ABSTRACT
We aimed to determine the efficacy of the 10-valent pneumococcal-Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in children aged 18-months to <18-years with recurrent protracted bacterial bronchitis (rPBB), chronic suppurative lung disease (CSLD) or bronchiectasis. In a multi-centre, double-blind randomised controlled trial, children received two doses, 2-months apart of the 10vPHiD-CV or quadrivalent meningococcal-ACYW135 conjugate vaccine. Active surveillance for acute exacerbations, respiratory symptoms and antibiotic use was undertaken through to 12-months after the second vaccine dose (clinical cohort only). Serum, saliva and nasopharyngeal swabs were collected to measure immunological and microbiological effects (immunology cohort). Between December 2012 and August 2015, 62 children were enrolled onto the clinical protocol (1 excluded from clinical analyses due to unblinding), while 74 contributed to the immunology cohort (overall mean age = 6.8-years (standard deviation = 3.7), 42 (56.8%) male). The absolute risk difference comparing the 10vPHiD-CV group (n = 31 children) to the MenACYW135 group (n = 30 children) for acute exacerbations was -0.5 exacerbations/100-weeks at risk (95% confidence interval (CI)
-2.0, 0.9). Compared to the MenACYW135 group, children who received the 10vPHiD-CV were less likely to have respiratory symptoms in each fortnight of surveillance (incidence density ratio (IDR) 0.82, 95%CI 0.61, 1.10) and required fewer short-course (<14-days duration) antibiotics (IDR 0.81, 95% CI 0.61, 1.09). The vaccine was immunogenic and no serious adverse events related to the vaccine were reported. In conclusion, 10vPHiD-CV might have a future role in managing children with rPBB, CSLD and bronchiectasis, but larger multicentre trials are needed to confirm or refute findings from this preliminary study.
Background
Persistent wet cough, a key feature in a group of paediatric chronic pulmonary disorders, is increasingly appreciated because of the burden it causes to the child, family and healthcare sector.Citation1,Citation2 These disorders, dominated by protracted bacterial bronchitis (PBB), chronic suppurative lung disease (CSLD) and bronchiectasis (Box 1), have in common impaired mucociliary clearance and lower airway bacterial infection and inflammation.Citation3,Citation4
Haemophilus influenzae (Hi) (usually unencapsulated or non-typeable (NTHi) strains) is consistently the most common bacterial pathogen isolated from the lower airways of children with PBB, CSLD or bronchiectasis, followed less often by Streptococcus pneumoniae (pneumococcus) and Moraxella catarrhalis.Citation5,Citation6 Despite childhood immunisation with pneumococcal and encapsulated Hi type b conjugate vaccines, these chronic disorders are commonly seen in paediatric respiratory practice.Citation5 Acute exacerbations are especially important as not only are they are associated with lower quality of life scores,Citation1 but the frequency of severe episodes leading to hospitalisation predicts pulmonary decline in children with bronchiectasis.Citation7 In PBB, recurrent exacerbations (>3/year, termed rPBB) and lower airway Hi infection are associated with future bronchiectasis.Citation3
Interventions targeting Hi more broadly may be efficacious in reducing exacerbations and improving clinical outcomes. Currently, the only licensed vaccine that may impact upon Hi is the 10-valent pneumococcal-H. influenzae protein D conjugate vaccine (PHiD-CV; Synflorix®, GlaxoSmithKline Biologicals, Belgium). The protein D (PD) component is an outer membrane lipoprotein, which is antigenically conserved, surface located and present in most Hi (encapsulated and NTHi) strains.Citation8 It is one of three Hi surface proteins (PD, P6 and OMP26) identified as potential vaccine antigens,Citation9 with PD and P6 showing the most promise. Vaccine-induced anti-PD antibodies are associated with protective efficacy against Hi infection in middle ear and pulmonary clearance in rat disease models.Citation10 To date, no studies have evaluated PD-based vaccines in children with rPBB, CSLD or bronchiectasis.
We aimed to determine the clinical efficacy, immune response, impact on nasopharyngeal carriage of Hi and safety of two doses of PHiD-CV in children with rPBB, CSLD or bronchiectasis. We tested the primary hypothesis that amongst children aged 18-months to <18-years with rPBB, CSLD or bronchiectasis, PHiD-CV reduces the incidence of respiratory exacerbations in the 12-months following two doses of vaccine compared to children who received a control conjugate vaccine (quadrivalent meningococcal ACYW135, MenACYW135; Menactra®, Sanofi Pasteur, Lyon).
Results
Study cohort
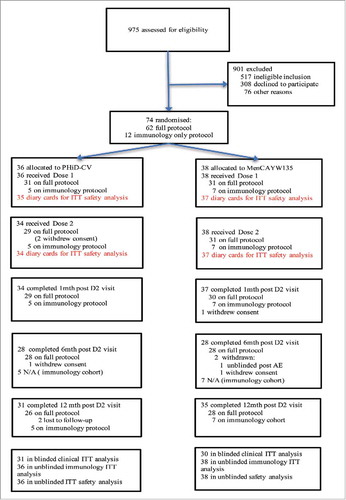
Between December 2012 and August 2015, 975 children were screened and 75 enrolled (63 onto the full clinical protocol and 12 onto the immunology only protocol) (). One child was a screen failure (clinical protocol) and thus 74 children were randomised. A participant on the clinical protocol was unblinded following dose-2 at the request of the ethics committee and following discussion with the Data Safety Monitoring Committee (DSMC). This was after a severe local reaction to the 23-valent pneumococcal polysaccharide vaccine (PPV23), which was deemed unrelated to the study. However, this child was not included in the clinical analyses. Six children were withdrawn from the study at parental request due to family commitments over the 14-months of the study and two were lost to follow-up ().
The mean age of the 74 children was 6.8 (standard deviation 3.7) years, 56.8% were male and at baseline the overall characteristics between the PHiD-CV and control vaccine groups
() were similar. Importantly, baseline prior pneumococcal vaccination history was comparable between the groups, in particular the proportion of those who had had at least three doses of a pneumococcal conjugate vaccine (PCV) and at least 1 dose of PPV23, as well as the median time in months since the last dose of either vaccine ().
