?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A cross-sectional study for poliovirus seroprevalence in Guangdong was carried out in 2014, just before a change in polio vaccine commenced in 2015. The aim of the study was to test whether polio immunity level was high enough to satisfy the polio vaccine switch. A total of 6339 people were tested for poliovirus neutralization antibodies (NA). Overall NA seropositivity for PV1, PV2 and PV3 were 95.2%, 94.9% and 88.7%, and the respective geometric mean titer (GMT) were 82.9, 55.8, and 26.3, respectively. There were statistically significant differences in the positive rates and GMT of the 3 serotypes and PV3 had relatively lower positive rates and GMT. The highest seropositivity and GMT were observed in the 1–9 year-old age group. The positive rates of NA and GMT for PV1, PV2 and PV3 in the western region of Guangdong were lower than those of other three regions. The results of this study showed that the population of Guangdong province had a high polio immunity level, a stable base for a polio vaccine switch.
KEYWORDS:
Introduction
Poliomyelitis (hereinafter referred to as polio) is a type of intestinal infectious disease caused by poliovirus (hereinafter referred to as PV). PVs are subdivided in three serotypes: poliovirus type1, 2 and 3 (hereinafter referred to as PV1, 2, 3). Since the global polio eradication initiative was launched in 1988, the number of polio cases has declined rapidly from over 350,000 cases in 1988 to 73 in 2015 and the list of polio endemic countries now includes only Pakistan and Afghanistan. Of the three types of wild PVs, the last case of type 2 was reported in 1999 and its eradication was declared in September 2015 and the most recent case of type 3 dates to November 2012.Citation1 Besides wild poliovirus (hereinafter referred to as WPV), the attenuated viruses in live OPV (Oral polio vaccine), which had been incorporated in EPI in most countries, may become vaccine-derived PV (hereinafter referred to as VDPV) that can cause paralytic poliomyelitis.Citation2,Citation3,Citation4 During 2011–2015, 230 VDPVs were reported worldwide.Citation1 To eradicate polio, WHO proposed the Polio Eradication and Endgame Strategic Plan 2013–2018.Citation5 In light of this plan, as eradication wild PV2, certified in 2015, trivalent OPV was substituted by bivalent OPV synchronously globally on May 1st 2016 and at least 1 dose of IPV (Inactivated polio vaccine) was introduced.
Since the surveillance of infectious diseases began in 1956, there has been a high rate of polio incidence in Guangdong province, with the highest morbidity of 11.76/100,000 in 1963. Oral attenuated polio vaccine (hereinafter referred to as OPV) was first used in the 1960s and was included into the expanded immunization program (EPI) in 1978. Along with the gradual improvement of the cold chain system in the 1980s, the vaccination coverage for polio increased significantly and the morbidity rate dropped rapidly, resulting in an annual polio morbidity of only 0.17-0.43/100,000 in 1989–1993. To eliminate polio, intensified large-scale supplementary immunization activities were carried out in the 1990s. No polio case has been reported in Guangdong since 1994 and China was certified polio free by the World Health Organization in 2000.Citation6
China is close to Pakistan and Afghanistan where polio is still endemic and Guangdong province has frequent economic cooperation with foreign countries. Although no WPV case was reported for 22 years in Guangdong, its population is still at risk of infection from imported WPV or vaccine-derived PV, as reported in Africa,Citation7 Europe,Citation8,Citation9 Xinjiang Uygur Autonomous Region in ChinaCitation10 and Amish community in USA.Citation11 Therefore, a polio NA seroprevalence survey is considered necessary to identify population-categories at risk of infection of polio, including WPV and VDPV, allowing for targeting of susceptible area.
