ABSTRACT
A novel Staphylococcus aureus 4-antigen vaccine (SA4Ag) is under development, comprising capsular polysaccharide serotypes 5 and 8 (CP5 and CP8) conjugated to CRM197, recombinant protein clumping factor A (rmClfA), and recombinant manganese transporter protein C (MntC). We evaluated SA4Ag safety, tolerability, and immunogenicity in Japanese adults aged 20 to 64 and 65 to 85 years.
A total of 136 healthy Japanese adults (68 per age group) were randomized 1:1 to receive single-dose SA4Ag or placebo intramuscularly (Day 1). Safety assessments included reactogenicity and adverse events. The ability of the vaccine to induce immune responses that are considered functional due to their ability to facilitate the killing of S. aureus or neutralize S. aureus virulence mechanisms was assessed using 5 different antigen-specific assays.
SA4Ag was well tolerated in both age groups, with no safety concerns. At Day 29, > 85% of SA4Ag recipients in each age group achieved predefined thresholds for each antigen. Antibody geometric mean-fold rises from baseline to Day 29 in SA4Ag groups were: > 80 and > 30 for CP5 and CP8 (opsonophagocytic activity assay), > 10 for ClfA (fibrinogen-binding inhibition assay), and > 15 and > 7 for ClfA and MntC (competitive Luminex® immunoassay), respectively. Antibody titers decreased through Month 12 but remained well above baseline and placebo levels.
SA4Ag had an acceptable safety profile and induced rapid and robust functional immune responses in both age groups. These results support ongoing development of SA4Ag for the prevention of invasive S. aureus disease in elective-surgery patients in Japan, North America, and Europe.
Introduction
Staphylococcus aureus (S. aureus) is a Gram-positive coccus causing 12% of all healthcare-associated infections in the United States (US).Citation1 S. aureus is the most frequent isolate from inpatients in Japan, comprising approximately 14% of all clinical isolates in 2016.Citation2 The annual incidence of methicillin-resistant S. aureus (MRSA) infection in Japan was 3.1 per 1,000 inpatients in 2016,Citation3 and hospitalized surgical and nonsurgical inpatients with MRSA infections had much higher hospitalization costs, length of stay, and inhospital mortality than those without MRSA.Citation4
S. aureus is the most common cause of surgical-site infections (SSIs) globally.Citation5 In Japan, S. aureus was the second leading cause of SSIs, accounting for approximately 9% of clinical isolates from SSIs in 2016,Citation6 with MRSA comprising approximately half (49% to 54%) of S. aureus SSIs.Citation6,Citation7 In Japanese university hospitals, the crude mortality rate of postoperative bloodstream infections due to S. aureus was reported to be as high as 25%.Citation8 Postoperative S. aureus infection therefore remains a common and serious burden on health systems globally, despite the preventive and therapeutic measures currently employed. Development of an efficacious prophylactic S. aureus vaccine would provide a novel mechanism for S. aureus disease prevention.Citation9
An investigational S. aureus 4-antigen vaccine (SA4Ag), currently in development, has been shown to elicit rapid and robust functional immune responses (demonstrated by the ability of antibodies to facilitate S. aureus killing and neutralize virulence mechanisms) in clinical studies in healthy US adults aged 18 to 85 years.Citation10–Citation12 SA4Ag includes 4 antigens targeting 3 virulence mechanisms: capsular polysaccharide serotypes 5 and 8 (CP5 and CP8) conjugated to the nontoxic mutant form of diphtheria toxin (cross-reactive material 197 [CRM197]), a recombinant mutated surface protein clumping factor A (rmClfA), and recombinant protein 305A (rP305A, a recombinant nonlipidated form of the S. aureus manganese transporter C [MntC] protein). SA4Ag is designed to be protective against both methicillin-susceptible S. aureus and MRSA. Among healthy adults (aged 18 to 85 years) who received a single dose of SA4Ag, peak levels of bacteria-killing antibodies were achieved 10 to 14 days after vaccination and remained higher than baseline levels through Month 36.Citation10,Citation11,Citation13 Antibody levels were persistently elevated throughout the first 3 months following vaccination.Citation11 This suggests that SA4Ag may be useful for preventing postoperative S. aureus infection, given that the risk of infection is highest in the first few months following surgery. In all studies, SA4Ag was well tolerated, with no safety concerns identified.Citation10–Citation12 In this first-in-Japanese study (NCT02492958), the safety, tolerability, and immunogenicity of a single dose of SA4Ag were evaluated in adults aged 20 to < 86 years.