Table 1. Baseline characteristics of children enrolled by vaccine group.
Clinical outcomes
Amongst the 61 children included in the clinical analyses, the overall incidence density of acute exacerbations following dose 2 was 4.4 episodes per 100 child-weeks at risk (95% confidence interval (CI) 3.7, 5.2); the incidence density ratio (IDR) between the groups was 0.92 (95%CI 0.68, 1.32) (). Compared with MenACYW135, children receiving PHiD-CV had lower incidence rates for fortnights with respiratory symptoms (IDR 0.82, 95%CI 0.61, 1.10) and antibiotic short courses (ie. <14-days duration) (IDR 0.81, 95%CI 0.61, 1.09) ().
Table 2. Clinical endpoints by vaccine group.
To assess whether clinical findings may be confounded by use of azithromycin, we compared number and duration of azithromycin courses between the two groups. Overall, 55 courses were administered to 26/61 (42.6%) children during the study; 29 in 12/31 (38.7%) children in the PHiD-CV group and 26 in 14/30 (46.7%) children in the MenACYW135 group. There was no difference in the median duration of azithromycin courses between the two groups (PHiD-CV = 13.5-days; MenACYW135 = 14-days). The proportion of long-term (>3-months) azithromycin use was also similar between groups (PHiD-CV = 8/29, 27.6% and MenACYW135 group = 5/26,19.2%).
Data on hospitalised exacerbations were available for all 74 children; overall eight episodes were reported (1 in PHiD-CV group, 7 in MenACYW135 group). All episodes occurred post-dose 2. The IDR for hospitalised exacerbations in the PHiD-CV group compared to controls was 0.15 (95%CI 0.01–0.95) across the entire period of follow-up and 0.16 (95%CI 0.01–1.02) for the period at risk post-dose 2. The single event in the PHiD-CV group occurred in a child with severe cerebral palsy; three of the seven events in the control group occurred in one child who also had severe cerebral palsy.
Immunological outcomes
The complete dataset for saliva and serum antibody titres at each study time-point by vaccine group are presented in Supplementary Tables 1–5.
Salivary antibodies to H. influenzae and pneumococcal proteins (Supp Table 1).
At baseline, there were no between-group differences in anti-PD saliva antibodies (p = 0.238; Supp Table 1). However, between-group differences were observed for IgG antibodies to Hi proteins P6 IgG and P26 IgG and for IgG antibodies to pneumococcal proteins PspA2 IgG and Ply IgG with higher titres observed in the MenACYW135 vaccine group for all four proteins at baseline.
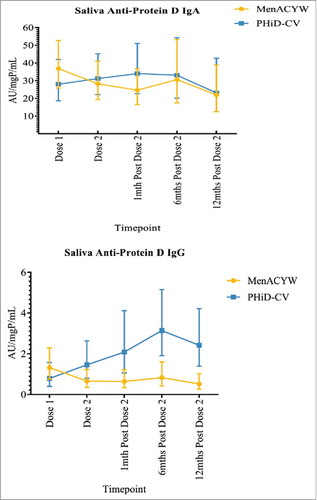
While no anti-PD saliva IgA response was observed at any time-point in the PHiD-CV group, anti-PD saliva IgG increased following both doses of vaccine and significant differences between the PHiD-CV and MenACYW135 groups were sustained to 12-months following the second dose (). In contrast, no saliva IgA or IgG responses were observed to Hi proteins P4, P6 and P26 or to any of the pneumococcal proteins.
Figure 2. Saliva anti-protein D IgA and IgG geometric mean antibody units per milligram of protein per millilitre (AU/mgP/mL), with corresponding 95% confidence intervals, by vaccine group and study time-point.
Salivary antibodies to pneumococcal polysaccharides (Supp Table 2).
At baseline significant between-group differences were observed for saliva anti-pneumococcal polysaccharide IgG antibodies to serotypes 4, 5 and 7F with higher levels present in the MenACYW135 group. High-levels of 19A and 19F IgA and IgG antibodies were observed in both groups relative to the other serotypes. Increased saliva IgA and IgG concentrations occurred in all PHID-CV vaccine type anti-pneumococcal polysaccharide antibodies in the PHiD-CV group, but not in the MenACYW135 group. There were no saliva antibody responses to the two non-PHiD-CV pneumococcal serotypes, 3 and 19A. Although a persistent response was achieved for several serotypes, the difference between the groups was no longer significant at the 6-months post-dose 2 time-point for serotypes 5, 6B, 9V, 19F and 23F for IgA, and 6B and 23F IgG antibodies respectively. Similarly, at
12-months post-dose 2, there were no differences between groups for serotypes 1, 5, 6B, 7F, 9V, 19F and 23F for IgA, and 6B, 9V and 23F IgG antibodies respectively.
Serum antibodies to H. influenzae and pneumococcal proteins (Supp Table 3).
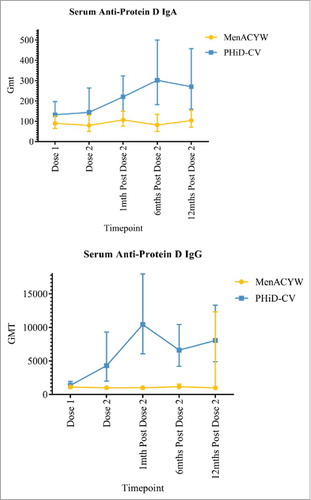
There were no baseline between-group differences in Hi and pneumococcal serum antibody geometric meant tires (GMT; Supp Table 3). Increases in serum anti-PD IgA were observed in the PHiD-CV group following the second dose and serum anti-PD IgG increased following the first dose (). GMTs for anti-PD IgA and IgG antibodies were higher in the PHiD-CV than MenACYW135 group at the 12-month post dose 2 time-point. There were no changes in serum IgA and IgG antibodies to P4, P6 and P26 nor to any of the pneumococcal proteins.