The oral attenuated poliovirus live vaccine (OPV) was the only EPI vaccine in Guangdong before the polio vaccine switch. Its schedule includes vaccine at 2, 3, 4 months and 4 years old. Inactivated polio vaccine (IPV) was first introduced in 2009 as as a type 2 (parent-pay) vaccine. In 2010–2014, vaccinated doses of IPV accounted for only 7.1% of all polio vaccine doses in Guangdong. In 2015, three cities incorporated a single dose of IPV into the EPI and since May 1st 2016, the whole province adopted an IPV-bOPV-bOPV-bOPV series in its EPI. NA seroprevalence of 2014 can be compared with that after the switch, providing a basis of efficacy comparison between different polio immunization schedules. Therefore, we carried out this cross-sectional hospital-based study to evaluate the NA seroprevalence to PV among broad age groups in Guangdong province of China in 2014.
Results
Characteristics of the study population
A total of 6394 people were enrolled and stratified into four age groups(0 year, 1 – 9 years, 10–39 years, 40 years above) and four regional groups (Pearl River delta region, east of Guangdong, west of Guangdong and north of Guangdong). Among the recruited individuals, 3392 were male and 3002 were female, with a sex ratio of 1.13:1.
Distribution of PV antibody levels by age
The NA positive rates for PV1, PV2 and PV3 were 95.2%, 94.9% and 88.7% respectively. 84.5% of subjects were NA positive for all 3 serotypes and 1.1% were all NA negative. There were significant differences in the positive rates of the 3 serotypes (p < 0.01), among which there were significant differences between the positive rates of PV1 and PV3, as well as PV2 and PV3 antibodies (p < 0.01). GMT for PV1, PV2 and PV3 were 82.9, 55.8, 26.3 respectively. The GMT differences between PV1 and PV2, PV1 and PV3, PV2 and PV3 were statistically significant (p < 0. 01).
The positive rates of NA for PV1, PV2 and PV3 were 93.2%-97.3%, 92.9%-97.2% 82.5%-93.2% in all age groups. There were significant differences in the positive rates of NA in different age groups (p < 0.01). The positive rate for PV1, PV2 and PV3 in the 1–9 years age group were higher than other three groups (p < 0. 01). The differences in positive rate for PV3 between the four age groups were statistically significant (p < 0. 05), among which the 1–9 years age group was the highest. GMT for PV1, PV2 and PV3 of the 0 years group and 1–9 years age group were higher than the 10–39 years age group and the 40 years and above group (p < 0.01) ( and ).
Distribution of PV antibody levels by location
The subjects were divided into four groups geographically: the Pearl River Delta, east Guangdong, west Guangdong and north Guangdong. The positive NA rates for PV1, PV2 and PV3 in west Guangdong were lower than those of the other three regions (p < 0.01). NA GMT for PV1 and PV2 for west Guangdong were lower than those of the other three regions (p < 0. 05). NA GMT for PV3 for west Guangdong were lower than those of Pearl River Delta and north Guangdong (p <0.05, p <0.01) ( and ).
Distribution of PV antibody levels by gender
There was no statistically significant difference in NA positive rates between genders, however, the NA GMT for PV1 and PV2 of males were higher than for females (p < 0.01) ( and ).
Discussion
In our study, overall seropositivity for PV1, PV2 and PV3 were 95.2%, 94.9% and 88.7%, and the respective GMT are 82.9, 55.8, 26.3. We compared the results with that of a previous study conducted in Guangdong in 2010,Citation12 finding similar general conclusions suggesting a sustained high seroprevalence and GMT in Guangdong.
