ABSTRACT
Nontyphoidal Salmonella (NTS) are important human enteric pathogens globally. Among the different serovars associated with human NTS disease, S. Newport (a serogroup C2-C3 Salmonella) accounts for a measurable proportion of cases. However, to date there are no licensed human NTS vaccines. NTS lipopolysaccharide-associated O polysaccharides are virulence factors and protective antigens in animal models. As isolated molecules, bacterial polysaccharides are generally poorly immunogenic, a limitation overcome by conjugation to a protein carrier. We report herein the development of a candidate serogroup C2-C3 glycoconjugate vaccine based on S. Newport Core-O polysaccharide (COPS) and phase 1 flagellin (FliC). S. Newport COPS and FliC were purified from genetically engineered reagent strains, and conjugated at the polysaccharide reducing end to FliC protein lysines with thioether chemistry. S. Newport COPS:FliC immunization in mice improved anti-polysaccharide immune responses, generated high anti-FliC IgG titers, and mediated robust protection against challenge with both the homologous serovar as well another serogroup C2-C3 serovar (S. Muenchen). Analyses of S. Newport COPS:FliC induced sera found that the anti-COPS IgG antibodies were specific for serogroup C2-C3 lipopolysaccharide, and could promote bactericidal killing by complement and uptake into phagocytes. These preclinical studies establish the protective capacity of serogroup C2-C3 OPS glycoconjugates, and provide a path forward for the development of a multivalent Salmonella vaccine for humans that includes serogroup C2-C3.
Introduction
Salmonella are facultative intracellular enteric pathogens that are important causative agents of diarrheal and invasive bacterial disease in humans worldwide. The genus Salmonella contains no less than 2500 different recognized serovars.Citation1 They are classified broadly into serogroups based on structural differences in the O polysaccharide (OPS) repeats of lipopolysaccharide (LPS), and subdivided into serovars based on unique epitopes of the flagellar filament subunit protein, flagellin. They are further distinguished by disease syndrome into typhoid, paratyphoid and nontyphoidal Salmonella (NTS) serovars. Whereas typhoid and paratyphoid serovars cause enteric fever, NTS infection generally results in gastroenteritis with invasive disease as a complication that occurs more often in individuals with immature, suppressed or aged immune systems. It is estimated that there are ∼1,000,000 Salmonella infections in the USA every year, and S. Typhimurium (serogroup B; O:4), S. Newport (C2-C3; O:8) and S. Enteritidis (serogroup D; O:9) are generally the most common etiological agents.Citation2-Citation6 Additionally, the rise in resistance to standard-of-care antibiotics used to treat invasive Salmonella infections has also spurred the US Centers for Disease Control and Prevention to identify NTS as a pathogen of serious concern.Citation7
While there are effective human vaccines to prevent typhoid fever, there are no available or imminent human NTS vaccines.Citation8 Immunization with S. Typhi Vi capsule polysaccharide vaccines prevents typhoid fever, whereby acquisition of a critical level of anti-Vi IgG correlates with protection.Citation9-Citation11 Vaccines that have induced antibodies against the OPS of S. Typhimurium and S. Enteritidis have protected against these pathogens in animal models.Citation8,Citation12–Citation15 Polyclonal vaccine-induced anti-Core-OPS (COPS) sera and monoclonal anti-OPS IgG antibodies have demonstrated functional bactericidal activity for Salmonella through activation of the classical complement pathway and promoting opsonophagocytic uptake and killing by oxidative burst.Citation16 Additionally, we have documented that antibodies against NTS flagellin also exhibit functional bactericidal activity through these mechanisms, and after passive administration protected mice against S. Typhimurium infection.Citation17 Hence, COPS of LPS, which constitutes the surface polysaccharide of serogroup C2-C3 serovars, and NTS flagellin proteins, represent compelling targets for the development of a vaccine against these pathogens.
As isolated molecules, bacterial polysaccharides are generally poor immunogens, failing to induce anamnestic immunological memory, antibody affinity maturation, or functional boost upon repeated vaccination.Citation18 Importantly, children less than two years of age do not respond to polysaccharide immunization. Conjugation to a protein carrier, however, overcomes these limitations and has allowed for the development of carbohydrate-based pediatric vaccines to prevent invasive bacterial infections.Citation19 We previously reported that glycoconjugates of S. Enteritidis and S. Typhimurium Core and O polysaccharide (COPS) with the homologous serovar phase 1 flagellin (FliC) proteins enhanced anti-COPS immune responses and protected against infection with the homologous pathogen in mice.Citation13,Citation14 We report herein the development and assessment of a candidate S. Newport COPS:FliC vaccine.
Results
Purification and characterization of S. Newport COPS, FliC and COPS:FliC glycoconjugate
S. Newport reagent strains for purification of COPS (O:6,8 epitopes) and FliC (e,h epitopes) for conjugate vaccine synthesis were generated using a strategy that has been successfully utilized previously to generate reagent strains for S. Enteritidis and S. Typhimurium glycoconjugate components.Citation20 S. Newport COPS was purified from a genetically engineered strain of S. Newport Chile 361, designated CVD 1962, that was mutated for the genes encoding guaBA (attenuating) and clpX (increased flagellin production) (). S. Newport FliC was purified from a derivative of CVD 1962, termed CVD 1964, that allowed for simple isolation of phase 1 flagellin monomers by additional deletion of the fliD (secretion of flagellin monomers) and fljB (removal of phase 2 flagellin) genes (). FliC purified from CVD 1964 produced a single predominant band by SDS-PAGE at the expected molecular weight based on the protein sequence (A, B). HPLC-SEC analyses of purified CVD 1962 COPS revealed distinct high molecular weight polysaccharide peaks (C). S. Newport COPS is documented to be O-acetylated at two distinct positions in the polysaccharide repeat,Citation1 and we found that O-acetyl groups were present at 1 μmol/mg COPS. Dionex HPAEC-PAD monosaccharide analysis of 1962 COPS was in agreement with the established saccharide composition for Salmonella serogroup C2-C3 OPS repeats (D). SDS-PAGE and HPLC-SEC analyses of the COPS:FliC conjugate confirmed that it was present at a higher molecular weight than either unconjugated FliC or COPS ( and ). Hestrin analysis of COPS:FliC polysaccharide further established an O-acetyl level equivalent to unconjugated COPS (1 μmol/mg).
