ABSTRACT
Background: Human papillomavirus (HPV) vaccination for young women up to age 26 is highly cost-effective and has been implemented in 65 countries globally. We investigate the cost-effectiveness for HPV vaccination program in older women (age > 26 years), heterosexual men and men who have sex with men (MSM).
Method: A targeted literature review was conducted on PubMed for publications between January 2000 and January 2017 according to the PRISMA guidelines. We included English-language articles that reported the incremental cost-effectiveness ratio (ICER) of HPV vaccination programs for women over age 26, heterosexual men, and MSM and identified the underlying factors for its cost-effectiveness.
Results: We included 36 relevant articles (six, 26 and four in older women, heterosexual men and MSM, respectively) from 17 countries (12 high-income (HICs) and five low- and middle-income (LMICs) countries). Most (4/6) studies in women over age 26 did not show cost-effectiveness ($65,000–192,000/QALY gained). Two showed cost-effectiveness, but only when the vaccine cost was largely subsidised and protection to non-naïve women was also considered. Sixteen of 26 studies in heterosexual men were cost-effective (ICER = $19,600–52,800/QALY gained in HICs; $49–5,860/QALY gained in LMICs). Nonavalent vaccines, a low vaccine price, fewer required doses, and a long vaccine protection period were key drivers for cost-effectiveness. In contrast, all four studies on MSM consistently reported cost-effectiveness (ICER = $15,000-$43,000/QALY gained), particularly in MSM age < 40 years and those who were HIV-positive. Countries’ vaccination coverage did not significantly correlate with its per-capita Gross National Income.
Conclusion: Targeted HPV vaccination for MSM should be next priority in HPV prevention after having established a solid girls vaccination programme. Vaccination for heterosexual men should be considered when 2-dose 4vHPV/9vHPV vaccines become available with a reduced price, whereas targeted vaccination for women over age 26 is unlikely to be cost-effective.
Introduction
Human Papillomavirus (HPV) infection is a common sexually transmitted infection (STI) and a necessary cause for cervical cancer in women.Citation1 It is also responsible for anal, vaginal, vulvar, oropharyngeal and penile cancers.Citation2 Cervical cancer was the fourth most common cancer among women globally, and second (only after breast cancer) in women in low- and middle- income countries (LMICs).Citation3 According to the World Health Organization (WHO), an estimated 530,000 cervical cancers were diagnosed in 2012, and approximately 270,000 women per year died from cervical cancer worldwide. More than 90% of deaths occur in low- and middle- income countries (LMICs) due to poor access to screening and treatment services.Citation4 However, HPV infection is vaccine-preventable, and currently approved vaccines have achieved an excellent safety and efficacy profile.Citation5
National HPV vaccination programs have been initiated over a decade ago, but there are large disparities in coverage and targeted populations of vaccination strategies between countries where the program has been introduced. By mid-2016, national HPV vaccination programs have been established in 65 countries globally, most of which are high-income countries (HICs). Strong momentum has been observed to expand HPV vaccination programs to LMICs, where the majority of HPV-related cancers occur.Citation6
The type of HPV vaccination program that countries choose to implement depends on the countries’ economic status, disease priorities, and the cost-effectiveness of the programs. Most HPV vaccination programs target 9–14 year old schoolgirls before sexual debut and it is cost-effective if more than 70% of young women are vaccinated.Citation7 There remain lots of debate around whether it is cost-effective to expand the existing vaccination programs to also include women older than 26 years, heterosexual men, and men who have sex with men (MSM). Unlike HPV vaccination for adolescent girls and women up to 26 years which has been shown to be highly cost-effective in many studies,Citation8–Citation13 relatively fewer cost-effectiveness analyses (CEA) on HPV vaccination have been conducted in other population groups. This study aims to investigate the cost-effectiveness of HPV vaccination program for women older than 26 years, heterosexual men and MSM and the factors that drive its cost-effectiveness through a literature review.
