ABSTRACT
Hepatitis A and B disease burden persists in the US. We assessed hepatitis A and hepatitis B vaccination series completion rates among 350,240 commercial/Medicare and 12,599 Medicaid enrollees aged ≥19 years. A vaccination series was considered as completed provided that the minimum interval between doses, as defined by the CDC, and the minimum number of doses were reached. We stratified completion rates by vaccine type (i.e. monovalent or bivalent) at initial vaccination for each cohort. In the commercial/Medicare cohort, the series completion rate was 32.0% for hepatitis A and 39.6% for hepatitis B among those who initiated with a monovalent vaccine, and it was 36.2% for hepatitis A and 48.9% for hepatitis B among those who initiated with a bivalent vaccine. In the Medicaid cohort, the series completion rate was 21.0% for hepatitis A and 24.0% for hepatitis B among those who initiated with a monovalent vaccine, and it was 19.0% for hepatitis A and 24.6% for hepatitis B among those who initiated with a bivalent vaccine. In conclusion, hepatitis A and B vaccination series completion rates were low, and appeared to be lower among Medicaid than among commercial/Medicare enrollees. Commercial/Medicare enrollees who initiated with a bivalent vaccine had higher series completion rates than those who initiated with monovalent vaccines – an observation that was not made among Medicaid enrollees.
FOCUS ON THE PATIENT
What is the context?
Hepatitis A and B are vaccine-preventable liver diseases. Although the reported incidence of hepatitis A and hepatitis B infections have dropped significantly since the introduction of hepatitis vaccines, surveillance reports indicate that hepatitis A and B infections persist in adults.
There is limited information on hepatitis A and hepatitis B vaccine series completion.
What is new?
In our large cohort study we found that commercial/Medicare and Medicaid enrollees had low rates of vaccination series completion for both hepatitis A and hepatitis B vaccines.
What is the impact?
These results demonstrate the need for interventions to increase hepatitis A and B vaccine series completion.
Introduction
Hepatitis A and B are vaccine-preventable liver diseases caused by hepatitis A (HAV) and hepatitis B viruses (HBV), respectively.Citation1,Citation2 Since the Centers for Disease Control and Prevention's (CDC) introduction of hepatitis A vaccination recommendations for infants in high-risk communities in 1996,Citation3 followed by a recommendation for routine pediatric vaccination nationwide in 2006,Citation4 and of routine hepatitis B vaccination recommendations for children in 1991,Citation5 the reported incidence of hepatitis A has dropped by more than 90% and that of acute hepatitis B by more than 80% in the United States (US).Citation6
Despite this decrease, the disease burden of hepatitis A and B persists. Consistently, the US National Viral Hepatitis Action Plan 2017–2020 states that hepatitis B is still a serious threat to the health of the US population despite a ‘coordinated national response’ initiated in 20117. In 2015, the estimated number of new HAV infections in the US was 2,800 (95% confidence interval [CI] = 1,900–3,100), while that for new HBV infections was 21,900 (95% CI = 12,500–53,600), representing increases over the previous year of 12% and 14%, respectively.Citation8,Citation9
For adults at high risk for infection, morbidity, or mortality, the US Advisory Committee on Immunization Practices (ACIP) issued specific recommendations for hepatitis ACitation4 and hepatitis B vaccinations.Citation10 In the US, the available types of hepatitis vaccines for adults are the monovalent hepatitis A (HepA) vaccines, the monovalent hepatitis B (HepB) vaccines, or a combined bivalent hepatitis A/hepatitis B (HepA-HepB) vaccine.Citation11,Citation12 The monovalent HepA vaccines are inactivated whole-virus vaccines, and the monovalent HepB vaccines are recombinant vaccines. The bivalent HepA-HepB vaccine contains one pediatric dose of monovalent HepA vaccine and one adult dose of monovalent HepB vaccine.Citation11,Citation12 Potential reasons for the persisting vaccine-preventable hepatitis disease burden may be suboptimal vaccination rates and poor vaccination coverage among adults, especially among adults at a high-risk for hepatitis A or B, compared to infants and children.Citation7,Citation13 In 2015, for adults aged ≥19 years, reported HepA vaccination coverage (≥2 doses) and HepB vaccination coverage (≥3 doses) was 9.0% and 24.6%, respectively.Citation13