Table 1. List of Salmonella strains used in the study
Figure 1. Biochemical and biophysical characterization of purified S. Newport COPS, FliC and end-linked glycoconjugate. (A) 5 μg of CVD 1964 FliC alone [F] and conjugated to CVD 1962 COPS [C] were assessed by SDS-PAGE with Coomassie staining. M = molecular weight marker. (B) 2 μg CVD 1964 FliC [F] detected by western blot using pan-Salmonella flagellin monoclonal IgG CB7IH2. M = molecular weight marker. (C) HPLC-SEC chromatogram with RI detection for CVD 1962 COPS (dash trace), CVD 1964 FliC (dot trace), and COPS:FliC conjugate (solid trace). (D) Dionex HPAEC-PAD analysis of depolymerized CVD 1962 COPS with the published Salmonella serogroup C2-C3 OPS structureCitation1 inlaid
![Figure 1. Biochemical and biophysical characterization of purified S. Newport COPS, FliC and end-linked glycoconjugate. (A) 5 μg of CVD 1964 FliC alone [F] and conjugated to CVD 1962 COPS [C] were assessed by SDS-PAGE with Coomassie staining. M = molecular weight marker. (B) 2 μg CVD 1964 FliC [F] detected by western blot using pan-Salmonella flagellin monoclonal IgG CB7IH2. M = molecular weight marker. (C) HPLC-SEC chromatogram with RI detection for CVD 1962 COPS (dash trace), CVD 1964 FliC (dot trace), and COPS:FliC conjugate (solid trace). (D) Dionex HPAEC-PAD analysis of depolymerized CVD 1962 COPS with the published Salmonella serogroup C2-C3 OPS structureCitation1 inlaid](/cms/asset/2cddf1ac-ffd9-484b-82f0-e64c9d804c5f/khvi_a_1483808_f0001_b.jpg)
Immunogenicity of S. Newport COPS:FliC conjugate in mice
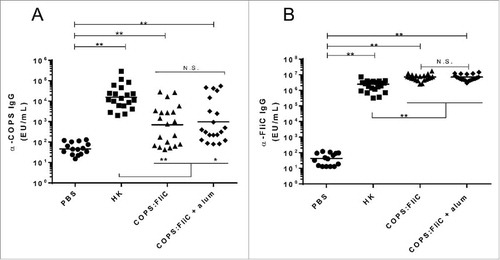
An initial experiment was conducted to assess the immunogenicity and kinetics for acquisition of antibodies to CVD 1962 COPS, when it was given in admixture or conjugated to CVD 1964 FliC. Mice immunized with S. Newport COPS:FliC produced significant anti-COPS IgG titers after three doses relative to PBS controls (Figure S1). By comparison, anti-COPS antibodies were not seen after intermediate doses of COPS:FliC, or when COPS was admixed with FliC. We next assessed the immunogenicity of the S. Newport COPS:FliC conjugate alone or formulated with aluminum hydroxide (alum) adjuvant. Negative control mice were administered PBS alone. As a positive control, a separate group of mice were given 108 CFU equivalent of whole cell heat-killed S. Newport bacteria (HK). Sera were collected from the mice 25 days after the final dose and assessed for IgG titers to the S. Newport COPS and FliC antigens by ELISA. Robust serum anti-COPS IgG titers were detected in mice after immunization with COPS:FliC relative to PBS controls (p < 0.0001), with seroconversion (≥4-fold increase over baseline) observed in 67.5% of the conjugate immunized mice (A). Formulation with alum induced a modest increase in the anti-COPS geometric mean titer (GMT) compared to mice immunized with the conjugate alone (10,573 vs. 4,677 ELISA Units [EU]/mL, respectively), however, this was not significant. Mice immunized with the HK preparation produced the highest anti-COPS GMTs that were significantly elevated relative to either PBS controls, or the conjugate immunized groups (p < 0.05). Mice immunized with conjugate alone or adjuvanted with alum, or with HK S. Newport, all manifested very high anti-FliC IgG titers, relative to PBS controls (B). Furthermore, levels of anti-FliC IgG among conjugate-immunized mice were significantly higher than HK-immunized mice (p<0.0001). Anti-FliC IgG GMTs were equivalent for groups receiving conjugate vaccine alone or formulated with alum.