Results
Study selection and characteristics
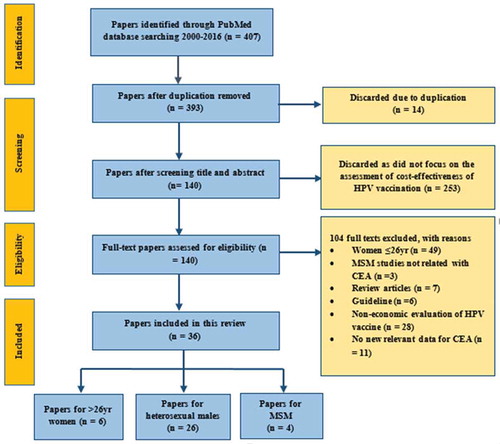
A total of 407 published articles were identified through PubMed (). Initial screening eliminated 14 duplicated articles and a further 253 articles were excluded because they were not cost-effectiveness analyses of HPV vaccination. The remaining 140 articles were reviewed in full-text for eligibility according to our inclusion and exclusion criteria. Another 104 articles were excluded and 36 papers were eventually selected for our literature review. Among these 36 studies, six reported on women over age 26, 26 on heterosexual men, four on MSM and one reported on both women over age 26 and heterosexual men. These studies were conducted in 17 countries (12 high-income countries (HICs) and five low- and middle- income countries (LMICs), ). Most (64%, n = 23) selected studies were published in 2011 or later.
Table 1. Gross National Income, HPV-related Disease Burden and Current HPV Prevention Programs in 17 countries.
Cost-effectiveness of HPV vaccination for ≥ 26-year-old women
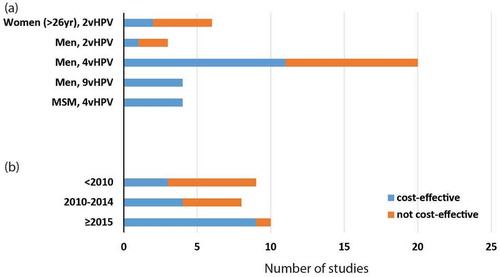
Six studiesCitation15-Citation20 evaluated the cost-effectiveness of 2vHPV vaccine in women > 26 years. Four studiesCitation15,Citation16,Citation18,Citation20 found the costs for targeted vaccination for women > 26 years (ICER = US$65,000–192,000/QALY gained, Table S1) were beyond their respective cost-effectiveness thresholds (~$50,000/QALY gained) (). Four studies assumed vaccination cost US$283–400/3-dose vaccination schedule and concluded the program as not cost-effective. However, one study from the UKCitation15 showed marginal cost-effectiveness when vaccine price was below £20/dose and life-time vaccine protection for women when no loss of immunity over time was considered. Another study from Lao PDRCitation19 showed the program to be cost-effective with a catch-up vaccination for women up to age 75 years and the existing schoolgirls vaccination program was strongly subsidised by GAVI, the Vaccine Alliance (US$8.5/dose). Only one Belgium studyCitation17 demonstrated their program to be very cost-effective with the 2vHPV for women age up to 33 years (Table S1). Both the Lao PDR and Belgium studies assumed high vaccination coverage (≥ 70%). All studies assumed 3-dose vaccination strategies and none compare it with a 2-dose vaccination strategy.
Cost-effectiveness of HPV vaccination for heterosexual men
Of 26 selected studiesCitation12,Citation21–Citation44 on gender-neutral vaccination (three in LMICs and 23 in HICs), two studies examined 2vHPV vaccine, 20 on 4vHPV, and four on 9vHPV vaccines. Sixteen studiesCitation22-Citation25,Citation27,Citation36–Citation46 demonstrated that HPV vaccination for heterosexual men with an existing female program was cost-effective (ICER = $19,600–52,800/QALY gained in HICs and $49–5,860/QALY gained in LMICs, Table S1) with respect to their respective cost-effectiveness thresholds ().