Literature on adherence to the CDC guidelines for HepA, HepB, and HepA-HepB vaccine series completion schedules among adults in the US is limited. One studyCitation14 determined that HepA and HepB series completion rates in the Vaccine Safety Datalink population ranged between 40% to 65% among those who received a first dose, depending on the hepatitis vaccine (HepA or HepB), with higher completion rates in those aged <18 years. However, this study was done prior to the US Food and Drug Administration approval of bivalent vaccine in 2001.Citation15 Bivalent vaccine introduction was expected to increase adherence to vaccination schedule, by offering fewer medical visits and by reducing the number of injections required by the separate administration of each monovalent vaccine.Citation16 Other studies assessing HepA, HepB or HepA-HepB series completion were either based on a select patient population, such as travelers,Citation17 or were conducted outside the US.Citation17,Citation18
In this context, we conducted a retrospective longitudinal cohort study using recent insurance claims data to assess HepA and HepB vaccination series completion rates among US adult enrollees of commercial/Medicare and Medicaid insurances, separately. We further stratified the results by type of vaccine, monovalent or bivalent, that was received when the vaccination series was initiated.
Results
The eligible population consisted of 350,240 commercial/Medicare patients and 12,599 Medicaid patients. summarizes patient demographics based on vaccination series; mean age ranged from 42.1 to 45.8 years among commercial/Medicare patients and 37.1 to 40.2 years among Medicaid patients; over half of commercial/Medicare patients (55.3-60.0%) were female and most Medicaid patients (66.9-75.1%) were female.
Table 1. Baseline characteristics of commercial/Medicare and of Medicaid enrollees who meet study selection criteria, by type of vaccine at initial vaccination dose.
As shown in , among commercial/Medicare patients who initiated with a monovalent initial dose, 32.0% and 39.6% of patients completed the full HepA and HepB vaccination series, respectively. With a bivalent initial dose, 36.2% and 48.9% of patients completed the full HepA and HepB vaccination series, respectively. Among patients who were initiated on a HepB series, 75.5% received ≥2 doses when the initial dose was with the bivalent vaccine and 65.1% when the initial dose was with a monovalent vaccine.
Figure 1. Rates of vaccination series completion among (a) adult commercial/Medicare (N = 350,240) and (b) adult Medicaid patients (N = 12,599)*
*Schedule of vaccine series was determined by type of vaccine (monovalent hepatitis A or B or bivalent hepatitis A/B) administered on index date. Completion schedules with a bivalent vaccine on the index date are shown stratified by whether series fulfilled hepatitis A or B coverage; Hepatitis A vaccine is a 2-dose series; however, discrepancies in series completion for hepatitis A allow for bivalent vaccine to be included in the measurement, thus needing three doses, as appropriate, to be complete. The bivalent inclusion required 3 doses for completion.
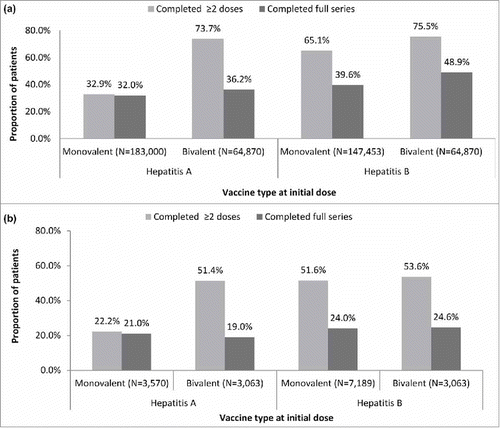
Among Medicaid patients (), full series completion rates were similar regardless of whether the initial dose was a monovalent or a bivalent vaccine in both the HepA and HepB groups and ranged from 19.0% to 24.6%. Among patients who were initiated on a HepB series, 53.6% received ≥2 doses of HepB vaccination when the initial dose was a bivalent vaccine and 51.6% when the initial dose was a monovalent vaccine.