Figure 2. Immunogenicity of S. Newport COPS:FliC in mice. Serum IgG titers for anti-S. Newport COPS (A) or FliC (B) from mice (n = 20/group) immunized with PBS, HK S. Newport or COPS:FliC conjugate, with or without aluminum hydroxide as an adjuvant. Each point represents an individual mouse. Solid bars indicate the GMT; comparisons between groups were accomplished by unpaired two-tailed Mann-Whitney. *P < 0.001, **P < 0.0001, N.S. – not significant
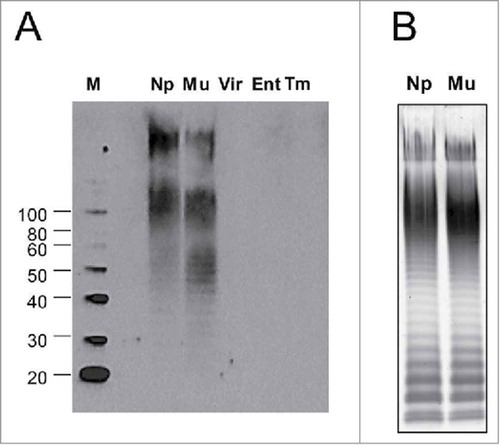
In order to assess the epitope-specific binding pattern for anti-COPS antibodies induced by S. Newport COPS:FliC, western blot analyses were conducted using sera from mice vaccinated with the COPS:FliC conjugate for LPS extracts from different Salmonella serovars (A). This included isolates within the same C2-C3 serogroup as S. Newport (S. Muenchen, O:6,8), a serogroup C1 serovar that shares O epitope 6 (S. Virchow, O:6,7,14), and heterologous serogroup serovars that have the same core polysaccharide but express different OPS epitopes (S. Enteritidis, O:1,9,12; S. Typhimurium: O:1,4,5,12). We found that S. Newport COPS:FliC-induced IgG detected high molecular weight O-antigen chains in LPS extracts from both serogroup C2-C3 strains (S. Newport and S. Muenchen) (A), corresponding to the analogous long and very-long OPS repeat containing molecules seen in the banding pattern for S. Newport and S. Muenchen LPS by SDS-PAGE/Pro-Q analysis (B). These sera displayed no measurable binding, however, for serogroup C1 OPS, or OPS from serogroups B or D. Additionally, there was no detectable binding to the core polysaccharide that could be seen as the low molecular weight band at the bottom of the SDS-PAGE gel after Pro-Q staining (B).
Figure 3. OPS antigen specificity of IgG antibodies in conjugate-immunized mice sera. (A) LPS extracts from various non-typhoidal Salmonella serovars described in (Np – Newport Chile 361, Mu – Muenchen ATCC 8388, Vir – Virchow Q23, Ent – Enteritidis R11, Tm – Typhimurium D65) were transferred to PVDF membranes and probed with S. Newport COPS:FliC immune antisera. M – Molecular weight marker. (B) SDS-PAGE with Pro-Q staining for LPS from S. Newport Chile 361 (Np) or S. Muenchen ATCC 8388 (Mu)
Immunization with COPS:FliC conjugate protects mice against challenge with wild-type S. Newport
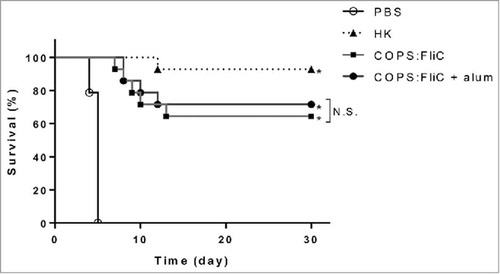
In order to assess the ability of the conjugate to serve as a protective vaccine against fatal infection with wild-type S. Newport, four weeks after the last immunization, mice were challenged intraperitoneally (IP) with a lethal dose of wild-type S. Newport Chile 361. While 100% mortality was observed in the PBS control group, robust protection (95% vaccine efficacy [VE]) was achieved in the positive control group immunized with HK S. Newport (). We found that mice immunized with COPS:FliC were also significantly protected against infection (p<0.0001) compared to PBS controls with comparable levels of protection observed for COPS:FliC alone or formulated with alum (VE of 64.3% and 71.4%, respectively). In a separate experiment, we found that immunization with CVD 1964 FliC alone produced a trend towards partial protection against S. Newport infection (VE = 37%), although this was not significant (Table S1).
Figure 4. Protection against S. Newport infection in mice immunized with S. Newport COPS:FliC conjugate with or without adjuvant. BALB/c mice (n = 14/group) as described in , were immunized with heat-killed S. Newport Chile 361 (HK), PBS or S. Newport COPS:FliC conjugate alone or with formulated with alum. Kaplan-Meier survival curves after challenge with 3 × 107 CFU (6xLD50) were compared using log rank analysis. *P <0.0001, N.S. – not significant
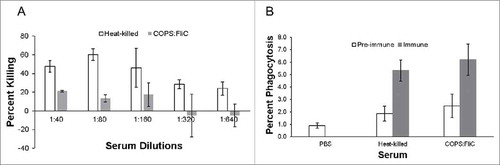
Functional activity of COPS:FliC sera for S. Newport
HK S. Newport immune serum was used as a positive control to assess complement-mediated bactericidal activity against S. Newport Chile 361. We found that this serum consistently killed approximately 50% of bacteria in the presence of baby rabbit complement (BRC) (A) until a dilution of 1:160 had been achieved, after which point the level of killing dropped to ∼30%. Sera from mice immunized with COPS:FliC conjugate were also able to kill S. Newport, albeit at a lower level compared to HK sera, whereby approximately 20% of S. Newport bacteria were killed out to a dilution of 1:160 after which point no further bactericidal activity could be detected. No serum bactericidal activity (SBA) was found for sera from PBS controls (data not shown). We further assessed functional antibody activity by investigating the capacity of immune sera to mediate phagocytosis by mouse macrophages. HK S. Newport immune sera was also used as a positive control in this assay and increased uptake of S. Newport Chile 361 two-fold compared to pre-immune sera. COPS:FliC vaccine-induced sera produced comparable levels of uptake compared to HK S. Newport immune sera where phagocytosis was increased two-fold compared to PBS or pre-immune sera controls (B).
