ABSTRACT
Background: To evaluate the one-year immunogenicity and safety of a subunit plague vaccine. Methods: In the initial study, 240 healthy adults aged 18–55 years were administrated with 2 doses of 15 or 30 µg plague vaccines at day 0 and 28, respectively. In this extended follow-up study, we evaluated the immunogenicity and safety of the plague vaccine up to one year. Results: For antibody to envelope antigen faction 1 (F1) antigen, titers were up to new peaks at month 6, then declined slowly to month 12, but remained at higher levels than those at day 56. Geometric mean titers (GMTs) of F1 were significantly higher in 30 µg group than those in 15 µg group at month 6 and 12 (P < 0.0001 and P < 0.001). However, approximate 100% seroconversion rates of F1 antibodies were found in both 15 and 30 µg groups at the both time points. For antibody to recombinant virulence (rV) antigen, titers and seroconversion rates were decreased sharply at month 6 and continue to decrease at month 12. GMTs and seroconversion rates were not significantly different between the 15 and 30 µg groups, respectively. No serious adverse events (SAEs) related to vaccine occurred. Conclusion: The new plague vaccine (F1+rV) induced a robust immune response up to 12 months and showed a good safety profile in adults aged 18–55 years.
Introduction
Plague caused millions of deaths over the course of human history, with the causative agent of Yersinia pestis.Citation1,Citation2 Although the advancements in public health and the advent of antibiotics have greatly reduced plague outbreaks in recent years, the epidemics are still breaking out sporadically in several areas of the world, including Asia, Africa, even developed country in the western United States and South America, and continue to infect thousands of people worldwide each year.Citation3 Plague is a very severe disease in people, with a case-fatality ratio of 30% to 60% for the bubonic type, which is always fatal for the pneumonic kind when left untreated.Citation4 The potential use of the bacteria in modern times as an agent of bioterrorism and the emergence of a Y. pestis strain resistant to antibiotics bring out severe potentially significant public health concerns.Citation5–Citation7 Therefore, prophylactic vaccination against this disease holds the brightest prospect for its long-term prevention.
Although live attenuated and killed whole cell plague vaccines have been developed and used since the last century, there were limited commercial availability now with some shortcomings including significant adverse reactions and limited evidence for efficacy to provide long-term protection. On the other hands, killed whole cell vaccines failed to protect against the pneumonic plague, and live attenuated whole cell vaccines with losing of some virulence factors was incomplete and unreliable to protect against plague.Citation3,Citation8,Citation9 In recent decade, development of vaccine has focused on subunit vaccine of envelope antigen faction 1 (F1), virulence antigen (V) and other types of new plague vaccines.Citation10–Citation11 F1 antigen, a kind of anti-phagocytic capsular protein, was known to be involved in protecting Y. pestis from phagocytosis by macrophages of the host. V antigen was part of a type III secretion system and acts as both virulence determinant and protective antigen of Y. pestis.Citation13–Citation15 The injected subunit vaccine has been demonstrated the best near-term immunogenicity that protects against both bubonic and pneumonic plague. The vaccine provides strong protective immunity in animal models such as mice, rats, rabbits, and nonhuman primates.Citation12,Citation16–Citation18 In the initial study, results showed that the plague vaccine comprised of F1 and rV antigens were safe and immunogenic in adults aged 18–55 years. The 30 µg formulation could induce a stronger immune response than did the 15 µg formulation. After that, an extended follow-up study was conducted to evaluate the immunogenicity of the new subunit (F1+rV) plague vaccine up to one year.
Results
In the initial study, 238 participants (119 in each group) received two doses of 15 or 30 µg plague vaccines at a 1:1 ratio. 237 (99.58%) of them (119 in 15 µg group, 118 in 30 µg group) completed the extended one-year follow-up and the blood collection at month 6 and 12.