Discussion
Assessing HepA and HepB vaccination series completion rates in a large sample from a US insurance claims dataset, we found that 32–36% of commercial/Medicare insurance enrollees received the full HepA vaccination series, and 40–49% received the full HepB vaccination series. The rate varied depending on whether they initiated on a monovalent or bivalent vaccine. Between 65% and 76% of patients initiating HepB vaccination received ≥2 doses. Although this analysis was not designed to compare completion rates between commercial/Medicare and Medicaid enrollees, the respective ranges for Medicaid enrollees were observed to be lower: 19–21% and 24–25% completed a full HepA and HepB vaccine series, respectively, and 52–54% received ≥2 HepB doses. In general, Medicaid enrollees face challenges in terms of gaps in access to certain providers because of provider shortages in low-income communities, lack of transportation, and lower physician fees and participation in Medicaid compared to commercial insurance.Citation19
This study updates previously published material on HepA and HepB vaccination series completion rates among adults in the US, which are limited to only one full publication and two announcements in 2017 conferences. Nelson et al. report the results of a retrospective population-based study of patient vaccination data recorded from 1996 to 2004 in the administrative databases of seven health care organizations participating in the Vaccine Safety Datalink Project.Citation14 It was found that within one year of the first dose in adults, completion rates ranged 25–44% for HepA and 41–62% for HepB according to the age group (18-29; 30–49; 50–64; ≥ 65 years old).Citation14 Their results also corroborate an observation of lower completion rates among Medicaid enrollees in the current study. The latest findings presented at 2017 congresses were derived from the same database as in our study.Citation20,Citation21 They showed that 27%, 51%Citation20 and 40%Citation21 of the patients received ≥2 doses of HepA, HepB, or HepA-HepB vaccines, respectively. No differentiation, however, was made between Medicaid and commercially insured individualsCitation20 who have different socioeconomic, disease and disability status that cannot be controlled for in this databaseCitation22; nor did the definition of series completion account for switching between the bivalent and monovalent hepatitis vaccines.Citation21
Finally, a number of studiesCitation13,Citation23–Citation27 used the National Health Interview Surveys (NHIS) to assess hepatitis vaccination coverage but none examined the series completion rates neither the type of vaccine (monovalent or bivalent) administered.
Potential barriers to vaccination series completion may include administrative or financial barriers, limited access to vaccination services, or lack of awareness of recommended schedules among patients. For example, travelers who were partially vaccinated with HAV and HBV, believed that they had received the recommended number of HepA and HepB vaccine doses.Citation17 In another study, medical visits prior to or after starting HepA or HepB vaccination series was found to facilitate series completion.Citation14,Citation28
Our results highlight the need for future research to determine the effectiveness of interventions to increase vaccination completion rates. Electronic health records with complete information on patient's immunization history are among the Standards for Adult Immunization Practice recommended by the National Vaccine Advisory Committee.Citation29 Previous investigations have shown that electronic medical record (EMR) reminders significantly improved the rates of influenza and pneumococcal vaccination in a population of patients over 65 years of age.Citation30 More recent analysesCitation31,Citation32 compared 3-dose HepB vaccine completion rates in patients with diabetes aged 19–59 years between two large US managed care organizations, Kaiser Permanente Southern California in which an electronic alert and a reminder were implemented in the EMR and Kaiser Permanente Northern California in which no such reminder was used.Citation31 The site with EMR reminders increased vaccination coverage rapidly from 7.6% in 2012 to 29.4% in 2015, while no increase was seen at the site with no EMR reminder.Citation31,Citation32 EMR are increasingly being adopted by health systems,Citation31 and might therefore be a valuable tool for improving vaccination completion.
Limitations
Selection of patients with a HepA, HepB, or HepA-HepB vaccine was based on CPT codes, and coding inaccuracies may lead to misclassification bias and misidentification of patients with a hepatitis vaccine. In addition, CPT codes cannot distinguish between brands of hepatitis vaccines and thus cannot differentiate between vaccination schedules for the two HepA vaccines. Completion windows were adjusted to enable completion of the longest time window within each vaccine series. Finally, our population was covered by employer-sponsored health care with supplemental Medicare plans, or Medicaid, and may not be generalizable to an uninsured population.
