZV IgG seropositivity was 60.82% and the GMC for VZV IgG was 5.17 ± 2.18 mIU/ml for the subjects without inoculation of VarV. For subjects wirh 1-dose of VarV, significantly higher rates of VZV IgG seropositivity and GMC were found in female compared with men, urban compared with rural(all P-values < 0.05). For subjects with 1-dose of VarV, the GMC for VZV IgG of children aged one year and aged 4–6 years were lower than other participants age groups (1-,2-,3-,7-,8-,9-,10-,11–14 years). In addition, For subjects without inoculation of VarV, both of the rate of VZV IgG seropositivity and the GMC for VZV IgG of children younger than 3 years and aged 4–6 years were lower than other age groups (3-,7-,8-,9-,10-,11–14 years) (all P-values <0.05) ().
Associations of seroepidemiology of VZV IgG and related variables via multivariate analysis
In multivariate analysis, overall, significantly higher rates of VZV IgG seropositivity and GMC were found in female (OR = 1.587, 95% CI: 1.166–2.159, P = 0.003; OR = 1.384, 95% CI: 1.083–1.766, P = 0.009) compared with male, subjects had one dose of VarV compared with who without inoculation of VarV (OR = 4.075, 95% CI: 2.108–7.877, P<0.001; OR = 2.593, 95% CI: 1.473–4.563, P = 0.001) (), Moreover, both the rate of VZV IgG seropositivity (OR = 1.119, 95% CI: 1.049–1.193, P = 0.001) and the GMC for VZV IgG (OR = 1.170, 95% CI: 1.106–1.239, P<0.001) were also significantly influenced by age. With vaccination age older and years after vaccination longer, both the rate of VZV IgG seropositivity and the GMC for VZV IgG were declining significantly (all P values <0.05) (). In short, both the rate of VZV IgG seropositivity and the GMC for VZV IgG were significantly influenced by gender, age and the immunization history of VarV.
Table 2. Association of seroepidemiology of VZV IgG and related variables via multivariate analysis.
Immunological response of VarV via 2 dose regimen immunization
Basic information
In all, 90 subjects aged 4–6 years were included after the 2nd dose immunization of VarV in the current study. There 30 subjects in every age group, and among them, there were 52 male and 38 female. The rate of VZV IgG seropositivity was 50% before the 2nd dose immunization of VarV, and 98.89% after the 2nd dose immunization. The GMC for VZV IgG before the 2nd dose immunization of VarV was 4.44 ± 1.61 mIU/ml, while 6.58 ± 1.14 mIU/ml after the 2nd dose immunization of VarV ().
Immunological response of VarV via 2 dose regimen immunization
Both the rate of VZV IgG seropositivity and the GMC for VZV IgG were significantly higher after the immunization of the second dose of VarV (all P-values <0.001). Before vaccination, females had a higher VZV seropositivity rate (46.15% vs. 55.26%) and after 2nd dose immunization, it goes up to 98.08% vs.100% respectively. (). The GMC for VZV IgG was increased from 4.36 ± 1.54 mIU/ml to 6.62 ± 1.21 mIU/ml after the 2nd dose immunization of VarV. Both the difference of the rate of VZV IgG seropositivity and the GMC for VZV IgG between male and female were significantly (all P-values <0.001). In view of the rate of VZV IgG seropositivity and the GMC for VZV IgG among three age groups, the difference between before and after the 2nd dose immunization of VarV were significantly (all P-values <0.001). After the 2nd dose immunization of VarV, the rate of VZV IgG seropositivity and the GMC for VZV IgG in aged 4–6 years groups were all increasing significantly (all P-values <0.001). When the interval years between 2 doses of VarV was shorter than 6 years, all of the rate of VZV IgG seropositivity and the GMC for VZV IgG were higher significantly after the 2nd dose immunization of VarV (all P-values <0.001).
Table 3. The rates of VZV IgG seropositivity and the GMC for VZV IgG after the 2nd dose immunization of VarV among various groups.