Results
Participants
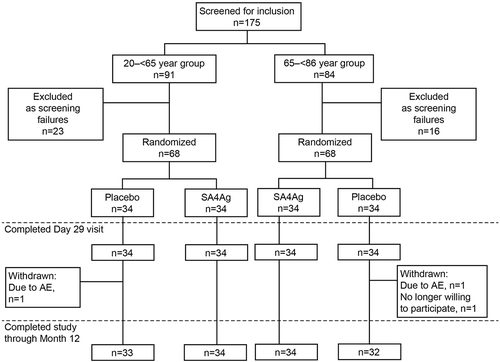
A total of 91 participants in the 20 to < 65 year age group and 84 participants in the 65 to < 86 year age group provided informed consent and were screened. In each age group, 68 participants were randomized to receive either placebo or SA4Ag, with 34 participants each in the placebo and SA4Ag groups. All participants (100%) in both age groups completed the study up to the Day 29 visit (for blood sampling) and were included in the evaluable, safety, and modified intention-to-treat (mITT) immunogenicity populations (). After the Day 29 blood sampling visit, 3 participants who received placebo withdrew from the study; 1 participant in each of the age groups withdrew owing to an adverse event (AE) (considered unrelated to investigational product); and 1 participant in the 65 to < 86 year age group withdrew, as they were no longer willing to participate in the study (). All participants were Japanese and 50% were female (). The mean age at vaccination was 47.0 (range 21 to 64) and 70.3 (range 65 to 80) years for the younger and older age groups, respectively. Participant demographics were generally well balanced across the SA4Ag and placebo groups for both age groups. However, in the older age group, the SA4Ag group had a higher proportion of males (61.8%) than the placebo group (38.2%) ().
Table 1. Participant demographics.
Immunogenicity
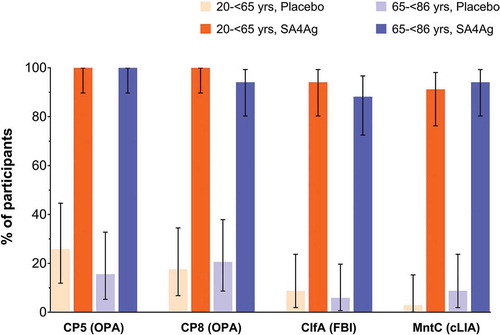
Prior to vaccination, less than 21% of placebo recipients and less than 34% of SA4Ag recipients had titers at or above the defined thresholds for CP5, CP8, ClfA, or MntC, regardless of participant age group. In both age groups, almost all participants (> 90%) receiving SA4Ag achieved the predefined opsonophagocytic activity (OPA) thresholds for CP5 and CP8 on Day 29 after vaccination (). Similarly, > 85% of participants receiving SA4Ag achieved the predefined thresholds for ClfA (measured using the fibrinogen-binding inhibition [FBI] assay) and MntC (measured using the competitive Luminex® immunoassay [cLIA]) on Day 29 after vaccination. In contrast, less than 30% of placebo recipients in each age group achieved the defined thresholds for CP5, CP8, ClfA, or MntC ().
Figure 2. Primary immunogenicity results, participants achieving predefined antibody thresholds to target antigens on Day 29 after vaccination.
OPA antibody titer thresholds were predefined for CP5 and CP8 at OPA titers ≥ 1,000 and ≥ 2,000, respectively. The cLIA antibody titer threshold of 4 × the LLOQ was set for the response to MntC (the LLOQ of the MntC cLIA was adjusted from 107.9 to 128 U/mL following assay method validation). The threshold for ClfA responses assessed using the FBI assay was set as 1 × the LLOQ (titer of 121). Footnote: ClfA, clumping factor A; cLIA, competitive Luminex® immunoassay; CP5, capsular polysaccharide serotype 5; CP8, capsular polysaccharide serotype 8; FBI, fibrinogen-binding inhibition; LLOQ, lower limit of quantification; MntC, manganese transporter protein C; OPA, opsonophagocytic activity; SA4Ag, Staphylococcus aureus 4-antigen vaccine.
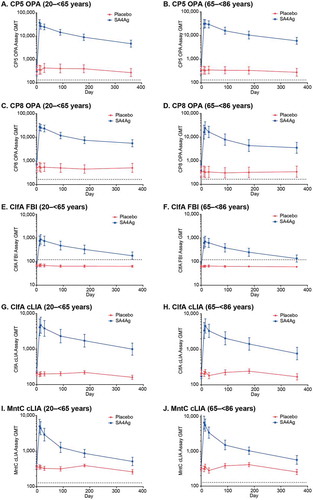
In both age groups, participants receiving SA4Ag showed a rapid increase in OPA (CP5 and CP8), FBI (ClfA), and cLIA (ClfA and MntC) geometric mean titers (GMTs), which peaked at Day 11 or 15 (). Elevated assay GMTs were observed for each antigen through Month 3 in participants receiving SA4Ag; although GMTs gradually declined through Month 12, they remained substantially higher than at baseline (). For placebo recipients in both age groups, the assay GMTs for all antigens remained close to baseline values at each time point from baseline through Month 12 (). Participants in both age groups receiving SA4Ag had substantial OPA, FBI, and cLIA geometric mean-fold rises (GMFRs) from baseline to each time point from Day 11 through Month 3 for all antigens; OPA GMFRs were > 80 for CP5 and > 30 for CP8; FBI GMFRs were > 10 for ClfA; and cLIA GMFRs were > 15 for ClfA and > 7 for MntC at Day 29 (). For all subjects receiving placebo, no substantial changes in GMFRs were observed from Day 11 through Month 3 ().