Immunogenicity
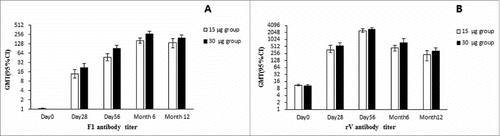
After two doses vaccination, the plague vaccine elicited substantial responses to the vaccine with geometric mean titers (GMTs) of anti-F1 and rV antibodies peaked at day 56 in 15 and 30 µg groups, which was reported in previous study.Citation20 Titers of anti-F1 antibody were increased from day 56 to month 6 and then decreased to month 12. However, titers of rV antibody were waned from day 56 throughout to month 12 ( A and B). Variation tendencies of antibodies to F1 and rV antigens during the extended follow-up were different in the study groups. However, for a same antibody, the variation tendencies were similar between the 15 and 30 µg groups.
Figure 1. Geometric mean titer of antibodies at various time points after the vaccination from day 0 through month 12. Error bars indicate 95% Confidence intervals (CI). The data of GMTs from day 0 to 56 have been showed in the initial study.Citation20
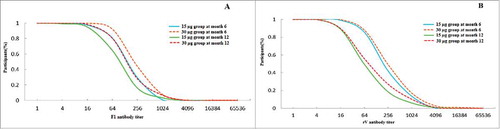
The distributions of anti-F1 antibodies titers are shown in A. The accumulation curves of anti-F1 antibodies showed that over 50% participants in 30 µg group and nearly 40% in 15 µg group with the titers up to 256 at month 6, more than 50% participants in the two groups with the titers over 64. The titers of antibodies in 30 µg group seemed higher than those in 15 µg group at month 6 and 12.
Figure 2. Accumulation curve of antibodies after vaccination at month 6 and 12. Figure A: Accumulation curve of antibodies to anti-F1 after vaccination in 15 and 30 groups at month 6 and 12. Figure B: Accumulation curve of antibodies to anti-rV after vaccination in 15 and 30 groups at month 6 and 12.
For the antibody to anti-F1, seroconversion rates in 15 and 30 µg groups were both 100% at month 6, with the GMT in 30 µg group was increased to 270.33 which was significant higher than that in 15 µg group (P < 0.0001). At month 12, the seroconversion rate slightly reduced to 99.16% (118/119) in 15 µg group, maintained at 100% in 30 µg group. Meanwhile, the GMTs were reduced to 198.80 and 140.10 with significant difference between the two groups (P < 0.001). Although seroconversion rates were similar between the two groups, the GMTs were significantly higher in 30 µg group. ()
Table 1. Immunogenicity of antibodies to anti-F1 and anti-rV after vaccination at month 6 and 12.
The distributions of anti-rV antibodies titers are shown in B. More than 50% participants had titers over 256 in 15 and 30 µg groups at month 6, and approximate 60% participants had titers of 64 in the two groups at month 12. The levels of antibodies in 30 µg group seemed higher than those in 15 µg group at month 6 and 12.
For the antibody to anti-rV, the seroconversion rates were reduced by approximately half (less than 50%) at month 6. The GMTs were 452.50 and 728.50 in 15 and 30 µg groups, respectively. At month 12, the seroconversion rates were 16.81% and 27.12% in the two groups. The GMTs were reduced to 225.60 and 323.50, respectively (). Although seroconversion rates and GMTs were not significantly different between the two groups, the values were numerically higher in 30 µg group.
Safety
From day 56 to month 6, no serious adverse events (SAEs) was observed. Only one participant reported a SAE during month 6 to 12 with chronic uterine myositis, disease of uterine gland and moderate hemorrhagic anemia. The SAE was not related to vaccination.