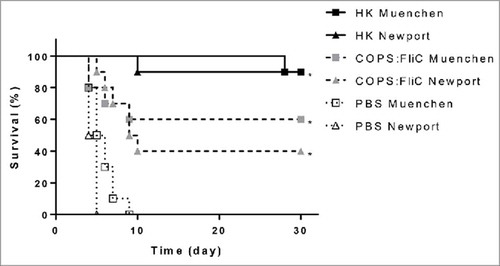
Cross protection by S. Newport COPS:FliC against other Salmonella C2-C3 strains
Since sera from S. Newport COPS:FliC-immunized mice bound the LPS of another serogroup C2-C3 serovar, and the ultimate goal of this work is to develop a pan-serogroup C2-C3 vaccine, we wanted to assess whether S. Newport COPS:FliC would also offer intra-serogroup protection. To investigate this, we assessed protection against S. Muenchen ATCC 8388, that has the same COPS epitopes, but expresses different phase 1 flagellin epitopes (H:d) compared to the S. Newport FliC (H:e,h) used as the conjugate carrier protein. For this, groups of mice (n = 20/group) were immunized with PBS or the S. Newport COPS:FliC conjugate, as described above. Positive control groups (n = 10/group) received 108 CFU equivalents of HK S. Newport Chile 361 or S. Muenchen ATCC 8388, respectively. Four weeks after the final immunization, half of the conjugate-immunized or PBS control groups, or the homologous serovar HK groups were infected with 6 x LD50 of either S. Newport Chile 361 or S. Muenchen ATCC 8388. Full mortality (10/10) was observed in the PBS control groups infected with S. Newport Chile 361 or S. Muenchen ATCC 8388, where the time to death was comparable after infection with either of the two pathogens (). Significant protection was achieved against the two challenge serovars in the groups immunized with the S. Newport COPS:FliC conjugate (VE = 40% and 60% against S. Newport and S. Muenchen, respectively, p<0.0001). As was seen previously, almost full protection (90% VE, p<0.0001) was observed for mice immunized with the homologous serovar HK preparation. Analyses of COPS and FliC IgG titers in post-immunization sera demonstrated comparable immune responses to these antigens as were seen previously (data not shown).
Figure 6. Intra-serogroup protection after S. Newport COPS:FliC immunization. Protection against challenge with 3 × 107 CFU S. Newport Chile 361 (triangles) or S. Muenchen ATCC 8388 (squares) in mice (n = 20/group) immunized with homologous heat-killed strain (solid lines, black shapes), PBS (dotted lines, open shapes) or S. Newport COPS:FliC (dashed lines, grey shapes). Kaplan-Meier survival curves were compared using log rank analysis. *P < 0.0001
Discussion
Salmonella serogroup C2-C3 is a major cause of salmonellosis in the USA, for which S. Newport is the most common etiological C2-C3 serovar.Citation2,Citation4 S. Newport invasive infections are also associated with measurable rates of invasive infection.Citation3 A commercially available serogroup C2-C3 vaccine comprised of siderophore receptors and porin proteins is available for livestock, however there are no licensed human C2-C3 vaccines.Citation21 This is the first study to our knowledge assessing a glycoconjugate vaccine against Salmonella C2-C3.
Whereas unconjugated S. Newport COPS was not immunogenic, conjugation to FliC induced robust anti-COPS IgG titers. These findings are similar to our previous observation for S. Enteritidis COPS, where negligible immune responses were seen in the absence of conjugation to a protein carrier, and significant antibody levels only occurred after three doses of vaccine.Citation14 The demonstration herein for the carrier function of S. Newport FliC is also in agreement with previous findings from our group, whereby other Salmonella flagellin proteins have also served as effective carrier proteins for NTS COPS molecules.Citation13,Citation14 Consistent with the documented OPS structure, CVD 1962 COPS was found to be highly O-acetylated. The conjugation strategy utilized herein retains polysaccharide O-acetyls, and is analogous to the approach we reported previously for an optimized S. Typhimurium COPS:FliC conjugate, for which the O-acetyl epitopes were immunodominant.Citation13 The importance of S. Newport OPS O-acetyls to protection is unknown however, and should be addressed in future studies.
We found that S. Newport COPS:FliC immunization induced bactericidal antibodies in sera and offered robust protection for both the homologous serovar, as well as for S. Muenchen that shares the same O type but bears different flagellin epitopes. These findings support serogroup C2-C3 OPS as a protective antigen. Immunization with S. Newport FliC alone provided a trend towards partial protection against fatal infection with S. Newport, however this was not significant. As the variable region of Salmonella flagellin proteins bear serovar specific epitopes, and form the antibody accessible surfaces of the flagella filament, it is not anticipated that FliC-induced antibodies would offer measurable cross protection against serovars expressing heterologous flagellin types.
Surprisingly, we found no cross reactivity for S. Newport COPS:FliC sera with S. Virchow, which is a C1 isolate and shares O epitope 6 with serogroup C2-C3. It is possible that O6 is a minor epitope and poorly immunogenic, and thus not a major target of the polyclonal immune response. Cross-protection studies will be needed, however, to verify whether this correlates with a lack of functional activity. The equivalent protection offered by S. Newport COPS:FliC against infection with S. Muenchen, another important cause of Salmonella infection in the USA,Citation5 provides important evidence to support the notion that this approach may provide broad protection against serogroup C2-C3 pathogens.
While the present study establishes the feasibility of an OPS-based Salmonella serogroup C2-C3 glycoconjugate vaccine approach, several limitations and caveats remain. Immunization herein was by the intraperitoneal route. Although this is well accepted in preclinical murine models, human parenteral vaccines are administered intramuscularly or subcutaneously. Immunogenicity following administration by these routes should thus be assessed in future studies. Additionally, while a single dose and interval between immunizations was utilized, variation of these parameters could improve anti-polysaccharide immune responses. Dose-ranging studies incorporating different immunization schedules will need to be conducted to verify optimal conditions for establishment of immunologic memory and associated anamnestic boost responses upon repeated glycoconjugate administration.