Methods
Study design
We used the Truven Health Analytics MarketScan claims database, which is a large US administrative claims database. Data retrieved included Q1 2007-Q3 2015 data from the Commercial Claims and Encounters database and the Medicare Supplemental and Coordination of Benefits database (“commercial/Medicare” cohort) and Q1 2007-Q4 2014 data from the Medicaid Multi-State database (“Medicaid” cohort). The Truven MarketScan database is de-identified and complies with the Health Insurance Portability and Accountability Act (HIPAA) to preserve patient anonymity and confidentiality.
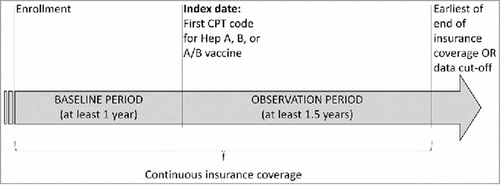
The date of the first Current Procedural Terminology (CPT) code for a HepA, HepB, or HepA-HepB vaccine was defined as the ‘index date’. More precisely, patients who initiated a series for HepA and another series for HepB vaccine with monovalent vaccines had distinct index dates that corresponded to the first dose of each vaccine series. However, once a patient had initiated a HepA or HepB series, their index date for each series was locked. Thus, patients who initiated with a monovalent vaccine could not have a later bivalent index date, and patients who initiated with a bivalent vaccine could not have a later monovalent index date. Identification of subsequent doses was also based on CPT codes. Study periods consisted of a ‘baseline period’ that was the period from the date of health plan enrollment up to and including the index date, and an ‘observation period’ that was the period from the index date to the end of insurance coverage or data cut-off ().
Figure 2. Study design scheme; Abbreviations: CPT, Current Procedural Terminology
*Hepatitis A adult dosage, CPT 90632; Hepatitis B adult standard 3 doses, CPT: 90740; Hepatitis B for dialysis or immunocompromised patients, 3 doses, CPT: 90746; Hepatitis B for dialysis or immunocompromised patients, 4 doses, CPT: 90747; Hepatitis A and B adult dosage, CPT: 90636.
Study population
Patients were included in the study if they had ≥1 claim with a CPT code for HepA, HepB, or HepA-HepB vaccine, were ≥19 years of age at index date, and had continuous insurance enrollment for ≥12 months before the index date (“baseline period”) and ≥18 months after the index date (“observation period”). This was to allow for the evaluation of vaccination series completion, because the minimum required time for the completion of a full series vaccination against HepA is 6 months and against HepB is 4 months (). Patients were excluded if they had ≥2 claims with HepA diagnoses before HepA or HepA-HepB index dates or ≥2 claims with HepB diagnoses before HepB orHepA-HepB index date.
Table 2. Hepatitis A, B, or A/B Vaccine Completion Adult Schedule GuidelinesCitation11,Citation12
Outcomes
HepA and HepB vaccine series completion was assessed separately for HepA and HepB and was defined as a series of either monovalent HepA or HepB vaccines, bivalent vaccines, or a combination of monovalent HepA or HepB vaccines with bivalent HepA-HepB vaccine, as shown in . In addition, only doses separated by minimum intervals based on schedules per CDC guidelines were included, and identified using CPT codes in the datasetsCitation11,Citation12 (). The proportion of patients in each cohort who received ≥2 doses of HepB vaccination was also assessed.
Statistical analyses
All analyses were stratified by commercial/Medicare and Medicaid patient cohorts. Descriptive statistics were generated to summarize patient baseline characteristics and series completion. Additionally, the number and proportion of patients completing ≥2 doses of HepB and the full HepA and HepB series were assessed and analyses were stratified according to whether the initial dose was a monovalent or bivalent vaccine.