Discussion
This study is the first report of one-year follow-up for immunogenicity and safety of a recombinant subunit plague vaccine (F1 + rV) in healthy adults aged 18–55 years. Only six studies had been registered in clinicaltrials.gov in terms of plague vaccines and all were completed since 2005. In 2005, a phase 1b one-year study from Pharm Athene UK Limited in the United Kingdom and United States was conducted at three doses of levels to evaluate three different adjuvanted doses of the recombinant plague vaccine (rF1 and rV Antigens). In 2006, a phase 1 clinical trials in United States were completed to estimate the safety, tolerability and immunogenicity of the recombinant plague vaccine rF1V. Then in 2008, a phase 2 trial was completed at the dosage of 80 µg and 160 µg rF1V vaccine, and the immunogenicity observation time was up to day 210 and 540, and the phase 2b trial was completed in 2013 from the same company at the dosage of 80 µg with the immunogenicity observation time of 182 days. However, only three studies published the results of immunogenicity and safety, one of them was our initial study.Citation19–Citation21 None has reported the long-term immunogenicity up to 12 months.
In 2005, Williamson et al showed the result within 90 days by antibody concentration which can't be directly compared with results in our study. In 2017, lower doses of 3, 6 and 10µg of plague vaccine were conducted during a period of 208 days, the peaks of antibody to anti-F1 and anti-rV in the highest group were lower compared to the result in our study. Titers at the final time point were declined to lower levels in the two studies, our results showed more robust immunogenicity. Comparing the three studies, they showed that immune response caused by rV antigens was more intense than F1 antigens, with higher titers of antibody to anti-rV. However, the stability of immunogenicity of F1 antigens was better than rV antigens, the decline of antibody to rV antigens was more early and sharply than F1 antigen. It suggested that rV antigens may have the potential risk to the good immunity durability related with the recombine form in our study, the natural extracted F1 antigen may have no disadvantages in those respects.Citation11,Citation22
Nazarova et al focused on personal immune response to plague vaccine, a proportion of person may respond weakly or remain non-responsive to vaccine. Mutation of genes for pattern recognition receptors (PPRs) and cytokines leads to amino acid substitution that may influence the immune response.Citation23 Seroconversion rates of antibody to anti F1 and anti-rV achieved almost 100% in our study, the decline of immunogenicity seemed have little relation with the effects of genes.
From day 56 to month 6, the antibodies to F1 antigen peaked to higher levels and reduced from month 6 to 12 still with higher GMTs than day 56. The result was interesting that the antibody titer reached to a new peak without the antigenic stimulation from day 56 to month 6. Maybe the timing we observed was inappropriate or uncomprehensive, the real peak of the antibody to anti-F1 was existed in the time between day 56 and month 6. The result reminded that more observed timing should be considered in the future study. The GMTs of antibody to the F1 antigen were significant higher in 30 µg group, suggested that the formulation of 30 µg F1 antigen showed more satisfactorily immunogenicity than 15 µg.
In our study, the GMTs of antibodies to anti-F1 was approximate 1 at baseline, but to anti-rV were approximate 11.00 in the two study groups. This suggested that participants had prior exposure to antigens of Y. pestis, even though this study was conducted in an area non-endemic for plague. Maybe because that the genus Yersinia includes 3 human pathogenic species with significant homology: Yersinia pestis, Yersinia pseudotuberculosis and Yersinia enterocolitica.Citation24 The pathogenicity of these 3 species is dependent on the presence of a 70-kb conserved virulence plasmid with polynucleotide sequence homology ranging from 55 to 90%.Citation24 Yersinia enterocolitica which was related with Yersiniosis and transmitted by an oral-fecal route, have been frequently reported in many countries over the world.Citation25 Thus, this may be explained that antibodies to V antigen may exist prior to vaccination.Citation26 Although GMT and seroconversion rate of antibody to anti-rV have no significant difference between the 15 and 30 µg groups, the values of GMT were about 1.5-2 folds in 30 µg group, the formulation of 30 µg rV antigen showed a little higher immunogenicity.
Limitations of the study included: (1) the serum at day 0, 28, 56 and month 6 and 12 were not tested at the same time and condition. In order to avoid affecting immunogenicity of anti-F1 and anti-rV antibodies with a long-term preservation, the serums were detected as soon as possible. The different testing time could affect the results of immunogenicity. (2) The ELISA kits for F1 and rV antigens of Yersinia pestis developed by the Lanzhou Institute of Biological Products Co., Ltd, maybe those were high affinity to the antibodies to antigens in the vaccine from the same company. However, there were no other ELISA kits available in China. (3) The visit time between day 56 and month 12 was a little less, maybe the reasonable and real variations of antibodies were not showed by the existing data in this study. Even needing more longer-term observation to provide information for development of the new plague vaccine, after all, little result was published.