All four studies that assessed 9vHPVCitation36,Citation38,Citation40,Citation42 vaccine concluded that the vaccine for both girls and boys was cost-effective (ICER = $8600–49,800/QALY gained, Table S1) in comparison with 2vHPV or 4vHPV vaccination for both women and/or men. The majority (2/3) of studies with 2vHPV vaccinationCitation24,Citation30 was not cost-effective, while 11/20 studies with 4vHPV vaccination were cost-effective. Interestingly, when stratified by five-year time periods (< 2010, Citation2010–2014 and ≥ 2015, ), increasing proportion of studies demonstrated cost-effectiveness of HPV vaccination for heterosexual men in recent years (p-value = 0.035).
The assumed price of HPV vaccines varied substantially across studies (US $10–130/dose), and our analysis did not show any correlation between vaccine price and program cost-effectiveness in heterosexual men. While 3-dose vaccination strategy showed mixed results (14 cost-effective and 11 not), both studies with a 2-dose vaccination strategy showed cost-effectiveness.Citation45,Citation46 Longer duration of vaccine protection (life time protection) and program evaluation (100 years horizon) led to lower ICERs in these studies.
Age was an important factor for vaccine cost-effectiveness. Eight studies showed it was cost-effective to expand existing schoolgirl program to cover schoolboys at the same age (< 15 years). However, a UK studyCitation28 and a Danish studyCitation31 demonstrated that in the presence of a schoolgirl program, catch-up vaccination for young women up to 26 was a more cost-effective option than expanding schoolgirl program to cover the same age schoolboys. Eight studies showed that vaccination program for schoolboys and heterosexual men was no longer cost-effective if the vaccination coverage in women was beyond 70–75%. There was no evidence that the countries’ economic development status and vaccine efficacy had any impact on the cost-effectiveness of vaccination program for heterosexual men.
Cost-effectiveness of HPV vaccination for MSM
Four studiesCitation47-Citation50 evaluated the cost-effectiveness of 4vHPV vaccine for MSM. All four studies demonstrated that the 4vHPV vaccine for MSM compared with no vaccination was cost-effective ($15,000–43,000/QALY gained) ( a, Table S1), and it showed lower ICERs, hence better cost-effectiveness, for vaccination against MSM at a young age (< 40 years) or against those who were HIV-positive. A good cost-effectiveness of HPV vaccination for MSM was also associated with a high vaccination coverage (at least 55–80%), a potent vaccine efficacy (50–90%), a low vaccine price of 4vHPV (US$180–360/3-doses), a long duration of evaluation (life-time/100 years’ time horizon) (). In all MSM studies, there was no evidence that the socio-economic development status of the countries and vaccine dosage influenced the cost-effectiveness of MSM vaccination.
Table 2. Summary of cost-effectiveness of targeted HPV vaccination program in MSM,women over age 26 and heterosexual men (all age) in the presence of a background vaccination program for young women.
Vaccination and cervical cancer screening in included countries
The HPV vaccination and cervical cancer screening programs from the selected studies were described in . The annual cervical cancer incidence was generally higher (9.4–23.7 versus 5.5–12.9 per 100,000) in women from LMIC than HIC, as was the age standardized mortality rate for cervical cancer (3.4–8.0 versus 1.4–2.1 per 100,000). Cervical cancer mortality rates were significantly and negatively correlated with Gross National Income (GNI) (Spearman, r = -0.75, p < 0.001). Cervical cancer screening coverage among targeted women in HIC was more than 50–70%. In contrast, among LMIC, only Brazil reached a similar screening coverage as in HIC, while other countries were consistently below 40%. All National HPV vaccination programs for schoolgirls (up to age 14) were introduced before 2011 in HIC, and some programs included a catch-up program for young women up to age 26. To date, Austria, Australia, Canada, and the US, have expanded the vaccination program to schoolboys (age 9–14 years). In contrast, HPV vaccination began much later in LMICs, typically between 2013 and 2015 and China and Vietnam did not implement any vaccination programs until 2017. Vaccination coverage for women ranged from 40–80% in developed countries, where Germany had the lowest (40%) and the United Kingdom the highest (80%) coverage. We found no significant correlation between GNI per capita and vaccination coverage (R = -0.0049, p = 0.9877).