The mouse model used herein assesses protection against invasive infection, which is a severe complication of S. Newport gastroenteritis and potentially fatal. Vaccines that induce antibodies against surface polysaccharides of enteric pathogens have been theorized to exert antibacterial activity at the mucosal surface. Indeed, a recent report documented protection against Campylobacter jejuni oral infection in non-human primates vaccinated with a C. jejuni capsule polysaccharide conjugate with CRM197.Citation22 It is thus possible that immunization with S. Newport COPS:FliC could offer protection against gastroenteritis caused by this organism. Further studies should assess this possibility. Prior research efforts from our group have focused on the development of optimized serogroup D (S. Enteritidis) and serogroup B (S. Typhimurium) COPS:FliC conjugate vaccines.Citation13,Citation14 Additional work should assess combining these vaccines with S. Newport COPS:FliC and a serogroup C1 vaccine to generate a formulation that would provide coverage against the majority of Salmonella infections in the USA.
Materials and methods
Bacterial strains, medium, and growth
The strains used in this study are shown in and were maintained on Hi-Soy (HS) bacteriological medium (5 g/L sodium chloride, 10 g/L soytone [Teknova, Cat # S9052], 5 g/L Hy-yest [Sigma Aldrich, Cat # 09182]) at 37°C. Growth and preparation of bacteria for in-vitro analyses and in-vivo infections were conducted as described.Citation13 Bacterial concentrations were calculated by OD600 with an experimentally determined correction factor and confirmed by viable count. Heat-killed (HK) bacteria were achieved by heating (80°C, 60 minutes), with inactivation of the inoculum confirmed by plating on HS agar and incubating overnight at 37°C. Bacterial fermentation cultures of S. Newport reagent strains were grown in fully chemically defined media supplemented with guanine as described.Citation13
Protein and polysaccharide purification
S. Newport FliC was purified from clarified CVD 1964 fermentation culture supernatants as described previously, by sequential cation exchange and anion exchange membrane chromatography (Sartorius, Cat # 96IEXS42E9BFF, 96IEXQ42E9BFF), with concentration and diafiltration into saline with 30-kDa tangential flow filtration (TFF) (Millipore, Cat # PXBO30A50).Citation23 Purified CVD 1964 FliC was monomerized by treatment at pH 2 and then brought to 0.1% Tween 20 (Sigma, Cat # P1379) and stored at −80°C until use. S. Newport COPS was purified from CVD 1962 fermentation culture biomass using the method described.Citation13 Briefly, COPS was extracted by boiling the CVD 1962 biomass in ∼9% acetic acid pH ∼3.7 for four hours. Insoluble material and cell debris were removed by centrifugation and 0.2 μm TFF microfiltration (GE Healthcare, Cat # CFP-2-E-6A). The bulk polysaccharide was then concentrated with 30-kDa TFF ultrafiltration (Hydrosart, Sartorius, Cat # 3061445901E–SW) and diafiltered first with 35 diavolumes of 1 M NaCl, and then 10 diavolumes of 20 mM Tris 50 mM NaCl pH 7. The retentate fraction was then subjected to negative chromatography with an anion exchange membrane (Sartorius, Cat # 96IEXQ42EUC11–A). Additional impurities in the flow through fraction were precipitated by ammonium sulfate (Sigma, Cat # A4418), after which COPS in the cleared supernatant fraction was then concentrated and diafiltered into deionized (DI) water with 10-kDa TFF (Hydrosart, Sartorius, Cat # 3081443902E–SW). The polysaccharide preparation was then lyophilized and stored at −80°C until use.
Protein and polysaccharide analyses
Purified CVD 1964 FliC proteins were assessed by 4–20% Tris-Glycine SDS-PAGE (Life Technologies, Cat #EC6028) with Coomassie staining for purity and integrity, and by western blot as described with pan-flagellin monoclonal antibody CB7IH2 for identity.Citation23 FliC protein concentration was determined by absorbance at 280 nm with the calculated extinction coefficient determined from the amino acid sequence and the ExPASy ProtParam tool (https://web.expasy.org/protparam/). CVD 1964 COPS yield was determined by dry weight after lyophilization. COPS monosaccharide composition was assessed by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) using a Dionex ICS4000 (Thermo) run on a CarboPac PA10 column (Thermo, Cat # 82320) at 0.01 mL/min in 18 mM KOH. For this, COPS samples were prepared by boiling for 1 hour in 2 M Trifluoroacetic acid (American Bioanalytical, Cat # AB02010) followed by lyophilization and reconstitution in DI water and composition determined relative to commercially available monosaccharide standards. Polysaccharide O-acetyl content for purified CVD 1962 COPS and COPS:FliC conjugates was assessed by the Hestrin method with acetylcholine chloride standards (Sigma, Cat # A6625) as described.Citation13,Citation24 Residual protein levels in COPS preparations were determined by bicinchoninic acid (BCA) assay (Thermo, Cat# 23225) using commercially available bovine serum albumin (BSA) standards (Thermo, Cat # 23209). Residual endotoxin in FliC and COPS preparations were assessed by limulus amebocyte lysate assay (LAL) with the Endosafe PTS system (Charles River). Molecular size analyses of CVD 1962 COPS, CVD 1964 FliC and COPS:FliC conjugates were assessed by high performance liquid size exclusion chromatography (HPLC-SEC) with a Waters Alliance system and a BioSep SEC-s4000 column (Phenomenex, Cat # 00H-2147-K0) run at 1 mL/min in PBS pH 7.4 with monitoring for refractive index (RI). Polysaccharide and protein concentrations in the final purified conjugate were assessed by resorcinol assay with purified CVD 1962 COPS standards and BCA assay with BSA standards respectively.