Conclusions
HepA and HepB vaccination series completion rates were low among US adults, and appeared to be generally lower among Medicaid than among commercial/Medicare enrollees. Adult commercial/Medicare patients starting hepatitis vaccination series with the bivalent vaccine had higher rates of completion than those starting with monovalent vaccines – an observation that was not made among Medicaid enrollees. Low rates of HepA and HepB vaccination series completion rates among adults in the US, demonstrate the need for interventions that increase series completion, thereby offering optimal protection for individual patients, and contributing to public health.
Clinical trial registration
Not applicable.
Prior presentation
ISPOR 22nd Annual International Meeting; May 20–24, 2017; Boston, MA, USA.
Abbreviations
| ACIP | = | Advisory Committee on Immunization Practices |
| CI | = | confidence interval |
| CDC | = | Centers for Disease Control and Prevention |
| CPT | = | Current Procedural Terminology |
| EMR | = | electronic medical record |
| HAV | = | hepatitis A virus |
| HBV | = | hepatitis B virus |
| HepA | = | hepatitis A |
| HepB | = | hepatitis B |
| US | = | United States |
Disclosure of potential conflicts of interest
BJP and PKG are employees of the GSK group of companies. BJP and PKG hold shares in the GSK group of companies. WYC, ED, MM, and MSD, are employees of Analysis Group, Inc., which has received research funds from the GSK group of companies, including one for the current study.
Author contributions
WYC, ED, MM, BJP, MSD were involved in the study design. All authors participated in the interpretation of the study results and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Acknowledgments
The authors would like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Marie Cloes coordinated manuscript development and provided editorial support. Athanasia Benekou provided medical writing support.
Additional information
Funding
References
- Matheny SC, Kingery JE. Hepatitis A. Am Fam Physician. 2012; 86(11):1027–34.
- Chang MS, Nguyen MH. Epidemiology of hepatitis B and the role of vaccination. Best Pract Res Clin Gastroenterol. 2017; 31(3):239–47. doi:10.1016/j.bpg.2017.05.008.
- Centers for Disease Control and Prevention. Prevention of hepatitis A throught active or passive immunization: recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 1996; 45(RR15):1–30.
- Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006; 55(RR-7):1–23.
- Centers for Disease Control and Prevention. Hepatitis B Virus: A comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: Recommendations of the immunization practices advisory committee (ACIP). MMWR Morb Mortal Wkly Rep. 1991; 40(RR13):1–19.
- Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitis – United States, 2007. MMWR Surveill Summ 2009; 58(3):1–27.
- The U.S. National Viral Hepatitis Action Plan for 2017–2020. Department of Health & Human Services,Division of Viral Hepatitis and National Center for HIV/AIDS; [accessed 12/8/2017]. https://www.hhs.gov/sites/default/files/National%20Viral%20Hepatitis%20Action%20Plan%202017-2020.pdf.
- Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States, 2015. [accessed 12/8/2017]. https://www.cdc.gov/hepatitis/statistics/2015surveillance/commentary.htm.
- Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States, 2014. [accessed 12/8/2017]. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#summary.
- Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward J, et al. Prevention of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018; 67(RR-1):1–31. doi:10.15585/mmwr.rr6701a1.
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: Hepatitis A. [accessed 9/9/2017]. https://www.cdc.gov/vaccines/pubs/pinkbook/hepa.html.
- Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases: Hepatitis B. [accessed 9/9/2017]. https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html.
- Williams WW, Lu PJ, O'Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of Vaccination Coverage among Adult Populations – United States, 2015. MMWR Surveill Summ. 2017; 66(11):1–28. doi:10.15585/mmwr.ss6611a1.
- Nelson JC, Bittner RC, Bounds L, Zhao S, Baggs J, Donahue JG, Hambidge SJ, Jacobsen SJ, Klein NP, Naleway AL, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a Vaccine Safety Datalink study. Am J Public Health. 2009; 99 Suppl 2:S389–S97. doi:10.2105/AJPH.2008.151332.
- U.S.Food & Drug Administration. TWINRIX [Hepatitis A & Hepatitis B (Recombinant) Vaccine]. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM110079.pdf.