Discussion
At present, the disease burden caused by varicella in China is mainly as much as other vaccine preventable diseases which have been incorporated into national immunization programme. In China, studies shows that the sampling incidence of varicella among children who were younger than 14 years was account for 95% of all cases in 2007.Citation1 It is important to investigate the seroepidemiology of VZV in Hangzhou City, for the outbreak of varicella was increasing. In the present study, the rate of VZV IgG seropositivity in Hangzhou was 65.59%, which was higher than the results of Shandong and other areas.Citation10 Both the rate of VZV IgG seropositivity and the GMC for VZV IgG were significantly influenced by age and regional distribution. It may be related to the inequalities of immunization coverage of VarV, the incidence of varicella, and different sample survey objects, etc. Varicella, with highly contagious, especially prevalent in children in kindergartens and primary schools. Parents of children voluntarily select and pay for the vaccine for their children since VarV is a private vaccine in China.Citation11 The inequalities of immunization coverage of VarV due to socioeconomic differences also exist in other countries.Citation12 This study also found that the rate of VZV IgG seropositivity and the GMC for VZV IgG were significantly influenced by gender, with which in female were both higher significantly than male subjects. It suggested that it may be meaningful to analyze the difference between the gender, such as the different incidence of varicella between male and female.
VarV vaccination is the most effective and reliable method to prevent varicella. Several countries have implemented routine childhood vaccination. Studies indicate that this intervention strategy may provide economic benefits for both the individual and the society. With the rise in vaccination rate of VarV, the incidence, mortality and health care costs associated with varicella declined dramatically[4]. In the present study, there was 70.06% subjects have immunization history of VarV. In spite of the vaccination rate of VarV in Hangzhou was not higher as other vaccine preventable diseases which have been incorporated into national immunization programme, the rate of VZV IgG seropositivity of subjects has immunization history of VarV (67.62%) was significantly higher than who were no immunization history (60.82%). Thus, it is important to improve the vaccination rate of one dose of VarV. The rate of VZV IgG seropositivity and the GMC for VZV IgG in children aged 4–6 years were lower than participants younger than 4 years and aged 7–14 years (1-,2-,3-,7-,8-,9-,10-,11–14 years). It may be related to the varies of the incidence of varicella, the vaccination rate of VarV, and different sample survey objects, etc. Children entered into kindergartens at 3 years old in Hangzhou. Changes in the environment and the aggregation of children may increase the risk of varicella. Study conducted by Xu Mingang et al. shows that the antibody to vaccination would decrease in the 3–5 years after vaccination, which was consistent with the results of this study.Citation13 A case-control study from 1997 to 2003 showed that the protection of 1 dose of VarV was 97% in the first year and 86% in the second year. The protection of the vaccine from the second year to the eighth year after inoculation remained stable at 81–86%. Studies show that the routine single dose of VarV vaccination could not provide the population enough immunity to prevent the outbreaks of varicella.Citation14-15 Thus, just as many other vaccines in the childhood immunization schedule, high 1-dose VarV vaccination coverage is effective in reducing varicella incidence, but not sufficient to prevent outbreak.
Based on the international general immunization strategy and combined epidemiological and laboratory data, 2 doses of the VarV schedule have been carried out in this study: In 12–18 months for first dose, 4–6 years for second dose. Results shows that positive conversion and 4 times growth of antibody after the 2nd dose of VarV was 98.89%, it demonstrated that 2 doses of VarV would increase the level of VZV antibody titer, which was consisted with other studies founded.Citation16-17 The study showed that after 2 doses of VarV, the positive rate of the antibody in healthy children increased to more than 99%, and the level of antibody titer produced higher, which reduced the risk of breakthrough varicella by 3.3 times.Citation18 Varicella incidence, hospitalizations, and outbreaks declined substantially during the first 5 years of the 2-dose varicella vaccination program.Citation19 According to a randomized controlled trial study, followed up for 10 years, the cumulative incidence in single dose of vaccine group was 7.3%, and vaccination 2 doses of VarV group is 2.4%.Citation20 Study on epidemiology of varicella during the 2-dose VarV Program in the United States shows that between the period 2005–2006 (before the 2-dose recommendation) and 2013–2014, overall varicella incidence declined 84.6%, with the largest declines reported in children aged 5–9 years (89.3%) and 10–14 years (84.8%).Citation21 The routine 2-dose varicella vaccination program appears to have significantly reduced the number, size and duration of varicella outbreaks in the USCitation22 A 2nd dose may deserve additional consideration to control varicella outbreak and for development of an adequate and persisting antibody response.Citation23
In conclusion, in order to prevent varicella transmission and outbreaks, 2 doses of secondary VarV immunization are preferred. Analysis shows that immunological response was better if the start age of 2nd dose immunization of VarV was at 4 years and vaccination as soon as possible.
Analysis of the limitations of this study: Firstly, results would be meaningful if it was analyzed combination of the incidence of varicella among children who were younger than 14 year in Hangzhou, which would be studied in future. Secondly, the sample size can be expanded in further studies, especially for the study on intervals between 2 doses of VarV, the sample size can be expanded in subsequent studies to further improve the inoculation procedure.