Figure 3. Serum anti-protein D antibody geometric mean titres (GMTs), with the corresponding 95% confidence intervals, by vaccine group and study time-point.
Serum antibodies to pneumococcal polysaccharides (Supp Table 4).
At baseline, the only between-group difference in anti-pneumococcal polysaccharide antibodies was for serotype 5 IgA, with higher levels in the MenACYW135 group. High levels of serum IgA antibodies to 19A and both IgA and IgG antibodies to 19F were observed relative to other serotypes. Specific IgA and IgG increases were observed following both doses in the PHiD-CV group for all PHiD-CV vaccine-type serotypes, which were not observed in the MenACYW135 group. These differences were sustained for the
12-months following dose 2 for all serotypes other than 6B, 19F and 23F IgA, and 6B and 23F IgG antibodies respectively. No between-group differences at any time-point were observed for non-vaccine serotypes 3 and 19A.
Microbiological outcomes (Supp Table 5). At baseline, nasopharyngeal swab (NPS) cultures grew Hi in 2/36 (5.6%) children from the PHiD-CV group and 4/38 (10.5%) from the MenACYW135 group. No between-group differences were observed in Hi prevalence at any time-point, although Hi was not isolated in the PHiD-CV group at the 6-month post-dose 2 time-point. The baseline prevalence of pneumococcus was 22.2% and 15.8% in the PHiD-CV and MenACYW135 groups respectively. Between-group differences of pneumococcus were observed only at the 2-months post-dose 1 time-point (8.8% and 27% in the PHiD-CV and MenACYW135 groups respectively. Interpreting culture data was complicated by the high antibiotic use throughout the study, particularly azithromycin () and subsequently no further culture analyses were undertaken.
Figure 4. Haemophilus influenzae and pneumococcal culture-positive nasopharyngeal swabs, and antibiotic use in 14-days prior to each swab, by vaccine group and study time-point. Hi: H. influenzae, CSpn: capsular pneumococci, 14DayAB: any antibiotics in the 14-days prior to nasopharyngeal swab,14DayAZM: azithromycin in the 14-days prior to nasopharyngeal swab
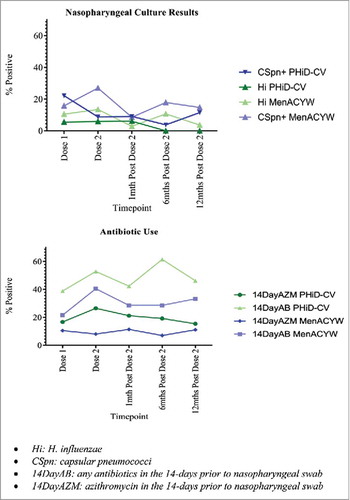
Of all swabs collected over the study period (n = 320), Hi was identified in just 25, including four (16%) that were beta-lactamase positive. Overall, 18 (72%) Hi isolates were hpd positive and 19 (76%) were fucP gene positive differentiating them from the commensal organism, H. haemolyticus. However, no differences in these characteristics existed between vaccine groups.
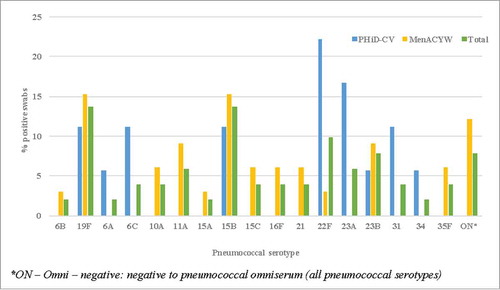
Of the 51 culture positive swabs for pneumococci (n = 18 in PHiD-CV group and n = 33 in MenACYW135 group), serotypes 19F and 15B (7/51 (13.7%) for each serotype respectively) were the most common overall. While they were also the most common serotypes identified in the MenACYW135 group (5/33 (15.2%) for each serotype respectively), 22F and 23A were the most common in the PHiD-CV group (4/18 (22.2%) and 3/18 (16.7%) respectively) (). Penicillin non-susceptible and macrolide-resistant isolates were each identified in 15/51 (29.4%) pneumococcal positive swabs and no differences in antibiotic resistance rates were observed between vaccine groups for either antibiotic.
Safety outcomes
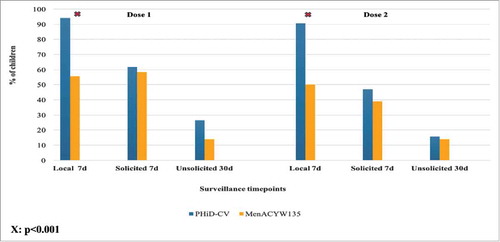
Parent-completed diary cards were not available for three vaccine doses (one in the PHiD-CV group following dose 1 and one each in the MenACYW135 group after doses 1 and 2). The proportions of children reporting any local reaction in the 7-days post each vaccine dose were higher in the PHiD-CV (94% and 91%) than the MenACYW135 group (56% and 50%) for each dose respectively (). There were no between-group differences in the 7-day general solicited and 30-day unsolicited symptoms. Of the local symptoms, pain was the most common (PHiD-CV 28/34 (82%) and 29/32 (91%); MenACYW135 16/36 (44%) and (14/36 (39%) following the first and second doses respectively), most of which was mild and self-limiting. Pain was the only symptom to increase in severity following the second vaccine dose and only occurred in the PHiD-CV group (1 (3%) and 5 (16%) after doses 1 and 2 respectively reported severe pain). None sought medical attention for these severe pain events.
Figure 6. 7-day local and general solicited reactions, and 30-day unsolicited symptoms, following each vaccine dose by study group. X: p<0.001.
Nineteen serious adverse events (SAEs) were reported over the course of the study (5 PHiD-CV group; 14 MenACYW135 group). None were considered causally related to study vaccines. Of these SAEs, eight were for exacerbations of the child's pre-existing illness (see above).