Discussion
Our targeted literature review indicated that HPV vaccine for women > 26 years would not be cost-effective, and this is consistent with current policy and practice. In contrast, HPV vaccination for heterosexual men demonstrated mixed results: programs proposing 9vHPV (compared with 4vHPV and 2vHPV), those assuming a long duration of vaccine effectiveness and those vaccinating young heterosexual men (< 26) demonstrated cost-effectiveness. Further, it suggested that targeted HPV vaccination for MSM is cost-effective in all four included studies. A previous systematic review on the cost-effectiveness of HPV vaccination among adolescent girls in LMICs has shown that vaccine price is one of the key determinant of vaccination cost-effectiveness.Citation51 Our review further confirms this is also true in heterosexual men and MSM. In addition, we also identified a broad genotype coverage (9vHPV), less required doses and longer vaccine protection are important determinants for cost-effectiveness.
Our findings suggests that targeted HPV vaccination for MSM should be a priority worldwide. Unlike heterosexual men, MSM may benefit to a lesser extent from the herd immunity that heterosexual men may receive from the female vaccination programs.Citation52 On the other hand, MSM are much more at-risk than heterosexual men to HPV infection in particular anogenital warts and anal cancer. In contrast to vaccination program in women where the vaccination coverage required (~ 70%) is well established, the vaccination coverage required in MSM to achieve the same level of herd immunity that heterosexual men may experience is not known. Since the reproductive rate of HPV infection in MSM is much greater than heterosexual men, it is likely that a higher level of vaccination coverage will be required.Citation53
Despite only 16 of 26 studies in heterosexual men demonstrating cost effectiveness, our data suggest that a gender neutral vaccination strategy may become increasingly cost-effective for a number of reasons. First, recent literatures reported that 1- or 2-doses vaccination is as effective as 3-doses vaccination for people age 9–14 years, which means a potential 30% cost reduction per head if this is implemented in any school age vaccination programs.Citation54–Citation56 Second, it is anticipated that the mean price of HPV vaccine for LMICs will continue to decline over time, especially with significant subsidies and influence from major international health organizations such as GAVI, UNICEF and Pan American Health Organization (PAHO).Citation57,Citation58
Our analysis shows no correlation between individual country’s socio-economic status and vaccination coverage. However, we argue that the rollout of a universal HPV vaccination program in LMICs may face more challenges. Given limited resources, LMICs generally have a lower willingness-to-pay threshold for a vaccination program. Therefore, vaccine cost needs to be substantially lowered in LMICs, not only for the consideration of cost-effectiveness, but also the upfront investment cost must not become an excessive financial burden to the country budget. The initial rollout of the program often require a one-time investment for health facilities, establishment of an efficient implementation system and training for healthcare staff. Further, in resource-poor settings, an efficient healthcare provision system is often absent to provide the scheduled vaccination program, which is an essential infrastructure for additional HPV vaccination programs. For these settings, resources from the international community should be directed to provide point-of-care vaccination where primary healthcare is absent, and 2-dose HPV vaccine should be promoted to improve vaccination coverage in the population.
A number of limitations need to be considered when interpreting our results. As a targeted literature review, we excluded studies not published in English and therefore, our study may be subject to publication bias. Second, we could not conduct a meta-analysis due to limited data available from targeted reviews. Similarly, we could not prove the robustness of outcomes because of the variations in models applied in the included studies where different assumptions and parameters were used. For instance, population impact was not reported in a consistent form across the studies, however, we emphasized that all cost-effectiveness studies included a baseline scenario and the analysis was conducted by comparing the scenarios in the presence and absence scenario. Therefore, we summarized the absolute number of studies and the factors influencing the cost-effectiveness instead. Despite these limitations, we believe our findings would be a springboard for further studies of the cost-effectiveness of HPV vaccination for these currently untargeted populations.
Conclusion
Targeted HPV vaccination for MSM should be next priority in HPV prevention after having established a solid girls vaccination programme. Vaccination for heterosexual men should be considered when 2-dose 4vHPV/9vHPV vaccines become available with a reduced price. Vaccination for women over age 26 may not be cost-effective until the vaccine price is further reduced.