Table 2. GMFRs through Month 3.
Figure 3. Kinetics of GMTs (95% CI) for each antigen through Month 12: (A) and (B) CP5 (OPA), 20 to < 65 and 65 to < 86 age groups, respectively; (C) and (D) CP8 (OPA), 20 to < 65 and 65 to< 86 age groups, respectively; (E) and (F) ClfA (FBI), 20 to < 65 and 65 to < 86 age groups, respectively; (G) and (H) ClfA (cLIA), 20 to < 65 and 65 to < 86 age groups, respectively; (I) and (J) rP305A (MntC) (cLIA), 20 to < 65 and 65 to < 86 age groups, respectively. Dotted lines indicate LLOQ for each assay. Data are from the evaluable immunogenicity population.
Footnote: CI, confidence interval; ClfA, clumping factor A; cLIA, competitive Luminex® immunoassay; CP5, capsular polysaccharide serotype 5; CP8, capsular polysaccharide serotype 8; FBI, fibrinogen-binding inhibition; GMT, geometric mean titer; LLOQ, lower limit of quantification; OPA, opsonophagocytic activity; rP305A (MntC), manganese transporter protein C; SA4Ag, Staphylococcus aureus 4-antigen vaccine.
Safety
Local reactions
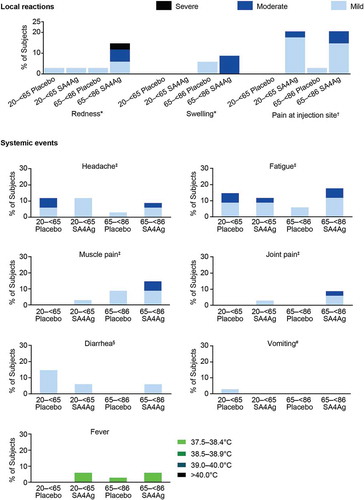
In both study groups, almost all local reactions reported in subjects’ electronic diaries (e-diaries) were mild or moderate in severity; only 1 severe local reaction (redness) was reported, by a subject in the 65 to < 86 year age group who received SA4Ag (). The most commonly reported local reaction was pain at the injection site, which was mild or moderate in severity and more frequently reported by subjects who received SA4Ag (). Local reactions were only reported by a small proportion (< 10%) of subjects who received placebo. The duration of the local reactions was 1 to 2 days in the 20 to < 65 year age group and 1 to 8 days in the 65 to < 86 year age group. In the SA4Ag group, both age groups reported local reactions with onset on Day 1 (5.9%) and Days 6 to 8 (2.9% to 11.8%). In the placebo group, onset of all local reactions was on Day 1 (in both age groups).
Figure 4. Proportion of participants with local vaccination-site reactions and systemic events within 14 days of vaccination.
20 to < 65 Placebo, 20 to < 65 year age group receiving placebo; 20 to < 65 SA4Ag, 20 to < 65 year age group receiving SA4Ag; 65 to < 86 Placebo, 65 to < 86 year age group receiving placebo; 65 to < 86 SA4Ag, 65 to < 86 year age group receiving SA4Ag. *For Redness and swelling: mild, 2.5 to 5.0 cm; moderate, 5.5 to 10.0 cm; severe, ≥ 10.5 cm. †For Pain at injection site: mild, no interference with activity; moderate, interfering with activity; severe, preventing daily activity. ‡For Headache, fatigue, muscle pain, and joint pain: mild, no interference with activity; moderate, some interference with activity; severe, preventing daily activity. §For Diarrhea: mild, 2 to 3 loose stools a day; moderate, 4 to 5 loose stools a day; severe, 6 or more loose stools a day. #For Vomiting: mild, 1 to 2 times a day; moderate: > 2 times a day, severe: requires intravenous hydration. Footnote: SA4Ag, Staphylococcus aureus 4-antigen vaccine.
Systemic events
A total of 20 (29.4%) subjects in the 20 to < 65 year age group and 18 (26.5%) subjects in the 65 to < 86 year age group reported at least 1 systemic event; all systemic events were mild or moderate in severity, and the incidence of systemic events was similar among subjects receiving SA4Ag and those receiving placebo (). Headache, fatigue, muscle pain, and diarrhea were the most commonly reported systemic events (), and the duration of all systemic events was 1 to 10 days. The onset of systemic events was from Day 1 and occurred intermittently throughout the 14-day reporting period, with no discernible pattern of onset among those receiving SA4Ag or placebo (in either age group).