The highest seropositivity and GMT were observed in the 1–9 years age group. This is likely attributable to the EPI, in which children receive a 3-dose primary vaccination series across 2, 3 and 4 months of age. It has been reported that the seropositivity and GMT of newborn infants and 2 month old infants were much lower than those of the pregnant women.Citation13,Citation14 In our study, a proportion of subjects aged below 1 years were not immunized or were tested not long after vaccination, which may explain the relatively lower seropositivity and GMT in this group. For the older subjects (10 years and above), seropositivity and GMT are lower than those of the 1–9 years age group, indicating immunity to PV wanes with time, which has been reported by other studies.Citation15,Citation16 While seroconversion is a reliable correlate of immunity against paralytic disease, there is no evidence that loss of detectable antibody puts immunocompetent individuals at risk of paralytic disease.Citation1
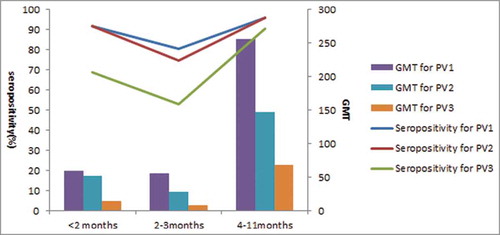
Our findings show that seroprevalence and GMT of PV3 was lower than PV1 and PV2. This phenomenon is also reported by other studies.Citation17 Researchers speculate that this may be attributed to lower immunogenicity of PV3 compared to PV1and PV2.Citation18 Our findings further confirm this. In our study, for subjects <2months old seropositivity of PV1, PV2 and PV3 were 91.9%, 91.9%, 68.7%; for subjects 2–3 months old they were 80.4%, 74.5%, 52.9% and for subjects 4–11 months old they were 95.8%, 96.1%, 90.6% (). PV3 seropositivity and GMT of new-born babies were quite low and this low level was sustained until 4 months old, when babies are often immunized with 2 doses of polio vaccine. But PV3 NA seropositivity and GMT decreased rapidly with increasing age, and dropped to 85% and 16.8 in GMT in the 10–39 years age group, which can also be observed in surveys done in Henan, Jiangsu ect.al.Citation19,Citation20 As PV GMT of neonates are correlated with that of their mothers,Citation12 low seropositivity in fertile women lead to low seropositivity in neonates. Therefore, we conclude that low seroprevalence of PV3 in population is not only from the low immunogenicity of PV3, but also attributable to the low sustainability of PV3.
As to the regional divergence differences in of polio NA seropositivity and GMT, our study shows that individuals in west Guangdong are at a relatively higher risk of WPV or VDPV, indicating vigilance remains essential in this area. For example, supplementary immunization campaigns and improvement of routine immunization and AFP surveillance may be needed most in this region of Guangdong.
External validity of our research is good as samples are taken from all province's 21 cities, with proportionate age groups sampled and a sufficient sample size to detect a statistically significant change. For the large sample size and balanced distribution, the results of the study can not only represent the polio immunity level of the whole province, but also reflects that of each city. Though samples were analyzed in provincial or prefecture laboratories, the results have high reliability because NA detection methods used are internationally accepted as “gold standard”. Further, staff were trained uniformly and reagent was sourced through a unified procurement batch.
Our study has some additional limitations. The sample population investigated in our study were from hospital outpatients, therefore the results may not be truly representative of the general population. Notwithstanding, we consider this bias will not be large because there have been no wild polio cases since 1994 in Guangdong Province and the polio immunity level mostly relates to vaccination and there is no significant differences in vaccination rates between outpatients and the general population.
Overall, the results of this study showed that the population of Guangdong province had a high polio immunity level implying a stable basis for a polio vaccine switch.
Methods
Study Design
Specimen collection and laboratory testing
In 2014, we conducted a serologic survey in all 21 prefectures throughout Guangdong province. We used multi-stage random sampling. Three county/districts in each prefecture were randomly selected and a general hospital and 2 township hospitals/community health service centers were randomly selected from each county/district. Patients who were admitted to selected health facilities for a blood draw were invited to participate. Individuals were excluded if they had a known immunodeficiency or had been treated with immunosuppressant drugs during the previous 12 months. On enrolment, personal information (sex, age and region) was collected, but the immunization history was unavailable. The main purpose of this study was to estimate the seroprevalence rate of Polio NA. Sample size was calculated based on Formula 1. According to a previous serosurvey of polio in 2010 in Guangdong of China,Citation12 PV3 had the lowest positive rate (90%) among all 3 types and hence 90% was used as the estimated seroprevalence rate p. δ was the permissible error (0.8%) and α was the significant level (5%).