Method
Search
The full electronic search was conducted in PubMed for related articles and reviews on February 15th 2017, which were published in the English language from January 1, 2000 to December 31, 2016. The search strategy was conducted using the following key words: “Human Papillomavirus” AND “Cost-effectiveness” AND “Vacc*” in MeSH terms AND “HPV” OR “Human Papillomavirus” AND “Cost-effective*” AND “Vacc*” in titles and abstracts AND “English” in language.
Eligibility criteria
This review included English-language articles (published between 2000–2016) that assessed the incremental cost-effectiveness ratio (ICER) of HPV vaccination to the female population older than 26 years, heterosexual men and MSM, in comparison with the cost-effectiveness of existing cervical cancer screening or vaccination in young adolescent girls with a catch-up program for women age up to 26 years. In this review, the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta- Analyses) statementCitation59 was followed (). Articles were excluded if they (1) were in a language other than English; (2) did not report ICER of the HPV vaccination program; and (3) only focused on young female vaccination program.
Data collection
We collected demographic data, HPV epidemiological data, impact and cost-effectiveness data from aforementioned literature review. In addition, based on the countries identified from the selected studies, we further collected data on country-specific HPV-related programs and country incomes that were not available in the literature research.
First, demographic data included age and sex of the targeted population, period of analysis (retrospective or prospective study) and country of the study population. Second, epidemiological data included status quo HPV disease burden, subtypes and vaccination coverage. Third, population impact data included the type of model used, reduction in HPV infections, number of genital warts, pre-cancerous lesions CIN-1, −2, and −3 cases, cervical cancer cases and mortality. Fourth, cost-effectiveness data included incremental cost associated with HPV vaccination programs; incremental cost-effectiveness ratios (ICERs); incremental life-years gained (LYGs) or Quality-adjusted life-years (QALYs) gained from a vaccination program. Fifth, we identified 17 countries from the selected 36 publications. For these 17 countries, we collected other HPV-related program and income data from these well-known online HPV databases: HPV Information Centre;Citation60 National Cancer Institute;Citation61 and International Agency for Research on Cancer.Citation62 Specific country data included: gross National Income per capita (GNI); age-standardized incidence rate of cervical cancer; age-standardized mortality rate of cervical cancer; existence of national cervical cancer screening and HPV vaccination programs; years of introduction of the national HPV vaccination program; targeted age and gender of current HPV vaccination program; vaccination coverage; and cervical cancer screening coverage. Double-entry was performed to extract these data by two independent investigators (NNS, FC). Microsoft excel 2013 was used to store and analyse these data.
Quality assessment
The quality assessment of each included study was conducted by two independent investigators (NNS, FC). Any conflicting opinions were resolved by a third reviewer (LZ). The quality check for each included study was assessed by three domains: study design, data collection, and analysis and interpretation of the results (Cost-effectiveness study quality checklist,Citation63 Table S2).
Data analysis
Descriptive statistics were conducted for each study population group (older women, heterosexual men and MSM) to inform HPV program, impact and cost-effectiveness indicators. First, for each population, we categorized the selected studies that showed proposed strategy was cost-effective according to their stated willingness-to-pay threshold, and those showed it was not cost-effective. Second, the major contributing factors influencing the cost-effectiveness, including vaccination age and coverage, vaccine efficacy, price and dosage, duration of vaccine protection, and the time horizon of evaluation, were identified in both cost-effective and non-cost-effective studies. A Spearman’s correlation test was used to analyse the correlation between the GNI and HPV-burden of the included countries. In addition, chi-square tests were conducted to investigate the time trend of cost-effectiveness of HPV vaccination for heterosexual males.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Supplemental Material
Download MS Word (136.3 KB)Supplemental data
Supplemental data for this article can be accessed here.
References
- Walboomers JMM, Jacobs MV, Mnos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Munoz N. Human Papillomavirus is a necessary cause of invasive cervical cancer worldwide. 1999; Sep;189(1):12-9.