In conclusion, the novel subunit plague vaccine (F1+rV) was demonstrated desirably safety. the immunogenicity results showed that two doses of F1 antigens had stable immunity which can provide long-term protection at least 12 months, and 30 µg was more satisfactory than 15µg. Two doses of 15 and 30 µg of rV antigen showed short-term but robust protection, the 30 µg of rV antigen has a little stronger immunogenicity than 15 µg. It will be important to explore appropriate formulations of the plague subunit vaccine for the long-term efficacy.
Methods
Study design and participants
The extended study was designed by the Jiangsu Provincial Center for Disease Control and Prevention (JSCDC) and Lanzhou Institute of Biological Products Co., Ltd. This study extended the follow-up period of the initial phase 2a clinical trial up to 12 months, which was approved by the institutional ethics committee of JSCDC and national regulatory agencies and performed in accordance with Good Clinical Practice (GCP) and Good Clinical Laboratory Practice (GCLP) in China (ClinicalTrials.gov: NCT02596308). The initial phase 2a clinical trial was conducted from October to December, 2014, all participants provided the written informed consent prior to enrollment. We enrolled 240 health adults aged 18–55 years whom were administered with 15 or 30 µg vaccines according to a two-dose schedule at day 0 and 28, respectively. After the initial trial, an extra follow-up study was conducted up to month 12. The initial study had been unblinded since day 56, so the extended follow-up was performed as an open study. During the extended follow-up, 5.0 ml blood sample was obtained from each participant at month 6 and 12, respectively. Serum were collected and tested for immunogenicity independently by the National Institute for Food and Drug Control (NIFDC, Beijing, China). Safety observation was proceeded for SAEs throughout the extended study.
Vaccine
The tested vaccine was formulated by mixing natural F1 protein (F1) and recombinant V protein (rV) at two formulations of 15 µg (containing 15 µg F1 and 15 µg rV) and 30 µg (containing 30 µg F1 and 30 µg rV). Each dose contained a volume of 1.0 ml of buffered saline and aluminum adjuvant.
Immunogenicity assessments
Antibodies to F1 and rV antigens were detected by the enzyme-linked immunosorbent assay (ELISA) method. For F1 antibodies, the serum of month 6 and 12 were 10-fold serially diluted and then measured with ELISA kit. For antibodies to rV, the serum was serially diluted with a threshold of 80-fold, then measured with plates coated with rV antigen. The kits and plates were all developed by Lanzhou Institute of Biological Products Co., Ltd. Seroconvesion for anti-F1 antibody was defined as the titer of antibody after vaccination was at least 4 folds increased compared to that of the preimmunization. Seroconvesion for anti-rV antibody was defined as titers of antibodies at least 1:320 in initially seronegative participants (rV antibody <1:80) and as at least 4 folds increased in those with positive antibody at baseline. Titer determination: the serum titer is designated as the maximum diluted multiples for the positive result of the tested samples.
Safety assessments
During the whole study period, any SAE reported by the participants were collected. The occurrence of SAEs in all the vaccinated population was assessed. The relationship between SAEs and vaccination was judged by the principal investigators.
Statistical analysis
Immunogenicity analysis was conducted in the per-protocol set (PPS), which consisted of the participants who received two doses vaccination and donated blood samples at day 0, 28, 56, month 6 and 12 during the protocol-specified time frames.
Statistical comparisons were made using two-sided tests with an alpha (α) value of 0.05. GMT were summarized with 95% confidence intervals (CIs) and compared by the Student's t test or t’ test, respectively. Comparisons of seroconversion rate was conducted by Chi-square test or Fisher's exact test. The statistical analyses were performed by an independent statistician using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA).