Discussion
Our study provides the first data on PHiD-CV in children with rPBB, CSLD and bronchiectasis. Despite not achieving the required sample size, and the consequent fragility of the data, we observed that compared with control children, those receiving PHiD-CV had fewer fortnights with respiratory symptoms and antibiotic courses although the difference was not statistically significant. Further, there were fewer hospitalised exacerbations in the PHiD-CV group, however the actual number of events and children were small. PHiD-CV also induced both serum and salivary anti-PD antibodies and was generally well-tolerated, despite higher proportions of recipients than controls experiencing local reactions following both vaccine doses.
There are no comparable data with which to compare our findings. A Cochrane Review of six randomised controlled trials (RCTs) involving an oral, whole cell NTHi vaccine in 557 adults with either chronic obstructive pulmonary disease or chronic bronchitis found no effect on exacerbations or hospitalisations.Citation11 However, a subgroup analysis of the largest trial in the review, which was restricted to 91 subjects aged <65-years, reported efficacy (p < 0.001) against all major endpoints, including reductions of 54% in exacerbations, 22% in antibiotic courses and 62% in corticosteroid use.Citation12 Notwithstanding the limitations associated with post-hoc analyses and the different patient populations, as a potential ‘proof of principle’ the point estimate for reduced antibiotic use is similar to the 19% we observed in our study. Given worldwide concerns over increasing antibiotic resistance,Citation13 our study provides promising preliminary data to suggest that PHiD-CV could decrease antibiotic use in children with chronic pulmonary infections.
We observed a reduction in fortnights with respiratory symptoms following both doses of the PHiD-CV vaccine. The symptoms we recorded are common to both upper and lower respiratory infections and hence may include milder viral illnesses. We have not yet tested for viruses, but interactions between both Hi and pneumococci may be important. Hi colonisation of the airways alters immune responses to subsequent viral infections, potentially increasing susceptibility to exacerbations.Citation14 Further, there is growing evidence viruses predispose to pneumococcal infection by disrupting epithelial barriers and altering innate and adaptive immune responses, and that these actions can be ameliorated by pneumococcal vaccination.Citation15 A caveat of finding a reduction in fortnights with respiratory symptoms is that the duration and consistency of symptoms were not measured and hence whether it was one or more events within and across fortnights was not accounted for in the analysis.
The serum and salivary immune responses to vaccination in the study children suggest that except for saliva IgA, two doses of PHiD-CV induce significant boosting to anti-PD antibody and to most vaccine-type pneumococcal polysaccharide antibodies that persists for
12-months following the second vaccine dose. IgA antibodies observed in saliva are indicative of stimulation of a mucosal immune response following vaccination, which may also be reflected at other mucosal sites, including the upper airways.Citation16 Importantly, in this highly vaccinated population, there was no indication of hyporesponsiveness to the common polysaccharide antigens, although the limited sample size precluded analyses by prior vaccination status. Data in children and adults with bronchiectasis suggest systemic deficiencies in IFN-ɣ production,Citation17,Citation18 especially in cytotoxic T-lymphocytes and natural killer cells. In children, this systemic deficiency correlated with increased lower airway inflammationCitation17 and importantly, the reduced IFN-ɣ production to Hi can be reversed following PHiD-CV vaccination.Citation19 These data, together with the systemic and mucosal antibody responses observed in our study, and the preliminary clinical findings, suggest vaccination against Hi may be an important adjuvant to managing children with rPBB, CSLD and bronchiectasis.
The NPS culture data in this study are difficult to interpret given the low proportion of positive specimens, and the frequent use of antibiotics during the study.Citation20 At baseline, only 8.1% of NPS were culture positive for Hi and 18.9% were pneumococcus culture positive. This compares with positive NPS culture results of 30.6% and 29.5% for Hi and pneumococci respectively in Queensland children undergoing bronchoscopy for chronic cough during the same time period.Citation21 The difference between the two studies, despite a similar distribution of children with PBB (not all recurrent), CSLD and bronchiectasis and the same laboratory performing the microbiology testing, is likely explained by children in the bronchoscopy study being newly-diagnosed with these chronic pulmonary infections, whereas children in our study were already receiving treatment for their disease. NPS collected while a child is anaesthetised for bronchoscopy may be of better specimen quality for culture. Azithromycin is used commonly in this patient populationCitation22 and in the current study over 40% of participants had received the antibiotic at least once during the trial. Azithromycin has long-term effects on carriage,Citation22,Citation23 lasting several months after ceasing therapy, and the small sample size and number of positive NPS cultures precluded differentiating trends in carriage between vaccine and antibiotic effects.
PHiD-CV was generally well tolerated with most adverse events being mild, self-limiting and within the ranges reported in other studies.Citation24 However, a higher proportion of children in the PHiD-CV group reported pain following both doses of vaccine compared to the MenACYW135 group and it was the only reaction that increased in severity following the second vaccine dose. The most plausible explanation for this finding is that PHiD-CV contains an alum adjuvant, as do other PCVs (PCV7 and PCV13), and PPV23, while the MenACYW135 vaccine used in this study does not. Increasing reactogenicity with cumulative exposure to alum adjuvants in several vaccines, as experienced by children in the study, has been reported elsewhere and may reflect both local tissue damage and systemic inflammation induced by innate immune receptor activation.Citation25
The major limitation of our study is the premature termination of the study, resulting in the small sample size and inability to adequately address the primary endpoint and perform important subgroup analyses of the data, such as further exploration of potential baseline differences in exposure to household tobacco smoke. The reason for the imbalance is unknown, but is likely a reflection of the small numbers in each group (two children exposed in the
PHiD-CV group and seven in the MenACYW135 group). The challenges of recruitment included confirming eligibility criteria (particularly exacerbation history), the reluctance of parents to subject their child to further, non-essential medical intervention (including blood tests and further clinical studies) and competing studies in the same patient population. However, the trial's experience provides valuable information for the design and implementation of further studies. Secondly, we did not have a group that received a PCV only vaccine and hence there is uncertainty over whether the clinical outcomes we observed may be due to effects on pneumococcal infection, particularly given the boosting of pneumococcal antibodies. Further exploration of this possibility is also limited by the low proportion of pneumococcal culture positive swabs. Finally, approximately 40% of participants received azithromycin during the conduct of the study and given its prolonged effect upon the airways and the inability to model the data, we cannot definitively conclude that the effects we observed were not due to azithromycin.Citation26 However, the number of children receiving azithromycin and the duration of the treatment courses was similar between the two study groups.