- Centers for Disease Control and Prevention. HPV and men- CDC fact sheet. 2015 Feb https://www.cdc.gov/std/hpv/hpv-and-men-factsheet-feb-2015.pdf.
- World Health Organization. Cervical cancer. 2018 http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/.
- World Health Organization. Human papillomavirus (HPV) and cervical cancer. 2016 June http://www.who.int/mediacentre/factsheets/fs380/en/.
- World Health Organization. Safety update of HPV vaccines. 2017 accessed. http://www.who.int/vaccine_safety/committee/topics/hpv/June_2017/en/.
- Stanley M. HPV vaccination in boys and men. Hum Vaccin Immunother. 2014;10(7):2109–2111. doi:10.4161/hv.29137.
- Canfell K, Harrell Chesson SL, Kulasingam JB, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine. 2012;30(Suppl 5):F157–67. doi:10.1016/j.vaccine.2012.06.091.
- Goldie SJ, Carol Levin N, Mosqueira-Lovón R, Ortendahl J, Kim J, O’Shea M, Sanchez MD, Araujo MAM. Health and economic impact of human papillomavirus 16 and 18 vaccination of preadolescent girls and cervical cancer screening of adult women in Peru. Rev Panam Salud Publica. 2012;32(6):426–434.
- Luttjeboer J, Westra TA, Wilschut JC, Nijman HW, Daemen T, Postma MJ. Cost-effectiveness of the prophylactic HPV vaccine: an application to the Netherlands taking non-cervical cancers and cross-protection into account. Vaccine. 2013;31(37):3922–3927. doi:10.1016/j.vaccine.2013.06.044.
- Uusküla A, Müürsepp A, Kawai K, Raag M, Jürisson M, Pillsbury M. 2013. The epidemiological and economic impact of a quadrivalent human papillomavirus (hpv) vaccine in Estonia. BMC Infect Dis. 13:304. doi:10.1186/1471-2334-13-304.
- Walwyn L, Janusz CB, Clark AD, Prieto E, Waight E, Largaespada N. Cost-effectiveness of HPV vaccination in Belize. Vaccine. 2015;33(Suppl 1):A174–81.
- Insingaa RP, Dasbach EJ, Elbasha EH, Puig A, Reynales-Shigematsu LM. Cost-effectiveness of quadrivalent human papillomavirus (HPV) vaccination in Mexico: a transmission dynamic model-based evaluation. Vaccine. 2007;26(1):128–139. doi:10.1016/j.vaccine.2007.10.056.
- Kawai K, de Araujo GTB, Fonseca M, Pillsbury M, Singhal PK. 2012. Estimated health and economic impact of quadrivalent HPV (types 6/11/16/18) vaccination in Brazil using a transmission dynamic model. BMC Infect Dis. 12:250. doi:10.1186/1471-2334-12-166.
- European Centre for Disease Prevention and Control. Introduction of HPV vaccines in EU countries – an update. Stockholm: ECD; 2012. doi: 10.2900/60814
- Turner HC, Baussano L, Garnett GP. Vaccinating women previously exposed to human papillomavirus: a cost-effectiveness analysis of the bivalent vaccine. PLoS One. 2013;8(9):e75552. doi:10.1371/journal.pone.0075552.
- Kim JJ, Ortendahl J, Goldie SJ. Cost-effectiveness of human papillomavirus vaccination and cervical cancer screening in women older than 30 years in the United States. Ann Intern Med. 2009;151(8):538–545.
- Demarteaua N, Van Kriekinge G, Simon P. Incremental cost-effectiveness evaluation of vaccinating girls against cervical cancer pre- and post-sexual debut in Belgium. Vaccine. 2013;31(37):3962–3971. doi:10.1016/j.vaccine.2013.06.008.
- Westra TA, Rozenbaum MH, Rogoza RM, Nijman HW, Daemen T, Postma MJ, Wilschut JC. Until which age should women be vaccinated against HPV infection? Recommendation based on cost-effectiveness analyses. J Infect Dis. 2011;204(3):377–384. doi:10.1093/infdis/jir281.