Preparation and characterization of S. Newport COPS conjugates with FliC
End-linked sun-type conjugates of CVD 1962 COPS with CVD 1964 FliC were generated as described.Citation13 Briefly, CVD 1962 COPS was brought to 20 mg/mL in 100 mM sodium acetate pH 5 and modified at the reducing end KDO carbonyl group with an aminooxy thiol linker by incubating with 2.75 mg/mL O-(3-mercaptopropyl)-hydroxylamine (Fina Biosolutions, MD) for 12–18 hours at room temperature. The reaction was then brought to 100 mM DTT (Roche, Cat # 10708984001) and labeled COPS was purified on Sephadex G25 in 1X PBS, 5 mM EDTA, pH 6.8. CVD 1964 FliC monomers were brought to ∼5 mg/mL in PBS pH 7.4 and incubated with a 30X molar excess of N-γ-maleimidobutyryl-oxysuccinimide ester (GMBS, Molecular BioSciences, Cat # 22324) for 1 h at room temperature at which point they were concentrated to 15 mg/mL with 10 kDa Biomax PES TFF membrane (Millipore, Cat # PXB010A50) and diafiltratered against 10 diavolumes of PBS / 5 mM EDTA, pH 6.8. COPS-thiol was then added to the FliC-maleimide at a ratio of 3:1 mg:mg, and incubated for 12–18 h at 4°C, followed by quenching with 0.1 mM 2-mercaptoethanol (GE Healthcare, Cat # 17-1317-01). COPS:FliC conjugates were separated from unreacted components by size exclusion chromatography on 16 × 600 HiLoad Pg Superdex 200 resin column with a NGC Chromatography System (BioRad) run at 1 mL/min in 20mM Tris pH 7 with monitoring for absorbance at 280 nm and 260 nm. Individual fractions were assessed by SDS-PAGE/Coomassie and fractions containing conjugates were pooled and filtered through 0.2 μm for use in animal immunization experiments.
Conjugate formulation with aluminum hydroxide
COPS:FliC formulation with aluminum hydroxide (alum) (InvivoGen, Cat# vac-alu-250) was accomplished as follows. Adsorption was assessed by adding increasing alum amounts to a fixed 0.1 mg/mL concentration of conjugate to produce alum:protein ratios of 1:1 – 30:1 wt:wt. Samples were vortexed and incubated for 30 minutes at room temperature with gentle rotating, followed by centrifugation for 5 min at 10,000 x g. Concentrations of unbound supernatant proteins were analyzed by BCA assay. The minimal condition of 5:1 (alum:protein) that allowed for ≥ 95% adsorption was selected for mouse immunization, which was accomplished with incubation at room temperature for 30 minutes with gentle rotating prior to immunization.
Ethics statement
All animal studies were performed in facilities that are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and were in compliance with guidelines for animal care established by the US Department of Agriculture Animal Welfare Act, US Public Health Service policies, and US federal law. All animal experiments were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Immunization, sera collection and challenge
Female BALB/c mice (8 to 10 weeks old) were purchased from Charles River Laboratories (Wilmington, MA) and maintained in the University of Maryland School of Medicine animal facility. Mice were immunized intraperitoneally (IP) at 0, 14 and 28 days with either sterile PBS (pH 7.4), 2.5 μg by polysaccharide weight of COPS:FliC alone or formulated with alum, or 108 CFU equivalent of HK S. Newport Chile 361 or S. Muenchen ATCC 8388. Sera were obtained by retro-orbital bleeding 25 days after the final immunization and stored at −20°C until use. Four weeks after the final immunization (day 56), mice were challenged IP with ∼6 x LD50 of S. Newport Chile 361 or S. Muenchen ATCC 8388 (1 LD50 = ∼5 × 106 CFU). Challenge bacteria were prepared using overnight bacterial cultures grown on solid media that were resuspended in PBS, adjusted to an OD600 of 0.2 – 0.3, and then diluted to the required concentration. Following challenge, mice were monitored daily for 30 days recording overall health, weight loss, and mortality. Mice that reached a moribund state (lethargy, non-responsiveness, dehydration, piloerection, and/or ≥ 20% weight loss) were euthanized and recorded as dead. Vaccine efficacy (VE) was calculated as ((proportional mortality in controls)-(proportional mortality in vaccine group))/(proportional mortality in controls).
ELISA analyses for polysaccharide antigenicity analyses and titration of serum antibodies
Titration of anti-COPS or -FliC serum IgG from PBS or vaccine-immunized mice was accomplished using an enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well, medium-binding, microtiter plates (Greiner bio-One # 655001) were coated with 100 µL/well of either CVD 1962 COPS or CVD 1964 FliC antigens at a concentration of 5 μg/mL and incubated overnight at 4°C. Plates were washed 3 times with 200 µL/well 0.05% Tween-20 in PBS (PBST) and blocked with PBST + 10% Omniblok non-fat dry milk (AmericanBio, Cat # AB10109) for 1 h at 37°C, then washed 3 times with 200 µL/well. Serum samples were serially diluted in PBST + 10% Omniblok, transferred to blocked ELISA plates, and incubated for 1 h at 37°C. Plates were washed 5 times with PBST, and incubated for 1 h at 37°C with horseradish peroxidase (HRP)-labeled anti-mouse IgG (Seracare, Cat # KPL-474-1802) diluted 1:2000 with PBST + 10% Omniblok. After 5 washes, 100 µL/well of substrate (3,3’,5,5’-tetramethylbenzidine, Seracare, Cat # 5120-0047) was added, and the plates were incubated with rocking at ambient temperature for 15 minutes in the dark. The reaction was stopped with the addition of 100 µL/well of 1 M H3PO4, and the absorbance at 450 nm was recorded using an Ascent microplate reader (Thermo Scientific). Endpoint titers, represented as ELISA units (EU) per mL, were defined and calculated as previously described.Citation13 Test and control sera were run in duplicate. Analyses were conducted with positive sera as plate controls with acceptance criteria of < 15% variance between plates. Titers were calculated by interpolation of absorbance values of test samples into the linear regression curve of a calibrated control (reference serum). The endpoint titers reported as ELISA units (EU) represent the inverse of the serum dilution that produces an absorbance value of 0.2 above the blank. Seroconversion in vaccinated mice was defined as a 4-fold increase in the antibody titer after immunization compared to control mice immunized with PBS. Any negative values ≤0.1 were assigned a titer of 6.25 EU/mL, and values of 0.1< and ≤0.2 were assigned a titer of 12.5 EU/mL.