Conclusion
Our study provides the first clinical and immunological data for a Hi vaccine, including one that also addresses pneumococcal infection, in children with rPBB, CSLD and bronchiectasis. It raises the possibility of important clinical effects warranting further investigation in this important group of children with chronic pulmonary disorders. However, the failure to achieve the required sample size and the lack of any statistically significant differences in our primary and secondary clinical outcomes means that our findings are instead hypothesis generating and require cautious interpretation. Consequently, these data will inform larger studies in a broader patient population, including adults, and serve to encourage future trials of candidate Hi vaccines.
Methods
Design
We conducted a multi-centre, parallel group, double-blind, RCT. The study protocol was published previously.Citation27 Briefly, children aged 18-months to <18-years with rPPB, CSLD or bronchiectasis were recruited from specialist respiratory clinics in tertiary paediatric hospitals in Brisbane, Perth, Sydney and Melbourne and allocated randomly (1:1) to receive either PHiD-CV or quadrivalent meningococcal ACYW135 conjugate vaccine in a schedule of two doses, 2-months apart. Key exclusion criteria included cystic fibrosis, immunosuppressive conditions and prior receipt of either of the two study vaccines. Active surveillance was implemented via fortnightly parent/caregiver contact to capture acute exacerbations, respiratory symptoms, antibiotic/steroid use, SAEs and medically-attended illnesses. Surveillance continued until 12-months following the second dose of vaccine. A respiratory exacerbation was defined as increased sputum volume or purulence, or ≥3-days of change in cough (>20% increase in cough scoreCitation28 or type [dry to wet]).Citation29 Exacerbations were classified by research staff following standardised parent/carer interviews. The trial was registered with the Australia and New Zealand Clinical Trials Registry (ACTRN12612000034831).
Published protocol changes
The original protocol limited the upper age of participants to <15-years.Citation27 Recruitment difficulties and limited funding meant the recruitment age was lifted to <18-years, another study site (Melbourne) was added in July 2014, and a change made to the primary objectives from the primary clinical endpoint of respiratory exacerbations to immunological endpoints (defined below). Children recruited from August 2014 entered the immunology cohort and no longer underwent active surveillance for non-hospitalised exacerbations. Their clinical specimens were reduced to baseline and 1-month post-dose 2 of study vaccine, with parents consenting to optional specimen taking at 12-months post-dose 2. Prior receipt of monovalent meningococcal C vaccine and a primary course of pneumococcal only conjugate vaccine were also removed from inclusion criteria. Hospitalisations for respiratory exacerbations were captured as part of SAE monitoring. Thus participants are those who followed either the full clinical protocol or the immunology protocol only. The independent DSMC approved secondary clinical endpoints (antibiotic use and frequency of any respiratory symptoms) being added to the pre-unblinding analysis plan for children on the clinical protocol.
Ethics
The Human Research Ethics Committees of all the participating institutions approved the study and all subsequent amendments. Parents/guardians and participants aged ≥12-years provided written informed consent. A DSMC was established and independent study monitors conducted regular study audits. The trial was conducted under the Australian Therapeutic Goods Administration Clinical Trial Notification scheme.
Objectives
The primary objective was to determine the efficacy of PHiD-CV in reducing respiratory exacerbations in children aged 18-months to <18-years with rPBB, CSLD or bronchiectasis. The secondary objectives were to:
a) evaluate the efficacy of PHiD-CV in reducing antibiotic use and the incidence of fortnights where parents reported their child had respiratory symptoms;
b) evaluate the serum and salivary antibody immune responses to PD, non-vaccine type Hi. proteins (P4, P6, P26) and PHiD-CV (1, 4, 5, 6B, 7F, 9V, 14, 18, 19F and 23F) and vaccine-related pneumococcal serotypes (3 and 19A) at 2-months post-dose 1, and then at 1, 6 and 12-months following the second vaccine dose;
c) evaluate the impact of the PHiD-CV vaccine on nasopharyngeal carriage of
Hi and pneumococcal vaccine-type and vaccine-related serotypes at
2-months post-vaccine dose 1 and then at 1, 6 and 12-months following the second vaccine dose;
d) evaluate local and systemic reactions to vaccine at 7 and 30-days after each dose and all SAEs for 14-months post-dose 1.
Randomisation and masking
An independent biostatistician prepared the randomisation code using a permuted blocking design (block size of 4) to maintain group balance.Citation27 Randomisation was stratified by site (n = 4), age (<6-years and ≥6-years), and rPBB vs CSLD/bronchiectasis. Allocation concealment was achieved by consecutively numbered opaque sealed envelopes. Since the vaccines differed in colour and packaging, preparation and delivery was performed by nurses independent of the study and without blinded staff present. Blinding of investigators, study staff and participants was maintained until children on the clinical protocol had completed follow-up and the clinical data had been cleaned and locked and blinded analyses of the clinical data had been completed. With the approval of the DSMC and the coordinating ethics committee, unblinding was then performed with the exception of ongoing participants in the immunology only cohort. The laboratories performing all immunology and microbiology procedures remained blinded for the complete duration of the study.