Adverse events
Between Day 1 and Day 29, no serious AEs (SAEs), severe AEs, life-threatening AEs, AEs leading to withdrawal, or deaths occurred in either age group. One event considered to be related to investigational product was reported in each study group for subjects aged 65 to < 86 years: 1 subject reported injection-site pain in the SA4Ag group, and 1 subject reported headache in the placebo group. Both subjects missed these entries in their e-diaries, and the events were subsequently reported as AEs by the investigator in the case report form.
From the Day 29 visit through Month 12, no vaccine-related AEs occurred, and no deaths or life-threatening AEs were reported in either age group. A subject in the 20 to < 65 year age group who received placebo had a severe SAE (bladder cancer, not related to the study vaccine) and withdrew from the study. No SAEs were reported for participants in the 20 to < 65 year age group receiving SA4Ag. For the 65 to < 86 year age group, SAEs were reported by 3 participants receiving SA4Ag (1 with cataract, macular fibrosis, and hyalosis asteroid; 1 with cerebral infarction, intracranial aneurysm, and carotid artery aneurysm; and 1 with prostatitis), and SAEs were reported by 2 participants receiving placebo (1 with contusion and rib fracture, the other with vocal cord disorder and breast cancer). The participant with breast cancer withdrew from the study.
The majority of Phase 1 participants had normal hematology, biochemistry, and coagulation parameters at baseline, Day 5, and Day 15 post-vaccination. No Grade 3 or 4 (see Supplementary Table 1 for definition) abnormalities were reported, and no consistent pattern of abnormal results was found. All abnormal laboratory values were not considered clinically significant (no associated AEs were reported) by the investigator.
Discussion
A single-dose administration of SA4Ag in Japanese adults aged 20 to < 65 and 65 to < 86 years elicited rapid and robust immune responses to CP5, CP8, ClfA, and MntC that persisted through Month 12 after vaccination. Peak antibody responses to each antigen were observed at Day 11 or Day 15 in participants receiving SA4Ag. Although antibody levels waned gradually through Month 12, persistent immune responses to each of the antigens were demonstrated; antibody levels at each post-vaccination time point through Month 12 remained substantially higher in the SA4Ag group compared with the placebo group or baseline. The proportions of participants in the placebo group who achieved the predefined thresholds for each antigen at Day 29 were low (< 30%) compared with the proportion of participants receiving SA4Ag (> 85%) and were similar at baseline and throughout the study. No increases in GMTs were observed in the placebo group.
SA4Ag was well tolerated, with an acceptable safety profile in healthy Japanese adults. Local reactions reported within the first 14 days after vaccination were more common in the SA4Ag group for both age groups and were mostly mild in severity. Systemic events were all mild or moderate in severity, and there were no clear differences in the incidences and severities of systemic events between SA4Ag and placebo recipients. Few AEs were reported in this study, and no notable differences in the frequency of AEs were observed between the participants receiving SA4Ag and those receiving placebo in either age group. Clinical laboratory evaluations suggest that SA4Ag did not have an impact on hematology parameters, coagulation profile, or blood chemistry.
SA4Ag targets highly conserved virulence factors of S. aureus that are expressed early during infection, regardless of the antibiotic resistance profile or geographic origin of the bacterium.Citation9,Citation14–Citation16 The results of this study are consistent with previous studies conducted in Australia with an initial 3-antigen vaccine formulation (SA3Ag)Citation17,Citation18 and studies with SA4Ag conducted in the US,Citation10–Citation12 which have demonstrated that after a single dose, functional immune responses can be elicited by SA3Ag or SA4Ag in populations from different geographic regions. There is currently no licensed S. aureus vaccine available, and no reliable natural history seroepidemiology studies to conclusively demonstrate that protection can be mediated by either opsonic antibodies to CP5 and CP8, or antibodies to the single adhesin (microbial surface component recognizing adhesive matrix molecule), ClfA, or to MntC. Demonstration that SA4Ag can induce an immune response that (i) increases the ability to recognize the pathogen, (ii) prevents the pathogen from binding to fibrinogen and from acquiring manganese, and (iii) subsequently enables the pathogen to be killed by neutrophils, is a positive step in the development of a vaccine to prevent invasive S. aureus disease. Ultimate validation of these S. aureus surface antigens as vaccine targets will be established after successful conclusion of an efficacy study with SA4Ag. Currently, the efficacy of SA4Ag is under assessment in an international, multicenter, randomized, placebo-controlled Phase 2b study in patients undergoing elective open posterior multilevel spinal fusion procedures in Japan, North America, and Europe (ClinicalTrials.gov identifier NCT02388165).