Materials and methods
Population and data collection
Population seroepidemiology of VZV IgG
The present study was performed in Hangzhou City in 2014–2016, which is the capital of the Zhejiang province and located in on the eastern coastline of China. The seroepidemiology of VZV IgG were compared among 11 various age groups: 1-,2-,3-,4-,5-,6-,7-,8-,9-,10-,11–14 years. A multi-stage stratified random sampling method was employed to select the participant. A sample size of at least 660 subjects was included for cross-sectional investigation based on the formula N = 400 [(1-P)/P] (P = 40%,P: the rates of VZV IgG seropositivity in Children <14 years of age group). First, 2 urban and 2 rural districts were randomly selected. Second, 15 subjects who were included via physical examination for children in the Community Health Centre at least per age group in each district were randomly selected. Basic socio-demographic information and vaccination history of all subjects derived from China Information System for Diseases Control and Prevention and Zhejiang Information System for Immunization Program respectively. Meanwhile, blood samples were collected and serums were isolated for antibody detection. In all, 895 subjects with available information were included in the present study.
Immunological response of 2 dose regimen immunization of VarV
According to the results of population Seroepidemiology of VZV IgG and epidemic characteristics of varicella, we conducted 2 dose regimen immunization of VarV on children who were in accordance with the following two conditions: have implemented based immune of VarV, and there is no history of varicella disease. A child was considered to have a missing vaccination status if no records were available to review and was considered vaccinated based on either affirmative historical documentation or data in the information system. 2 doses of the VarV schedule have been carried out in this study: in 12–18 months for first dose, 4–6 years for second dose. We included subjects aged from 4–6 years for three groups:,4-,5-,6-years. The vaccine used in the current study is a live attenuated VarV. There is no limit to vaccine manufacturers, dose was according to the vaccine instruction. A multi-stage stratified random sampling method was employed in the present study to select the participant. A sample size of at least 78 subjects was included for field-test with nor-continuous variable sample size calculation method based on the formula (p1 = 65%; p2 = 90%;
= (p1 + p2)/2; Zα = 1.96, Zβ = 1.28). The basic data of all subjects were collected. Blood samples were collected before and after the 2nd dose immunization, and the serum antibody levels were determined respectively. In all, 90 subjects with available information were included in the present study. All of the participants were given a brief oral description of the aims of the present study, and verbal consent was obtained from the participants. This program was approved by the ethics committee of the Hangzhou CDC.
Experimental approach and results calculation
Enzyme Linked Immunosorbent Assay (ELISA) was used to detect VZV IgG antibody quantitatively and the kit SERION ELISA classic Varicella-Zoster Virus IgG was distributed by Virion-serion Biological Technology co., LTD, Germany. OD-values evaluated by software SERION and results were calculated according to the mathematical formulas provided by the kit instruction. OD-values>high cutoff value was positive, and between low-high cutoff value was the critical value (i.e., gray area), <low cutoff value was negative.VZV IgG antibody positive means past VZV infection, successful vaccination or immunoglobulin recipient. Results of the gray area were re-examined, if check results remain in the gray zone, label as gray area which should be treated as a negative response. The serum antibody levels before and after the 2nd dose immunization were determined respectively. Positive conversion rate of antibody and 4 times growth rate of IgG antibody were used to evaluate the immunological effects of 2 dose regimen immunization.
Statistical analysis
Quantitative variables with normal distributions were expressed as means ± standard deviations (SD), and those with non-normal distributed variables were expressed as medians (inter-quartile range). Statistical significant between groups was examined by two independent variable t-test or one-way anova test for normally distributed variables or by Kruskal-Wallis tests for non-normally distributed ones. Categorical variables were expressed as frequencies (percentages), and Pearson's chi-square test or Fisher's exact test was used to determine group differences. Multivariate analysis including gender and age groups were used: Logistic regression was used to analyze the associations between the changes of the rate of VZV IgG seropositivity and the related factors. Linear regression was used to analyze the associations between the changes of the GMC levels and the related factors. Data were double entered independently and checked for accuracy using Epidata software, Version 3.1. All statistical analyses were performed using SPSS statistical software for Windows, version 17.0 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 (2-sided) was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Yin DP, Song LZ, Zhang XS, et al. Survey of the incidence of varicella in three provinces of LU GAN and XIANG in 2007. Chinese J Vaccines Immunization. 2009;15(02):119–22.
- Guillen JM, Gil-Prieto R, Alvaro A, Gil A. Burden of adult varicella hospitalizations in Spain (2001–2007). Hum Vaccin. 2010;6(8):659–63. doi:10.4161/hv.6.8.12014. PMID:20523119
- Centers for Disease Control and Prevention. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996;45(RR-11):1–36.