From the formulation, we calculated the minimum sample size is 5403. To account for withdrawal or missing data (13%), the total sample size was finally set to 6300. An 2ml blood sample was collected from each subject by venipuncture and kept in a sterile serum tube. Samples were immediately placed in an ice box and transported to the local laboratory. After centrifugation of blood, the serum was separated and then stored at −20°C. 11 of the prefecture have laboratories capable of doing the testing and the remaining samples were transported to a polio laboratory at the Guangdong provincial Center for Disease Control and Prevention (GDCDC) for testing. Besides, a common internal standard that is used for quantifying the result in each laboratory. All reagents were purchased by the provincial polio laboratory and then distributed. Neutralization antibodies (NA) against P1, P2, and P3 were determined by a standard microneutralization assay (MNA) in accordance with WHO guidelines.Citation21 In brief, before testing each serum sample was inactivated at 56°C for 30 minutes, and then diluted from 1:4 to 1:1,024 in two-fold serial dilutions. Each sample was incubated in duplicate wells for 3 hours at 36°C with 100 50% tissue culture infective doses (TCID50) of poliovirus antigen. After incubation for 7 days, the highest dilution of serum that protected 50% of the cultures was recorded. Serum with a titer of more than 1:4 was considered positive. Cell controls and a reference serum were included in each test to examine reproducibility of results.
Ethical approval and informed consent
Approval to conduct this study was granted by the relevant departments of GDCDC. All information was collected after the permission of the participants. Verbal informed consent was sought from each participant. Investigators explained to participants that their information would be only used to estimate the seroprevalence of polio NA.
Data Analysis
Statistical tests were performed using SPSS 19.0 software. Seropositivity rates and geometric mean titer (GMT) of antibody was calculated for each group. Chi-square test was used to determine the association among demographic characteristics (sex, age, and region) and seropositivity. GMT was logarithmically transformed and analysis of variance was done to compare the difference among groups with different demographic characteristics. The Bonferroni test was used to compare the difference between each group. Differences were regarded significant with p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the staff of the 21 prefectural CDCs of Guangdong for conducting volunteer enrolment and specimen collection. We salso thank the staff of 11 prefecture laboratories for conducting the respective testing.
Funding
This work was supported by Guangdong Medical Science and Technology Research Foundation: Study on the immune effect of different Vaccine Immunization Strategies in Polio Vaccine Transformation stage (Grant No. A2017028).
Table 1. NA positive rates for different ages, regions and genders.
Table 2. NA GMT for different ages, regions and genders.
References
- World Health Organization. Polio vaccine: WHO position paper-March 2016.
- Estívariz CF, Watkins MA, Handoko D, Rusipah R, Deshpande J, Rana BJ, et al. A large vaccine-derived poliovirus outbreak on Madura Island–Indonesia. J Inf Dis. 2005;197:347–54. doi:10.1086/525049.
- Jenkins HE, Aylward RB, Gasasira A, Donnelly CA, Mwanza M, Corander J, Garnier S, Chauvin C, Abanida E, Pate MA, et al. Implicastions of a circulating vaccine-derived poliovirus in Nigeria. N Eng J Med. 2010;362:2360–9. doi:10.1056/NEJMoa0910074.
- Duintjer Tebbens RJ, Pallansch MA, Kim JH, Burns CC, Kew OM, Oberste MS, Wassilak SG, Cochi SL, Thompson KM. Oral poliovirus vaccine evolution and insights relevant to modeling the risks of circulating vaccine-derived polioviruses (cVDPVs). Risk Anal. 2013;23(4):680–702. doi:10.1111/risa.12022.