Abbreviations
| CI | = | confidence interval |
| ELISA | = | enzyme-linked immunosorbent assay |
| F1 | = | envelope antigen faction 1 |
| GMT | = | geometric mean titer |
| NIFDC | = | National Institute for Food and Drug Control |
| PPR | = | pattern recognition receptor |
| rV | = | recombinant virulence antigen |
| SAE | = | serious adverse event |
| Y. pestis | = | Yersinia pestis |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
F-Y Meng, Y-M Hu, K Chu, F-C Zhu, L Jiao, Z-Y Wu and B-X Wang contributed to conception and design of the study; Y-M Hu and K Chu coordinated the clinical aspects of the study; F-Y Meng contributed to collecting data and managing participants; W Dong contributed to testing of antibody; T-S Li analyzed the data; F-Y Meng, J-L Hu, Y-M Hu, K Chu, and J-X Li wrote the paper; All authors read and approved the final manuscript.
Trial registration
ClinicalTrials.gov: NCT02596308
Acknowledgments
The authors would like to thank the volunteers who participated in this extended follow-up study, and also thank all the investigators who were responsible for collecting data and managing participants.
Additional information
Funding
References
- Prentice MB, Rahalison L. Plague. Lancet 2007;369:1196–207. doi:10.1016/S0140-6736(07)60566-2.
- Williamson ED. Plague. Vaccine 2009;27:D56–60. doi:10.1016/j.vaccine.2009.07.068.
- Butler T. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. The American journal of tropical medicine and hygiene 2013;89:788–93. doi:10.4269/ajtmh.13-0191.
- World Health Organization (WHO) Plague.Democratic Republic of the Congo. http://www.who.int/mediacentre/factsheets/fs267/en/
- Galimand M, Carniel E, Courvalin P. Resistance of Yersinia pestis to antimicrobial agents. Antimicrobial agents and chemotherapy 2006;50:3233–6. doi:10.1128/AAC.00306-06.
- Welch TJ, Fricke WF, McDermott PF, White DG, Rosso ML, Rasko DA, et al. Multiple antimicrobial resistance in plague: an emerging public health risk. PloS one 2007;2:e309. doi:10.1371/journal.pone.0000309.
- Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, et al.. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283:2281–90.
- Titball RW, Williamson ED. Yersinia pestis (plague) vaccines. Expert opinion on biological therapy 2004;4:965–73. doi:10.1517/14712598.4.6.965.
- Feodorova VA, Corbel MJ. Prospects for new plague vaccines. Expert review of vaccines 2009;8:1721–38. doi:10.1586/erv.09.129.
- Sha J, Kirtley ML, Klages C, Erova TE, Telepnev M, Ponnusamy D, Fitts EC, Baze WB, Sivasubramani SK, Lawrence WS. A Replication-Defective Human Type 5 Adenovirus-Based Trivalent Vaccine Confers Complete Protection against Plague in Mice and Nonhuman Primates. Clinical and vaccine immunology 2016;23:586–600. doi:10.1128/CVI.00150-16.
- Liu L, Wei D, Qu Z, Sun L, Miao Y, Yang Y, Lu J, Du W, Wang B, Li B. A safety and immunogenicity study of a novel subunit plague vaccine in cynomolgus macaques. Journal of applied toxicology 2017;14:1–10.
- Arnaboldi PM, Sambir M, D'Arco C, Peters LA, Seegers JF, Mayer L, McCormick AA, Dattwyler RJ. Intranasal delivery of a protein subunit vaccine using a Tobacco Mosaic Virus platform protects against pneumonic plague. Vaccine 2016;34:5768–76. doi:10.1016/j.vaccine.2016.09.063.
- Du Y, Rosqvist R, Forsberg A. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infection and immunity 2002;70:1453–60. doi:10.1128/IAI.70.3.1453-1460.2002.
- Sebbane F, Jarrett C, Gardner D, Long D, Hinnebusch BJ. The Yersinia pestis caf1M1A1 fimbrial capsule operon promotes transmission by flea bite in a mouse model of bubonic plague. Infection and immunity 2009;77:1222–9. doi:10.1128/IAI.00950-08.