Specimen collection and laboratory procedures
In children enrolled in the full clinical protocol, serum, saliva and NPS were collected at enrolment and at 2-months post-vaccine dose 1, and then at 1, 6 and 12-months following the second vaccine dose. These specimens were also collected at enrollment and 1-month post-vaccine dose 2, with optional collection at 12-months post-dose 2 from children on the immunology protocol. Although parents and/or the child could refuse specimen collection, extra emphasis was placed on ensuring at least the baseline and 1-month post-dose 2 specimens were obtained. Specimens were processed using published proceduresCitation27 and stored locally at participating centres at -80oC before being transported frozen to the research laboratories.
Outcomes
The primary clinical outcome was the incidence of respiratory exacerbations in the
12-months following the second vaccine dose. Child-time at risk of an exacerbation commenced 14-days following receipt of the second vaccine dose and the denominator for calculating incidence densities also excluded the time when the child had an exacerbation. At least 14-days following the child's cough returning to baseline was required before a subsequent increase in symptoms was classified as a new exacerbation. The secondary clinical outcomes were the antibiotic courses prescribed for cough (courses <14-days or
<28-days) and the frequency of fortnights when a parent/guardian reported respiratory symptoms during child-time at risk. Respiratory symptoms collected by nurses interviewing parents/carers at fortnightly surveillance contacts included any cough (ie wet or dry), parent-reported fever (±thermometer recording), nasal discharge/congestion, sore throat, wheeze, and difficulty breathing. Parents were educated on these symptoms at enrolment. As part of SAE surveillance, hospitalised exacerbations were recorded for all participants.
The primary immunological outcomes were serum and saliva IgA and IgG antibody titres to PD at 1-month post-dose 2 for all participants. The secondary immunological outcomes were serum and saliva IgA and IgG antibody titres at 1-month post-dose 2 to Hi proteins P4, P6 and P26; pneumococcal proteins Ply, PspA1, PspA2 and CbpA; and pneumococcal polysaccharides 1, 3, 4, 5, 6B, 7F, 9V, 14, 18, 19A, 19F and 23F. Where available, these Hi and pneumococcal antibody titres were also evaluated at 2-months post-dose 1, and at 6 and 12-months post-dose 2. The primary microbiological outcomes were culture-confirmed Hi and vaccine-type and vaccine-related pneumococci from NPS at each time-point.
The co-primary safety outcomes were solicited local and general symptoms for 7-days following each vaccine dose, unsolicited reactions for 30-days following each vaccine dose and SAEs for 14-months following dose 1.
Sample size
Prospective data collected in Brisbane children with bronchiectasis attending a specialist respiratory clinic indicated a mean annual incidence of 2.1 (standard deviation 1.046) exacerbations requiring hospital clinic attendance or hospitalisation (Chang, unpublished data). Based on these data and results from the POET study (30% reduction in otitis media),Citation30 our trial was powered to detect a 30% reduction from 2.1 to 1.47 exacerbations in the 12-months following the second vaccine dose. Assuming a Poisson distribution, 93 children per group would have provided 90% power (α = 0.05, 2-sided) to detect a 30% reduction in exacerbations in the PHiD-CV group and 80% power to detect a 25% reduction. Assuming a 10% loss to follow-up, we aimed to recruit 206 children (103 per group). Differences <20% were considered unlikely to change clinical practice without additional supporting evidence. However, given recruitment difficulties and exhaustion of grant funds, the study was stopped after enrolling 75 children.
Analysis
Demographic data were tabulated and expressed as proportions and/or means or medians of the selected characteristics by vaccine group with the corresponding 95%CI or interquartile ranges. The T-test for comparisons of means, Wilcoxon signed-rank test for medians and χ2 tests for comparison of proportions assessed between-group differences.
Poisson regression was employed to calculate the primary endpoint and antibiotic use rate ratios and CIs, following assessment of distributional assumptions and using robust standard errors. The primary analysis compared the incidence density of respiratory exacerbations between the two groups with child-time at risk commencing 14-days following the second vaccine dose. As children are not at risk of an event while they are experiencing an event, the total duration of each event (in days) was subtracted from the total time of observation to obtain the child-time at risk denominator used in calculating incidence density rates. Absolute risk differences and IDRs were calculated to compare exacerbation incidence in the PHiD-CV and control groups respectively and presented with their 95% CIs. The same comparisons were performed for courses of antibiotics. Fortnights with respiratory symptoms were counted on the basis of a “yes” or “no” parent response to whether or not the child had had any symptoms in that fortnight; individual symptoms and duration of symptoms within and between fortnights were not analysed for this paper. A log-binomial model, incorporating a robust standard error and Bernoulli random variable was employed to calculate rate ratios for respiratory symptom fortnights. All analyses were performed on an intention-to-treat basis.
Group-specific serum and saliva antibody responses to Hi and pneumococcal proteins and pneumococcal polysaccharide antigens were compared at each sampling time-point. Analyses were first performed using non-parametric methods (Wilcoxin signed-rank test). Data were then log-transformed to assess normality and if normality assumptions were met, T-tests were used to compare transformed data, otherwise non-parametric methods were employed. Log-transformed data were then back-transformed and presented as GMTs with the corresponding 95%CIs. Proportions of children with Hi and pneumococcal nasopharyngeal carriage at each time-point were compared between vaccine groups and presented with their corresponding
p-values. Safety data were analysed descriptively at each time-point with χ2 tests used to evaluate differences in the proportions of participants with solicited and unsolicited symptoms and SAEs at each study time-point. No adjustment was made for multiple testing.
Role of the funding source
The Australian National Health and Medical Research Council (NHMRC) funded this study (GNT1019904). The NHMRC had no role in the conception, design and implementation of the study, nor in the analysis and interpretation of data, the writing of the report and in the decision to submit the paper for publication. The vaccine manufacturers had no role in the study and vaccines were purchased at wholesale market prices from the distributors.