- Roush SW, Murphy TV. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298(18):2155–63. doi:10.1001/jama.298.18.2155. PMID:18000199
- Chaves SS, Gargiullo P, Zhang JX, Civen R, Guris D, Mascola L, Seward JF. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007;356(11):1121–9. doi:10.1056/NEJMoa064040. PMID:17360990
- Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics. 2007;120(1):221–31. doi:10.1542/peds.2007-1089. PMID:17606582
- Marin M, Güris D, Chaves SS, Schmid S, Seward JF, Advisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(No. RR-4);1–40. PMID:17585291
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: A meta-analysis. Pediatrics. 2016;137(3):e20153741. doi:10.1542/peds.2015-3741. PMID:26908671
- Evans R. European centre for disease prevention and control. Nurs Stand. 2014;29(9):30. doi:10.7748/ns.29.9.30.s34.
- Zhao Y, Li M, Yao ZY, et al. Survey of antibody levels against varicella-zoster virus in children aged 1–12 years in Licheng district, Jinan city. Chinese J Vaccines Immunization. 2017;23(03):298–301.
- Yue CY, Li Y, Wang YM, Liu Y, Cao L, Zhu X, Martin K, Wang H, An Z. The varicella vaccination pattern among children under 5 years old in selected areas in china. Oncotarget. 2017;8(28):45612–8. doi:10.18632/oncotarget.17317. PMID:28487494
- Joe W. Intersectional Inequalities in Immunization in India, 1992–93 to 2005–06: a Progress Assessment. Health Policy Plan. 2015;30:407–22. doi: 10.1093/heapol/czu023. doi:10.1093/heapol/czu023. PMID:24740707
- Xu MG, Huang J, Tang SW, et al. Cross-sectional study on varicella vaccination efficacy in children [J]. Shanghai J Preventive Med. 2011,23(3):97–99.
- Sosa LE, Hadler JL. Epidemiology of varicella in Connecticut, 2001–2005. J Infect Dis. 2008;197 Suppl 2:S90–3. doi:10.1086/522128. PMID:18419416
- Parker AA, Reynolds MA, Leung J, Anderson M, Rey A, Ortega-Sanchez IR, Schmid DS, Guris D, Gensheimer KF. Challenges to implementing second-dose varicella vaccination during an outbreak in the absence of a routine 2-dose vaccination requirement–Maine, 2006. J Infect Dis. 2008;197 Suppl 2:S101–7. doi:10.1086/522134. PMID:18419381
- Liu SK, Wang F, Zhang HB, et al. Study on Immunogenicity of Booster Immunization of Chinese Varicella Vaccine [J].Chinese J Vaccines Immunization. 2015;21(6):620–2.
- Ma M, Liu WM. Immune Persistence from Primary Immunization and Effectiveness of Booster Immunization for Varicella Attenuated Live Vaccine [J]. Chinese J Vaccines Immunization. 2016;22(2):183–6.
- Marin M, Meissner HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics. 2008;122(3):e744–51. doi:10.1542/peds.2008-0567. PMID:18762511
- Bialek SR, Perella D, Zhang J, Mascola L, Viner K, Jackson C, Lopez AS, Watson B, Civen R. Impact of a routine two-dose varicella vaccination program on varicella epidemiology. Pediatrics. 2013;132(5):e1134–40. doi:10.1542/peds.2013-0863. PMID:24101763
- Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, LaRussa PS, Gershon AA. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis. 2011;203(3):312–5. doi:10.1093/infdis/jiq052. PMID:21208922
- Lopez AS, Zhang J, Marin M. Epidemiology of Varicella During the 2-Dose Varicella Vaccination Program – United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2016;65(34):902–5. doi:10.15585/mmwr.mm6534a4. PMID:27584717
- Leung J, Lopez AS, Blostein J, Thayer N, Zipprich J, Clayton A, Buttery V, Andersen J, Thomas CA, Del Rosario M. Impact of the US Two-dose Varicella Vaccination Program on the Epidemiology of VaricellaOutbreaks: Data from Nine States, 2005–2012. Pediatr Infect Dis J. 2015;34(10):1105–9. doi:10.1097/INF.0000000000000821. PMID:26186103
- Fu J, Wang J, Jiang C, Shi R, Ma T. Outbreak of varicella in a highly vaccinated preschool population. Int J Infect Dis. 2015;37:14–8. doi:10.1016/j.ijid.2015.06.003. PMID:26072038