The 2 S. aureus vaccines previously under development (StaphVAX, Nabi; V710, Merck) did not address multiple virulence factors associated with S. aureus infection and there were limited or no data in humans demonstrating that the vaccines could induce functional antibodies that could kill the pathogen or neutralize the antigen-associated virulence mechanism. In addition, a post hoc analysis of safety data in the V710 study showed an increased risk of death (in some cases associated with multi-organ failure) in vaccinated participants who developed invasive S. aureus infections.Citation19 A causal link to the observed potential safety signal has not been established. Of note, V710 was a single antigen iron-binding protein that shares no antigens in common with SA4Ag, and importantly, no such safety signal was observed in the studies of StaphVAX.Citation20,Citation21 Functional antibodies were rapidly elicited by SA4Ag in both younger and older Japanese adults. This is important given the higher burden of S. aureus disease in the elderly,Citation22 and the potential for vaccines to have lower immune responses and efficacy in the elderly.Citation23
Conclusion
SA4Ag was well tolerated in healthy Japanese adults aged 20 to < 86 years, with no safety concerns observed. SA4Ag elicited rapid (11 to 15 days to peak response) and robust functional immune responses in all participants, and the immune responses persisted through Month 12. Results are consistent with Australian and US studiesCitation10-Citation12,Citation17,Citation18 and support further development of SA4Ag in Japan for the prevention of invasive S. aureus disease in adults. The efficacy of SA4Ag in adults undergoing elective spinal fusion procedures is being investigated in study B3451002 (STRIVE, NCT02388165), an ongoing study currently enrolling participants in Japan, North America (US and Canada), and Europe.
Materials and methods
Study design
This was a first-in-Japanese Phase 1/2a, multicenter, parallel-group, placebo-controlled, randomized, double-blind, sponsor-unblinded study, which included Phase 1 and Phase 2 components. The study was carried out between June 2015 and September 2016 at 2 study sites in Japan. Approximately 136 participants in 2 age groups (20 to < 65 years and 65 to < 86 years) were planned to be enrolled. The younger age group was further divided into 2 age substrata (20 to < 50 years and 50 to < 65 years), with approximately 34 participants in each substratum, while the 65 to < 86 year age group had 68 participants.
An interactive web response system was used for randomization of all participants in this study. The Phase 1 component of the study included approximately 24 participants (20 to < 50 years, 6 participants; 50 to < 65 years, 6 participants; and 65 to < 86 years, approximately 12 participants). Beginning with the youngest age substratum, participants were randomized in a 1:1 ratio to receive a single injection of SA4Ag or placebo. An internal review committee evaluated at least 14 days of post-vaccination safety data from all Phase 1 participants before Phase 2 enrollment began. Eligible participants enrolled in Phase 2 were to be randomized in parallel in a 1:1 ratio to receive a single injection of either SA4Ag or placebo.
Study visits took place on Days 1 (vaccination visit), 11, 15, and 29, and at Months 3, 6, and 12 (End of Study). Participants in Phase 1 of the study had additional visits, consisting of a screening visit 2 to 14 days prior to the vaccination visit and a visit on Day 5. This study was approved by the institutional review board of the individual investigational centers. It was conducted in compliance with the Declaration of Helsinki and all International Council for Harmonisation (ICH) Good Clinical Practice (GCP) guidelines. In addition, all local regulatory requirements were followed. Written informed consent was obtained from all participants prior to study enrollment and any study-related procedures.
Study population
Healthy Japanese male and female adults aged 20 to < 86 years at enrollment were eligible for participation in the study. Participants capable of having children were eligible if they agreed to use a highly effective contraception method from the day of vaccination to 3 months after vaccination. Several exclusion criteria were used to determine eligibility for participation in the study: (1) an unstable chronic medical condition or worsening disease within 3 months before vaccination; (2) serious chronic medical disorders, such as metastatic malignancy, severe chronic obstructive pulmonary disease, end-stage renal disease, or clinically unstable cardiac disease; (3) donation of ≥ 250 mL of blood or plasma within 3 months prior to enrollment; (4) bleeding diathesis or any condition associated with prolonged bleeding time; (5) any contraindication to vaccination or vaccine components; (6) immunocompromised, with current or past immunosuppressive therapy, immune-modifying drugs, or immune response modifiers; (7) planned surgical procedure within 30 days following vaccination; (8) previous administration of an S. aureus vaccine; (9) any proven or suspected S. aureus infection within 6 months before vaccination; (10) receipt of blood products or immunoglobulins within 12 months before enrollment; (11) participation in other trials involving investigational drug(s) within 30 days before enrollment; (12) pregnant or breastfeeding female participants; (13) resident in a nursing home or long-term care facility or requirement for semiskilled nursing care; and (14) known active disease with human immunodeficiency virus (HIV), hepatitis B virus (HBV), and/or hepatitis C virus (HCV), or other severe acute or chronic medical or psychiatric condition or laboratory abnormality.
Additional exclusion criteria were applied to the participants in the Phase 1 component of the study; participants with abnormality in screening hematology, coagulation, and/or blood chemistry laboratory values were not eligible for the study. However, participants with stable abnormality in lactate dehydrogenase, stable Grade 1 (Supplementary Table 1) abnormality in creatine kinase level, or decreased white blood cell and absolute neutrophil count within normal limits could be considered eligible at the discretion of the investigator. Participants who had positive results in the screening tests for HIV, HBV, and/or HCV were also excluded.