Abbreviations
| CI | = | confidence interval |
| CSLD | = | chronic suppurative lung disease |
| DSMC | = | Data Safety Monitoring Committee |
| GMT | = | geometric mean titre |
| Hi | = | Haemophilus influenzae |
| IDR | = | incidence density ratio |
| MenACYW135 | = | quadrivalent meningococcal conjugate ACYW135 vaccine |
| NPS | = | nasopharyngeal swab |
| NTHi | = | non-typeable Haemophilus influenzae |
| PBB | = | protracted bacterial bronchitis |
| PCV | = | pneumococcal conjugate vaccine; |
| PD | = | protein D |
| PHiD-CV | = | 10-valent pneumococcal-Haemophilus influenzae protein D conjugate vaccine |
| PPV23 | = | 23-valent pneumococcal polysaccharide vaccine |
| PV | = | pneumococcal vaccines |
| RCT | = | randomised controlled trial |
| Rpbb | = | recurrent protracted bacterial bronchitis |
| SAE | = | serious adverse event |
Disclosure of potential conflicts of interest
KOG was supported by a National Health & Medical Research Council (NHMRC) Career Development Fellowship (1045157), and a Queensland Government Smart Futures Fellowship and was the lead investigator on the NHMRC grant that funded this study. ABC and HSV have previously received a grant from GSK to evaluate the impact of PHiD-CV on bronchoalveolar lavage fluid in children. AC is supported by a NHMRC practitioner fellowship (grant 1058213) and holds multiple grants awarded from the NHMRC related to diseases associated with pediatric cough. AWC has previously been a member of the GlaxoSmithKline Advisory Panel for the PHiD-CV and presented at Symposia. He received an honorarium from GlaxoSmithKline to undertake these activities. He has also chaired a Symposium on pneumonia for Pfizer Australia. No honorarium was accepted. He is a member of the Seqirus Pneumococcal Expert Panel and has received an honorarium for attending Panel meetings. HSV is supported by a Fellowship under the NHMRC-funded HOT NORTH collaboration (1131932). NW was supported by a NHMRC Career Development Fellowship. The Sydney Children's Network where he works has received financial support from the GlaxoSmithKline for investigator initiated pertussis vaccine trials. RT is supported by a BrightSpark Foundation Research Fellowship. VG was supported by a NHMRC post-graduate scholarship (1075119). PM has received research grants from the NHMRC and funding from GlaxoSmithKline for an investigator-initiated study unrelated to this study. KG received grants from the NHMRC during the course of the study. EM, PT, MD, AC, SR, KP and DA have nothing to declare.
The views expressed in this publication are those of the authors and do not reflect the views of the NHMRC.
Box 1: Definitions of recurrent protracted bacterial bronchitis, chronic suppurative lung disease and bronchiectasis.4
Author contributions
KOG, ABC, KG, AWC, HSV, NW, RT, AW, PT, PM, PR and MD conceived and designed the trial, obtained grant funding and all made a substantial contribution to protocol development and amendments. SR, DA, AC, VG and TS all made substantial contributions to the conduct of the trial including leading recruitment sites, medical monitoring, data collection and management, data analysis, data quality control and compliance with Good Clinical Practice. KO wrote the first draft of the manuscript and all authors contributed to the revision and approval of the final manuscript.
Supplemental Material
Download Zip (894.5 KB)Acknowledgments
The authors thank: the members of the Data Safety Monitoring Committee (Tilman Ruff, Helen Marshall, Deborah Lehmann and Peter Jacoby); the study coordinators and research officers at each site (Jane Pooley, Rebecca Doyle, Stephanie Jeffares, Marita Kefford, Janet Briggs, Laura Rost and Jane Ryrie); the Departments of Respiratory Medicine at each participating site; the study monitors (Annmarie McEvoy and Clare Brophie); Anita Champion (Clinical Trial Pharmacist) at the Lady Cilento Children's Hospital; Jemima Beissbarth (microbiology manager); Terry Nolan at the Vaccine & Immunisation Research Group, Murdoch Children's Research Institute; Amber Revel for her contribution to design of the study, and the Respiratory infection Outreach and Research Team (study coordinating site) in Brisbane.
Additional information
Funding
References
- Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in pediatric non-cystic fibrosis bronchiectasis. Chest. 2012;141(4):1018–24. doi:10.1378/chest.11-0679.
- Chang AB, Redding GJ, Everard ML. Chronic wet cough: Protracted bronchitis, chronic suppurative lung disease and bronchiectasis. Pediatr Pulmonol. 2008;43(6):519–31. doi:10.1002/ppul.20821.
- Wurzel DF, Marchant JM, Yerkovich ST, Upham JW, Petsky HL, Smith-Vaughan H, Masters B, Buntain H, Chang AB. Protracted bacterial bronchitis in children: natural history and risk factors for bronchiectasis. Chest. 2016;150(5):1101–8. doi:10.1016/j.chest.2016.06.030.
- O'Grady K-AF, Grimwood K. The likelihood of preventing respiratory exacerbations in children and adolescents with either chronic suppurative lung disease or bronchiectasis. Front Pediatr. 2017;5:58.
- Kantar A, Chang AB, Shields MD, Marchant JM, Grimwood K, Grigg J, Priftis KN, Cutrera R, Midulla F, Brand PLP, Everard ML. ERS statement on protracted bacterial bronchitis in children. Eur Respir J. 2017;50(2):1602139 doi:10.1183/13993003.02139-2016.
- Goyal V, Grimwood K, Marchant J, Masters IB, Chang AB. Pediatric bronchiectasis: No longer an orphan disease. Pediatr Pulmonol. 2016;51(5):450–69. doi:10.1002/ppul.23380.
- Kapur N, Masters IB, Chang AB. Longitudinal growth and lung function in pediatric non-cystic fibrosis bronchiectasis: what influences lung function stability? Chest. 2010;138(1):158–64.
- Forsgren A, Riesbeck K, Janson H. Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin Infect Dis. 2008;46(5):726–31. doi:10.1086/527396.
- Khan MN, Kaur R, Pichichero ME. Bactericidal antibody response against P6, protein D, and OMP26 of nontypeable Haemophilus influenzae after acute otitis media in otitis-prone children. FEMS Immunol Med Microbiol. 2012;65(3):439–47. doi:10.1111/j.1574-695X.2012.00967.x.