- Van Damme P, K. Van Herck. A review of the efficacy, immunogenicity and tolerability of a combined hepatitis A and B vaccine. Expert Rev Vaccines 2004; 3(3):249–67. doi:10.1586/14760584.3.3.249.
- Heywood AE, Nothdurft H, Tessier D, Moodley M, Rombo L, Marano C, De Moerlooze L. Pre-travel advice, attitudes and hepatitis A and B vaccination rates among travellers from seven countries. J Travel Med. 2016; 24(1):1–8. doi:10.1093/jtm/taw069.
- Jacob L, Kostev K. Compliance with vaccination against hepatitis A virus in Germany: A retrospective analysis. Int J Clin Pharmacol Ther. 2017; 55(9):740–5.
- Paradise J. Data note: three findings about access to care and health outcomes in Medicaid. [accessed 2/11/2018]. https://www.kff.org/medicaid/issue-brief/data-note-three-findings-about-access-to-care-and-health-outcomes-in-medicaid/.
- Trantham L, Kurosky S, Zhang D, Johnson KD. Adherence to multidose hepatitis A and hepatitis B vaccine schedules in the US. October 4–8, San Diego, CA: IDWeek; 2017.
- Trantham L, Kurosky S, Zhang D, Lu IX, Johnson KD. Hepatitis A and hepatitis B recombinant vaccine adherence in the United States. Value in Health. 2017; 20(9):A791. doi:10.1016/j.jval.2017.08.2326.
- Paradise J. 10 Things to Know about Medicaid: Setting the Facts Straight. [accessed 2/11/2018]. https://www.kff.org/medicaid/issue-brief/10-things-to-know-about-medicaid-setting-the-facts-straight/.
- Lu PJ, Byrd KK, Murphy TV, Weinbaum C. Hepatitis B vaccination coverage among high-risk adults 18–49 years, U.S., 2009. Vaccine. 2011; 29(40):7049–57.
- Byrd KK, Lu PJ, Murphy TV. Hepatitis B vaccination coverage among health-care personnel in the United States. Public Health Rep. 2013; 128(6):498–509. doi:10.1177/003335491312800609.
- Byrd KK, Lu PJ, Murphy TV. Baseline hepatitis B vaccination coverage among persons with diabetes before implementing a U.S. recommendation for vaccination. Vaccine. 2012; 30(23):3376–82. doi:10.1016/j.vaccine.2012.03.055.
- Lu PJ, Byrd KK, Murphy TV. Hepatitis A vaccination coverage among adults 18–49 years traveling to a country of high or intermediate endemicity, United States. Vaccine. 2013; 31(19):2348–57. doi:10.1016/j.vaccine.2013.03.011.
- Williams WW, Lu PJ, O'Halloran A, Bridges CB, Kim DK, Pilishvili T, Hales CM, Markowitz LE, Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination – United States, 2013. MMWR Morb Mortal Wkly Rep. 2015; 64(4):95–102.
- Ramirez JC, Ackerman K, Strain SC, Ahmed ST, de los Santos MJ, Sears D. Hepatitis A and B screening and vaccination rates among patients with chronic liver disease. Hum Vaccin Immunother. 2016; 12(1):64–9. doi:10.1080/21645515.2015.1068484.
- Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014; 129(2):115–23. doi:10.1177/003335491412900203.
- Loo TS, Davis RB, Lipsitz LA, Irish J, Bates CK, Agarwal K, Markson L, Hamel MB. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011; 171(17):1552–8. doi:10.1001/archinternmed.2011.394.
- Hechter RC, Qian L, Luo Y, Ling Grant DS, Baxter R, Klein NP, et al. Impact of an electronic medical record reminder on hepatitis B vaccine initiation and completion rates among insured adults with diabetes mellitus. Vaccine. Manuscript accepted for publication (on June 16, 2018).
- Hechter RC, Qian L, Luo Y, Im T, Baxter R, Valdez-Nunley K, et al. Effect of electronic reminders on hepatitis B Vaccine completion in diabetic adults. Diabetes. 2017; 66(Supplement 1):A449.