Vaccination
Participants received a single 0.5-mL dose of SA4Ag (containing 30 μg each of CP5-CRM197 and CP8-CRM197, 60 μg of rmClfA, and 200 μg of rP305A [MntC]) or placebo into the deltoid muscle in the upper arm at the Day 1 visit. The placebo contained the same lyophilized constituents as the vaccine, without the active ingredients (antigens). No other vaccines were allowed to be administered concurrently. If medically necessary, the administration of a licensed vaccine at least 28 days after or at least 28 days before study vaccination was permitted. Medications required for the treatment of preexisting stable conditions, inhaled, topical, or localized injections (e.g., intra-articular or intrabursal administration) of corticosteroids and low-dose aspirin (≤ 300 mg per day), as well as antipyretics and other pain medication to treat symptoms associated with study vaccination were permitted.
Immunogenicity assessments
Immune responses were measured by immunoassays that monitor functional responses to each antigen: the OPA assayCitation12,Citation14 for CP5 and CP8; the FBI assayCitation12,Citation24 for ClfA; and the cLIACitation17,Citation25 for ClfA and MntC. These assays were performed on blood samples collected during each study visit. The lower limit of quantitation (LLOQ) for the OPA assays were OPA titers of 132 for CP5 and 165 for CP8. The LLOQ for the ClfA FBI assay was a titer of 121. Antigen-specific serum antibody levels against ClfA and MntC were measured using a competitive-binding 2-plex cLIA. The LLOQ values for ClfA and MntC were 11 U/mL and 128 U/mL, respectively.
Study endpoints
The primary immunogenicity endpoint was the proportion of participants achieving antibody responses to each vaccine antigen 1 month after vaccination (Day 29 visit). Antibody responses were assessed using antibody thresholds previously established for the preceding Phase-1/2 trials conducted in the US.Citation10 The secondary and exploratory immunogenicity endpoints included GMTs at each time point for each antigen and GMFRs from baseline (Day 1) to each time point up to Month 3 for each antigen. Safety endpoints included the number and proportion of participants reporting specific events: (1) local reactions (size of redness and/or swelling and severity of pain at the injection site) and their severity; (2) systemic events (fever, fatigue, headache, vomiting, diarrhea, muscle pain, and joint pain) and their severity; and (3) AEs, newly diagnosed chronic medical disorders, and SAEs. The additional safety endpoints were the number and proportion of participants in Phase 1 who had a shift in grade or an abnormality in hematology, coagulation, and blood chemistry laboratory assessments.
Safety assessments
Participants assessed redness, swelling, and pain at the injection site and recorded any symptoms in their e-diaries from Day 1 to Day 14 post-vaccination. Systemic events reportable in the e-diary from Day 1 to Day 14 were fever, vomiting, diarrhea, headache, fatigue, muscle pain, and joint pain. Axillary temperature was measured using a digital thermometer daily at bedtime for 14 days following vaccination and at any time during the 14 days when fever was suspected.
AEs were recorded from the date of informed consent to the Day 29 visit and were categorized according to the Medical Dictionary for Regulatory Activities (MedDRA) Version 19.0. From the Day 29 visit to the completion of study participation, only AEs of newly diagnosed chronic medical disorders were collected. SAEs were collected from date of informed consent through completion of participant study participation. For all Phase 1 participants, hematologic, coagulation, and blood chemistry assessments were performed at the screening visit prior to vaccination and at the Day 5 and Day 15 visits at a local laboratory at each study site (see Supplementary Table 1 for each assessment).
Statistical methods
Sample size
The sample sizes for the study were not based on statistical hypothesis testing considerations, and all statistical analyses were descriptive. The administration of SA4Ag to 34 participants in each age group provides more than an 80% probability of observing at least 1 occurrence of any AE with a true incidence rate of 4.7%. For the evaluable immunogenicity population, assuming approximately 10% dropout, 30 participants were to be evaluated per study group in each age group. Assuming 30 evaluable participants in each age group and the true probability of participants achieving the threshold value or higher being 95%, the probability of ≥ 90% of the participants achieving the threshold value or higher was 93.9%.
Population
The evaluable immunogenicity population was considered the primary population for the analysis of immunogenicity data and included participants who were eligible for the study and who had no other major protocol deviations as determined by the clinicians from Day 1 to Day 29. The mITT immunogenicity population included all participants who were randomized and had at least 1 valid and determinate assay result for at least 1 antigen after vaccination. All participants who received at least 1 dose of SA4Ag or placebo and had any safety data after vaccination were included in the safety population, which was used for the safety analyses.