- Poolman JT, Bakaletz L, Cripps A, Denoel PA, Forsgren A, Kyd J, Lobet Y. Developing a nontypeable Haemophilus influenzae (NTHi) vaccine. Vaccine. 2000;19:S108–S15. doi:10.1016/S0264-410X(00)00288-7.
- Teo E, House H, Lockhart K, Purchuri SN, Pushparajah J, Cripps AW, van Driel ML. Haemophilus influenzae oral vaccination for preventing acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(9):CD010010.
- Clancy RL, Dunkley ML, Sockler J, McDonald CF. Multi-site placebo-controlled randomised clinical trial to assess protection following oral immunisation with inactivated non-typeable Haemophilus influenzae in chronic obstructive pulmonary disease. Intern Med J. 2016;46(6):684–93. doi:10.1111/imj.13072.
- Tillotson GS. Where in the world? The role of geography in antibiotic resistance and the potential impact in pulmonary infections. Postgraduate Med. 2016;128(5):449–50. doi:10.1080/00325481.2016.1185375.
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–71. doi:10.1056/NEJMoa012561.
- Moore DP, Dagan R, Madhi SA. Respiratory viral and pneumococcal coinfection of the respiratory tract: implications of pneumococcal vaccination. Expert Rev Respir Med. 2012;6(4):451–65. doi:10.1586/ers.12.32.
- Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Annals N York Acad Sci. 2007;1098:288–311. doi:10.1196/annals.1384.012.
- Pizzutto SJ, Upham JW, Yerkovich ST, Chang AB. High pulmonary Levels of IL-6 and IL-1beta in children with chronic suppurative lung disease are associated with low systemic IFN-gamma production in response to non-typeable Haemophilus influenzae. PLoS One. 2015;10(6):e0129517. doi:10.1371/journal.pone.0129517.
- King PT, Ngui J, Farmer MW, Hutchinson P, Holmes PW, Holdsworth SR. Cytotoxic T lymphocyte and natural killer cell responses to non-typeable Haemophilus influenzae. Clin Exp Immunol. 2008;152(3):542–51. doi:10.1111/j.1365-2249.2008.03667.x.
- Pizzutto SJ, Yerkovich ST, Upham JW, Hales BJ, Thomas WR, Chang AB. Improving immunity to Haemophilus influenzae in children with chronic suppurative lung disease. Vaccine. 2015;33(2):321–6. doi:10.1016/j.vaccine.2014.11.024.
- Hare KM, Leach AJ, Morris PS, Smith-Vaughan H, Torzillo P, Bauert P, Cheng AC, McDonald MI, Brown N, Chang AB, et al. Impact of recent antibiotics on nasopharyngeal carriage and lower airway infection in Indigenous Australian children with non-cystic fibrosis bronchiectasis. Int J Antimicrob Agents. 2012;40(4):365–9. doi:10.1016/j.ijantimicag.2012.05.018.
- Hare KM, Smith-Vaughan HC, Chang AB, Pizzutto S, Petsky HL, McCallum GB, Leach AJ. Propensity of pneumococcal carriage serotypes to infect the lower airways of children with chronic endobronchial infections. Vaccine. 2017;35(5):747–56. doi:10.1016/j.vaccine.2016.12.059.
- Hare KM, Singleton RJ, Grimwood K, Valery PC, Cheng AC, Morris PS, Leach AJ, Smith-Vaughan HC, Chatfield M, Redding G, et al. Longitudinal nasopharyngeal carriage and antibiotic resistance of respiratory bacteria in indigenous Australian and Alaska native children with bronchiectasis. PLoS One. 2013;8(8):e70478. doi:10.1371/journal.pone.0070478.
- Hare KM, Grimwood K, Chang AB, Chatfield MD, Valery PC, Leach AJ, Smith-Vaughan HC, Morris PS, Byrnes CA, Torzillo PJ, et al. Nasopharyngeal carriage and macrolide resistance in Indigenous children with bronchiectasis randomized to long-term azithromycin or placebo. Eur J Clin Microbiol Infect Dis. 2015;34(11):2275–85. doi:10.1007/s10096-015-2480-0.
- Silfverdal SA, Coremans V, Francois N, Borys D, Cleerbout J. Safety profile of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Expert Rev Vaccines. 2017;16(2):109–21. doi:10.1586/14760584.2016.1164044.
- Petrovsky N. Comparative safety of vaccine adjuvants: A summary of current evidence and future needs. Drug Saf. 2015;38(11):1059–74. doi:10.1007/s40264-015-0350-4.
- Valery PC, Morris PS, Byrnes CA, Grimwood K, Torzillo PJ, Bauert PA, Masters IB, Diaz A, McCallum GB, Mobberley C, et al. Long-term azithromycin for Indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2013;1(8): 610–20. doi:10.1016/S2213-2600(13)70185-1.
- O'Grady KA, Grimwood K, Cripps A, Mulholland EK, Morris P, Torzillo PJ, Wood N, Smith-Vaughan H, Revell A, Wilson A, et al. Does a 10-valent pneumococcal-Haemophilus influenzae protein D conjugate vaccine prevent respiratory exacerbations in children with recurrent protracted bacterial bronchitis, chronic suppurative lung disease and bronchiectasis: protocol for a randomised controlled trial. Trials. 2013;14:282. doi:10.1186/1745-6215-14-282.
- Chang AB, Newman RG, Carlin JB, Phelan PD, Robertson CF. Subjective scoring of cough in children: parent-completed vs child-completed diary cards vs an objective method. Eur Respir J. 1998;11(2):462–6. doi:10.1183/09031936.98.11020462.
- Kapur N, Masters IB, Morris PS, Chang AB. Defining exacerbation in children with non-CF bronchiectasis. Pediatr Pulmonol. 2012;47:68–75 doi:10.1002/ppul.21518.
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367(9512):740–8. doi:10.1016/S0140-6736(06)68304-9.