- Weening EH, Cathelyn JS, Kaufman G, Lawrenz MB, Price P, Goldman WE, Miller VL. The dependence of the Yersinia pestis capsule on pathogenesis is influenced by the mouse background. Infection and immunity 2011;79:644–52. doi:10.1128/IAI.00981-10.
- Anderson DM, Ciletti NA, Lee-Lewis H, Elli D, Segal J, DeBord KL, Overheim KA, Tretiakova M, Brubaker RR, and Schneewind O. Pneumonic plague pathogenesis and immunity in Brown Norway rats. The American journal of pathology 2009;174:910–21. doi:10.2353/ajpath.2009.071168.
- Qiu Y, Liu Y, Qi Z, Wang W, Kou Z, Zhang Q, Liu G, Liu T, Yang Y, Yang X. Comparison of immunological responses of plague vaccines F1+rV270 and EV76 in Chinese-origin rhesus macaque, Macaca mulatta. Scandinavian journal of immunology 2010;72:425–33. doi:10.1111/j.1365-3083.2010.02456.x.
- Chichester JA, Musiychuk K, Farrance CE, Mett V, Lyons J, Yusibov V. A single component two-valent LcrV-F1 vaccine protects non-human primates against pneumonic plague. Vaccine 2009;27:3471–4. doi:10.1016/j.vaccine.2009.01.050.
- Williamson ED, Flick-Smith HC, Lebutt C, Rowland CA, Jones SM, Waters EL, Gwyther RJ, Miller J, Packer PJ, and Irving M. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infection and immunity 2005;73:3598–608. doi:10.1128/IAI.73.6.3598-3608.2005.
- Chu K, Hu J, Meng F, Li J, Luo L, Xu J, Yuan Z, Li Z, Chen W, Jiao L, et al. Immunogenicity and safety of subunit plague vaccine: A randomized phase 2a clinical trial. Human vaccines & immunotherapeutics 2016;12:2334–40. doi:10.1080/21645515.2016.1175261.
- Frey SE, Lottenbach K, Graham I, Anderson E, Bajwa K, May RC, Mizel SB, Graff A, Belshe RB. A phase I safety and immunogenicity dose escalation trial of plague vaccine, Flagellin/F1/V, in healthy adult volunteers (DMID 08–0066). Vaccine 2017;35:6759–65. doi:10.1016/j.vaccine.2017.09.070.
- Chalton DA, Musson JA, Flick-Smith H, Walker N, µgregor A, Lamb HK, Williamson ED, Miller J, Robinson JH, Lakey JH. Immunogenicity of a Yersinia pestis vaccine antigen monomerized by circular permutation. Infection and immunity 2006;74:6624–31. doi:10.1128/IAI.00437-06.
- Nazarova EL., Dyatlov IA, Pozdeev NM, Demyanova VT, Paramonov IV, Rylov AV, Khramov MV, Borzilov AI, Somov AN, Ablamunits VG, AnisimovGeneticmarkersofimmuneresponsetoYersiniapestisF1andVantigens-loadedmicrospheresvaccineagainstplague. Russian Biomedical research 2017;2:19–28.
- Roggenkamp A, Geiger AM, Leitritz L, Kessler A, Heesemann J. Passive immunity to infection with Yersinia spp. mediated by antirecombinant V antigen is dependent on polymorphism of V antigen. Infect Immun 1997 65:446–51.
- Ranjbar R, Afshar D. Development of a loop-mediated isothermal amplification assay for rapid detection of Yersinia enterocolitica via targeting a conserved locus. Iran J Microbiol 2015;7:185–90.
- Tomaso H, Mooseder G, Al Dahouk S, Bartling C, Scholz HC, Strauss R, Treu TM, Neubauer H. Seroprevalence of anti-Yersinia antibodies in healthy Austrians. Eur J Epidemiol 2006; 21:77–81. doi:10.1007/s10654-005-5047-z.