LPS extraction, Pro-Q staining and immunoblotting
Bacterial LPS minipreparations were generated as described.Citation13 Briefly, bacteria were grown overnight in HS broth then adjusted to an OD600 = 1.0. LPS was isolated by pelleting 1 mL of culture at 12,000 x g for 10 min then boiling pellets in lysis buffer (2% SDS, 10% glycerol, 400 mM Tris HCl pH 7, supplemented with 1/25 [vol/vol] of β-mercaptoethanol) for 10 min. Lysates were treated with Proteinase K (ThermoFisher, Cat # EO0491) to a final concentration of 0.5 µg/mL and incubated at 60°C for 1 hour. Lysates were mixed with Laemmli buffer (Bio-Rad # 171–0737) and separated on 4–12% Bis-Tris gels (ThermoFisher, Cat # NP0321) in 1 × 3-(N-morpholino)propanesulfonic acid (MOPS) running buffer (ThermoFisher Scientific, Cat # NP0001). LPS was visualized by staining the gel with the ProQ Emerald system (Life Technologies, Cat # P21857), per the manufacturer's instructions. Binding patterns of anti-COPS antibodies with LPS were assessed by immunoblotting of LPS extracts from strains as previously described.Citation13 Briefly, gels were transferred to Polyvinylidene fluoride (PVDF) membranes (BioRad, CA, Cat # 170–4274) overnight (35V, 4°C), blocked with 5% BSA-TBST then probed overnight at 4°C with pre-immune mouse serum, or sera collected from animals vaccinated with COPS:FliC conjugate (diluted 1:500 in 5% BSA-TBST). Blots were incubated with HRP-conjugated goat anti-mouse IgG for 1h at room temperature and developed with Clarity ECL substrate (BioRad, # 170–5060).
Serum bactericidal activity (SBA) Assay
The SBA assay was adapted from the basic serum bactericidal activity assay format described by Boyd et al.Citation25 Briefly, heat-inactivated mouse sera were diluted 1:10 in PBS and serially diluted two-fold in a 96-well plate. Overnight cultures of S. Newport Chile 361 were diluted 1:1000 in HS broth and incubated at 37°C with shaking at 115 rpm until the OD600 reached 0.3 to 0.4. Bacteria were serially diluted to 10−4 in PBS. Baby rabbit complement (BRC) (Pel-Freez Biologicals, Cat # 31061-1) was diluted in PBS so that the final concentration in each well would be 5%. Forty microliters of the complement mix was added to each well followed by 10 µl of the 10−4 dilution of bacterial suspension containing approximately 150–250 CFU of S. Newport. Plates were incubated at 37°C with shaking at 115 rpm for 1 h. Viable counts were performed on the serum-treated samples and on the bacterial inoculum. Colonies were counted, and percent killing was calculated as ((mean colony count per dilution)/(mean colony count of complement-only control)) x 100.
Opsonophagocytic uptake assay (OPA)
Serum-mediated bacterial uptake into phagocytes was conducted as previously described.Citation14 Briefly, log-phase cultures of S. Newport Chile 361 were diluted to 4 × 106 CFU/mL then incubated with normal or immune serum at a final concentration of 10% for 20 min at room temperature. Opsonized bacteria were added to confluent monolayers of J774 cells in antibiotic-free medium (DMEM [Cellgro, Cat # 10-013-CV] with 10% FBS [Sigma, Cat # F2442]) at a 1:1 ratio of bacteria to macrophage cells and centrifuged at 1,000 x g at room temperature for 10 min then incubated at 37°C with 5% CO2. After 1 hour, growth medium was replaced with DMEM+FBS supplemented with 100 µg/mL gentamicin (Gibco, Cat # 15750-060). Cell monolayers were incubated for another hour at 37°C, 5% CO2 then washed three times with sterile PBS. Bacteria were recovered with PBS, 0.5% Triton X-100 (Sigma, Cat # X100) and assessed for viable CFU by plating on solid agar medium. Results are expressed as the percent phagocytosis calculated by the ((number of recovered CFU)/(number of CFU added to J774 cells)) x 100.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 6 (GraphPad Software). Statistical significance were assessed using the two-tailed unpaired Mann-Whitney Rank-Sum test (α = 0.05), comparing individual sera from mice immunized with conjugate or HK bacteria relative to the levels obtained with control sera. Survival analysis after active immunization was assessed by the log-rank test or Fisher's exact test (alpha = 0.05). P values of ≤ 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
R. Simon and S.M. Tennant are co-inventors on the following patents that describe COPS:FliC conjugate and live attenuated NTS vaccines: US patent 9,050,283, “Broad spectrum vaccine against non-typhoidal Salmonella”; US patent 9,011,871, “Broad spectrum vaccine against typhoidal and non-typhoidal Salmonella disease”; and European Patent Number 2387417, “Broad spectrum vaccine against non-typhoidal Salmonella”.