Immunogenicity analysis
The proportion of participants achieving the predefined antibody thresholds was calculated, along with exact 2-sided 95% confidence intervals (CIs), using the F distribution (Clopper-Pearson). Titers were logarithmically transformed for analysis. Two-sided 95% CIs for GMTs were back-transformations of the CIs for the mean of the logarithmically transformed assay results, computed using the Student t distribution. GMFRs from Day 1 to each respective time point were back-transformations of the mean of the difference of the log-transformed measures. Two-sided 95% CIs for GMFRs were back-transformations of CIs based on the Student t distribution for the mean difference of the log-transformed measures. Missing assay results were not replaced or imputed. Values below the LLOQ or denoted as below LLOQ were set to 0.5 × LLOQ for analysis.
Safety analysis
The presence and maximum severity of each local reaction and systemic event, any local reactions, and systemic events at any day (Days 1 to 14) were summarized with exact 2-sided 95% CIs using the F distribution (Clopper-Pearson). Any events that were confirmed as entry errors in the e-diary were excluded from the summary tables. AEs, SAEs, and severe and life-threatening AEs were also summarized.
Abbreviations
| AE | = | Adverse event |
| CI | = | Confidence interval |
| ClfA | = | Clumping factor A |
| cLIA | = | Competitive Luminex® immunoassay |
| CP5 | = | Capsular polysaccharide serotype 5 |
| CP8 | = | Capsular polysaccharide serotype 8 |
| CRM197 | = | Cross-reactive material 197 |
| FBI | = | Fibrinogen-binding inhibition |
| GCP | = | Good Clinical Practice |
| GMFR | = | Geometric mean-fold rise |
| GMT | = | Geometric mean titer |
| HBV | = | Hepatitis B virus |
| HCV | = | Hepatitis C virus |
| HIV | = | Human immunodeficiency virus |
| ICH | = | International Council for Harmonisation |
| LLOQ | = | Lower limit of quantitation |
| MedDRA | = | Medical Dictionary for Regulatory Activities |
| mITT | = | Modified intention-to-treat |
| MntC | = | Manganese transporter protein C |
| MRSA | = | Methicillin-resistant Staphylococcus aureus |
| OPA | = | Opsonophagocytic activity |
| rmClfA | = | Recombinant protein clumping factor A |
| rP305A | = | Recombinant protein 305A |
| SA3Ag | = | Staphylococcus aureus 3-antigen |
| SA4Ag | = | Staphylococcus aureus 4-antigen |
| SAE | = | Serious adverse event |
| SSI | = | Surgical-site infection |
| US | = | United States |
Acknowledgments
Editorial support was provided by Shuang Li, PhD, and Chu Kong Liew, PhD, of Engage Scientific Solutions.
Disclosure statement
James Baber, Yasuko Shoji, Masakazu Aizawa, David Cooper, William C. Gruber, Kathrin U. Jansen, Annaliesa S. Anderson, and Alejandra Gurtman are employees of Pfizer. Joseph Eiden was an employee of Pfizer when the study was being conducted. Megumi Inoue and Takuma Yonemura have no conflicts of interest to declare.
Additional information
Funding
References
- Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, Edwards JR, Sievert DM.. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37:1288–1301. doi:10.1017/ice.2016.174.
- Ministry of Health, Labor and Welfare, Japan Nosocomial Infections Surveillance (JANIS) Clinical Laboratory Division open report 2016. https://janis.mhlw.go.jp/english/report/open_report/2016/3/1/ken_Open_Report_Eng_201600_clsi2012.pdf ( accessed 20 February 2018).
- Ministry of Health, Labor and Welfare, Japan Nosocomial Infections Surveillance (JANIS) Antimicrobial-Resistant Bacterial Infection Division open report 2016. https://janis.mhlw.go.jp/report/open_report/2016/3/2/zen_Open_Report_201600.pdf ( accessed 20 February 2018).
- Uematsu H, Yamashita K, Kunisawa S, Fushimi K, Imanaka Y. Estimating the disease burden of methicillin-resistant Staphylococcus aureus in Japan: retrospective database study of Japanese hospitals. PLoS One. 2017;12:e0179767. doi:10.1371/journal.pone.0179767.
- Lenz AM, Fairweather M, Cheadle WG. Resistance profiles in surgical-site infection. Future Microbiol. 2008; 3:453–462. doi:10.2217/17460913.3.4.453.
- Ministry of Health, Labour and Welfare, Japan Nosocomial Infections Surveillance (JANIS) Surgical Site Infection open report 2016. https://janis.mhlw.go.jp/report/ssi.html ( accessed 20 February 2018).
- Takesue Y, Kusachi S, Mikamo H, Sato J, Watanabe A, Kiyota H, Iwata S, Kaku M, Hanaki H, Sumiyama Y, et al. Antimicrobial susceptibility of pathogens isolated from surgical site infections in Japan: comparison of data from nationwide surveillance studies conducted in 2010 and 2014-2015. J Infect Chemother. 2017;23:339–348. doi:10.1016/j.jiac.2017.03.010.