- World Health Organization. 2013. Polio Eradication and Endgame Strategic Plan 2013–2018.
- Zheng H-Z, Deng H-H. Practice of immunization program in Guangdong province; 2011.
- CDC. Wild poliovirus type 1 and type 3 importations—15 countries, Africa, 2008–2009. MMWR Morb Mortal Wkly Rep. 2009 Apr 17;58(14):357–62.
- CDC. Outbreaks following wild poliovirus importations—Europe, Africa, and Asia, January 2009-September 2010. MMWR Morb Mortal Wkly Rep. 2010 Nov 5;59(43):1393–9.
- Yakovenko ML, Gmyl AP, Ivanova OE, Eremeeva TP, Ivanov AP, Prostova MA, Baykova OY, Isaeva OV, Lipskaya GY, Shakaryan AK, et al. The 2010 outbreak of poliomyelitis in Tajikistan: epidemiology and lessons learnt. Euro Surveill. 2014 Feb 20;19(7):20706. doi:10.2807/1560-7917.ES2014.19.7.20706.
- World Health Organization. Progress toward interruption of wild poliovirus transmission-worldwide, 2008. Morb Mortal Wkly Rep. 2009;58(12):308–12.
- Alexander JP, Ehresmann K, Seward J, Wax G, Harriman K, Fuller S, et al. Transmission of imported vaccine-derived poliovirus in an under vaccinated community in minnesota. J Inf Dis. 2009;199(3):391–7. doi:10.1086/596052.
- Guo X, Liu L, Deng W, Xu Y, Zheng H-Y, Zhu S-Y, et al. Analysis of Poliomyelitis neutralizing antibody level in healthy population in Guangdong Province in 2010. Chinese Journal of Vaccines and Immunization. 2013;19(1):27–30.
- You E-K, Yang Q-Z, Yang Q-L. Serum epidemiological study on the vertical distribution of polio neutralizing antibodies. Chinese Journal of epidemiology. 1994;15(3):158–61.
- Hu F-J, Zhang S-M, Lou L-B. Check and measure of poliomyelitis antibody in newborn babies. Chinese Journal of Expanded Immunization programme. 2000;6(2):75–76.
- Li Xiaomei, Zhang Herun, Wang Yumei, Liu Donglei, Zou Yanjie, Wang Dongmei. Analysis of polio antibody levels in healthy population in Beijing in 2007. Chinese Journal of Vaccines and Immunization. 2009;15(3):245–8.
- Gamage D, Palihawadana P, Mach O, Weldon WC, Oberste SM, Sutter RW. Achieving high seroprevalence against polioviruses in Sri Lanka-Results from a serological survey, 2014. J Epidemiol Glob Health. 2015 Dec;5(4 Suppl 1):S67–71. doi:10.1016/j.jegh.2015.06.004.
- Claudia R, Imke F, Holger F, Hans WD. Deficiency of immunity to poliovirus type 3:a lurking danger? BMC Infectious Diseases. 2012;12(1):12–24.
- Roivainen M, Montagnon B, Chalumeau H, Murray M, Wimmer E, Hovi T. Improved distribution of antigenic site specificity of poliovirusneutralizing antibodies induced by a protease-cleaved immunogen in mice. J Virol 1990;64(2):559–62.
- Chang Z, Feng D-X, Xu J, Feng Z-J, Chen M-G. Surveillance of Immune Level of Poliomyel itis in Healthy Population of Henan Province in 1998 Henan Provincial Epidemic Prevent ion Station, Zhengzhou 450003, China.
- Wu Y, Leng H-Y, Ye X, Hu Y, Deng X-Y, Lu P-S. Analysis of polio antibody levels in healthy population in Jiangsu province in 2011. Chinese Primary Health Care. 2012;26(5):76–77.
- World Health Organization. Manual for the virological investigation of polio. WHO. 1997 EPI/GEN/97.01.EPI. World Health Organization, Geneva, Switzerland.