KHVI_A_1483808_Supplemental.zip
Download Zip (69.2 KB)Additional information
Funding
References
- Lindberg AA, Le Minor L. Serology of Salmonella. In: Bergan T, ed. Methods in Microbiology: Academic Press, 1984. p. 1–141.
- Fuche FJ, Sow O, Simon R, Tennant SM. Salmonella serogroup C: current status of vaccines and why they are needed. Clin Vaccine Immunol. 2016;23:737–45. doi:https://doi.org/10.1128/CVI.00243-16.
- Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin-D'Angelo M, Hurd S, et al. Salmonellosis outcomes differ substantially by serotype. J Infect Dis. 2008;198:109–14. doi:https://doi.org/10.1086/588823.
- Vugia DJ, Samuel M, Farley MM, Marcus R, Shiferaw B, Shallow S, et al. Invasive Salmonella infections in the United States, FoodNet, 1996–1999: incidence, serotype distribution, and outcome. Clin Infect Dis. 2004;38 Suppl 3:S149–56. doi:https://doi.org/10.1086/381581.
- CDC. Foodborne Diseases Active Surveillance Network (FoodNet): FoodNet Surveillance Report for 2012 (Final Report). Atlanta, Georgia: U.S. Department of Health and Human Services, CDC, 2014.
- Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–9. doi:https://doi.org/10.1086/650733.
- CDC. Antibiotic Resistance Threats in the United States, 2013. Atlanta, Georgia: U.S. Department of Health and Human Services, CDC, 2013.
- Simon R, Levine MM. Glycoconjugate vaccine strategies for protection against invasive Salmonella infections. Hum Vaccin Immunother. 2012;8:494–8. doi:https://doi.org/10.4161/hv.19158.
- Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella Typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine. 1996;14:435–8. doi:https://doi.org/10.1016/0264-410X(95)00186-5.
- Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, et al. The efficacy of a Salmonella Typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–9. doi:https://doi.org/10.1056/NEJM200104263441701.
- Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: a randomised controlled, phase 2b trial. Lancet. 2017;390:2472–80. doi:https://doi.org/10.1016/S0140-6736(17)32149-9.
- Watson DC, Robbins JB, Szu SC. Protection of mice against Salmonella Typhimurium with an O-specific polysaccharide-protein conjugate vaccine. Infect Immun. 1992;60:4679–86.
- Baliban SM, Yang M, Ramachandran G, Curtis B, Shridhar S, Laufer RS, et al. Development of a glycoconjugate vaccine to prevent invasive Salmonella Typhimurium infections in sub-Saharan Africa. PLoS Negl Trop Dis. 2017;11:e0005493. doi:https://doi.org/10.1371/journal.pntd.0005493.
- Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, et al. Salmonella enterica serovar Enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. Enteritidis. Infect Immun. 2011;79:4240–9. doi:https://doi.org/10.1128/IAI.05484-11.
- Svenson SB, Lindberg AA. Artificial Salmonella vaccines: Salmonella Typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981;32:490–6.
- Goh YS, Clare S, Micoli F, Saul A, Mastroeni P, MacLennan CA. Monoclonal antibodies of a diverse isotype induced by an o-antigen glycoconjugate vaccine mediate in vitro and in vivo killing of African invasive nontyphoidal Salmonella. Infect Immun. 2015;83:3722–31. doi:https://doi.org/10.1128/IAI.00547-15.
- Ramachandran G, Tennant SM, Boyd MA, Wang JY, Tulapurkar ME, Pasetti MF, et al. Functional activity of antibodies directed towards flagellin proteins of non-typhoidal Salmonella. PLoS One. 2016;11:e0151875. doi:https://doi.org/10.1371/journal.pone.0151875.
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–20. doi:https://doi.org/10.1038/nri2494.
- Knuf M, Kowalzik F, Kieninger D. Comparative effects of carrier proteins on vaccine-induced immune response. Vaccine. 2011;29:4881–90. doi:https://doi.org/10.1016/j.vaccine.2011.04.053.
- Tennant SM, Wang JY, Galen JE, Simon R, Pasetti MF, Gat O, et al. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun. 2011;79:4175–85. doi:https://doi.org/10.1128/IAI.05278-11.
- Dodd CC, Renter DG, Thomson DU, Nagaraja TG. Evaluation of the effects of a commercially available Salmonella Newport siderophore receptor and porin protein vaccine on fecal shedding of Salmonella bacteria and health and performance of feedlot cattle. Am J Vet Res. 2011;72:239–47. doi:https://doi.org/10.2460/ajvr.72.2.239.
- Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, et al. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect Immun. 2009;77:1128–36. doi:https://doi.org/10.1128/IAI.01056-08.
- Simon R, Curtis B, Deumic V, Nicki J, Tennant SM, Pasetti MF, et al. A scalable method for biochemical purification of Salmonella flagellin. Protein Expr Purif. 2014;102:1–7. doi:https://doi.org/10.1016/j.pep.2014.07.005.
- Hestrin S. The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem. 1949;180:249–61.
- Boyd MA, Tennant SM, Saague VA, Simon R, Muhsen K, Ramachandran G, et al. Serum bactericidal assays to evaluate typhoidal and nontyphoidal Salmonella vaccines. Clin Vaccine Immunol. 2014;21:712–21. doi:https://doi.org/10.1128/CVI.00115-14.
- Fuche FJ, Sen S, Jones JA, Nkeze J, Permala-Booth J, Tapia MD, et al. Characterization of Invasive Salmonella Serogroup C1 Infections in Mali. Am J Trop Med Hyg. 2018;98:589–94. doi:https://doi.org/10.4269/ajtmh.17-0508.