- Nagao M. A multicentre analysis of epidemiology of the nosocomial bloodstream infections in Japanese university hospitals. Clin Microbiol Infect. 2013; 19:852–858. doi:10.1111/1469-0691.12083.
- Mohamed N, Wang MY, Le Huec JC, Liljenqvist U, Scully IL, Baber J, Begier E, Jansen KU, Gurtman A, Anderson AS. Vaccine development to prevent Staphylococcus aureus surgical-site infections. Br J Surg. 2017;104:e41–e54. doi:10.1002/bjs.10454.
- Frenck RW Jr., Creech CB, Sheldon EA, Seiden DJ, Kankam MK, Baber J, Zito E, Hubler R, Eiden J, Severs JM, et al. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine. 2017;35:375–384. doi:10.1016/j.vaccine.2016.11.010.
- Creech CB, Frenck RW Jr., Sheldon EA, Seiden DJ, Kankam MK, Zito ET, Girgenti D, Severs JM, Immermann FW, McNeil LK, et al. Safety, tolerability, and immunogenicity of a single dose 4-antigen or 3-antigen Staphylococcus aureus vaccine in healthy older adults: results of a randomised trial. Vaccine. 2017;35:385–394. doi:10.1016/j.vaccine. 2016.11.032.
- Begier E, Seiden DJ, Patton M, Zito E, Severs J, Cooper D, Eiden J, Gruber WC, Jansen KU, Anderson AS, et al. SA4Ag, a 4-antigen Staphylococcus aureus vaccine, rapidly induces high levels of bacteria-killing antibodies. Vaccine. 2017;35:1132–1139. doi:10.1016/j.vaccine. 2017.01.024.
- Frenck R, Creech CB, Fiquet A, Feldman R, Kankam M, Pathirana S, Baber J, Radley D, Cooper D, Eiden J, et al. Persistence of immune responses through 36 months after vaccination with a novel Staphylococcus aureus 4-antigen vaccine (SA4Ag). Vienna (Austria): 27th European Congress of Clinical Microbiology and Infectious Diseases. 2017.
- Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, Scully IL, McNeil LK, Aste-Amezaga JM, Cooper D, et al. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother. 2013;9:480–487.
- Scully IL, Timofeyeva Y, Keeney D, Matsuka YV, Severina E, McNeil LK, Nanra J, Hu G, Liberator PA, Jansen KU, et al. Demonstration of the preclinical correlate of protection for Staphylococcus aureus clumping factor A in a murine model of infection. Vaccine. 2015;33:5452–5457. doi:10.1016/j.vaccine.2015.08.029.
- Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, Nunez L, Carriere M, Singer C, Dilts DA, et al. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. Aureus and Staphylococcus Epidermidis. J Infect Dis. 2012;205:1688–1696. doi:10.1093/infdis/jis272.
- Nissen M, Marshall H, Richmond P, Shakib S, Jiang Q, Cooper D, Rill D, Baber J, Eiden J, Gruber W, et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine. 2015;33:1846–1854. doi:10.1016/j.vaccine.2015.02.024.
- Marshall H, Nissen M, Richmond P, Shakib S, Jiang Q, Cooper D, Rill D, Baber J, Eiden J, Gruber WC, et al. Safety and immunogenicity of a booster dose of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults: A randomized phase 1 study. J Infect. 2016;73:437–454. doi:10.1016/j.jinf.2016.08.004.
- Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, Corey GR, Carmeli Y, Betts R, Hartzel JS, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–1378. doi:10.1001/jama.2013.3010.
- Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: phase III randomized study. Hum Vaccin Immunother. 2015;11:632–641. doi:10.4161/hv.34414.
- Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–496. doi:10.1056/NEJMoa011297.
- Kaye KS, Schmit K, Pieper C, Sloane R, Caughlan KF, Sexton DJ, Schmader KE. The effect of increasing age on the risk of surgical site infection. J Infect Dis. 2005;191:1056–1062. doi:10.1086/428626.
- Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008;7:467–479. doi:10.1586/14760584.7.4.467.
- Hawkins J, Kodali S, Matsuka YV, McNeil LK, Mininni T, Scully IL, Vernachio JH, Severina E, Girgenti D, Jansen KU, et al. A recombinant clumping factor A-containing vaccine induces functional antibodies to Staphylococcus aureus that are not observed after natural exposure. Clin Vaccine Immunol. 2012;19:1641–1650. doi:10.1128/CVI.00354-12.
- Rozemeijer W, Fink P, Rojas E, Jones CH, Pavliakova D, Giardina P, Murphy E, Liberator P, Jiang Q, Girgenti D, et al. Evaluation of approaches to monitor Staphylococcus aureus virulence factor expression during human disease. PLoS One. 2015;10:e0116945. doi:10.1371/journal.pone.0116945.