- Chanthavilay P, Reinharz D, Mayxay M, Phongsavan K, Marsden DE, Moore L, White LJ. The economic evaluation of human papillomavirus vaccination strategies against cervical cancer in women in Lao PDR: a mathematical modelling approach. BMC Health Serv Res. 2016;16(1):418. doi:10.1186/s12913-016-1662-5.
- Liu Y-J, Zhang Q, Shang-Ying H, Zhao F-H. 2016. Effect of vaccination age on cost-effectiveness of human papillomavirus vaccination against cervical cancer in China. BMC Cancer. 16:164. doi:10.1186/s12885-016-2207-3.
- Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis. 2004. Nov;10(11):1915-23.
- Giuliano AR. Human papillomavirus vaccination in males. Gynecol Oncol. 2007;107(2 Suppl 1):S24–6. doi:10.1016/j.ygyno.2007.07.075.
- Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi:10.3201/eid1301.060438.
- Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: a cost-effectiveness analysis in a low-resource setting. Br J Cancer. 2007;97(9):1322–1328. doi:10.1038/sj.bjc.6604023.
- Kulasingam S, Connelly L, Conway E, Hocking JS, Myers E, Regan DG, Roder D, Ross J, Wain G. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian National Cervical Cancer Screening Program. Sex Health. 2007;4(3):165. doi:10.1071/SH07043.
- Pearson AL, Kvizhinadze G, Wilson N, Smith M, Canfell K, Blakely T. 2014. Is expanding HPV vaccination programs to include school-aged boys likely to be value-for-money: a cost-utility analysis in a country with an existing school-girl program. BMC Infect Dis. 14:351. doi:10.1186/1471-2334-14-351.
- Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867. doi:10.1016/j.vaccine.2010.08.030.
- Chessona HW, Ekwueme DU, Saraiyab M, Dunnea EF, Markowitza LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29(46):8443–8450. doi:10.1016/j.vaccine.2011.07.096.
- Zechmeister I, de Blasio BF, Garnett G, RaeNeilson A, Siebert U. Cost-effectiveness analysis of human papillomavirus-vaccination programs to prevent cervical cancer in Austria. Vaccine. 2009;27(37):5133–5141. doi:10.1016/j.vaccine.2009.06.039.
- Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. Bmj. 339;2009:b3884.
- Olsen J, Jepsen MR. Human papillomavirus transmission and cost-effectiveness of introducing quadrivalent HPV vaccination in Denmark. Int J Technol Assess Health Care. 2010;26(2):183–191. doi:10.1017/S0266462310000085.
- Jit M, Choi YH, John Edmunds W. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ (Clinical research ed) 2008; 337: a769.
- Laprise J-FO, Drolet M, Boily M-C, Jit M, Sauvageau C, Franco EL, Lemieux-Mellouki P, Malagón T, Brisson M. Comparing the cost-effectiveness of two- and three-dose schedules of human papillomavirus vaccination: a transmission-dynamic modelling study. Vaccine. 2014;32(44):5845–5853. doi:10.1016/j.vaccine.2014.07.099.
- Isaranuwatchai W, Graham DM, Siu LL, Hoch JS. Could the human papillomavirus vaccination be cost-effective in males for the prevention of oropharyngeal cancer? Expert Rev Pharmacoecon Outcomes Res. 2014;14(6):763–765. doi:10.1586/14737167.2014.946012.
- Sinisgalli E, Bellini I, Indiani L, Sala A, Bechini A, Bonanni P, Boccalini S. HPV vaccination for boys? A systematic review of economic studies. Epidemiol Prev. 2015;39(4 Suppl 1):51–58.
- Durhama DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci U S A. 2016;113(18):5107–5112. doi:10.1073/pnas.1515528113.
- Graham DM, Isaranuwatchai W, Habbous S, de Oliveira C, Liu G, Siu LL, Hoch JS. A cost-effectiveness analysis of human papillomavirus vaccination of boys for the prevention of oropharyngeal cancer. Cancer. 2015;121(11):1785–1792. doi:10.1002/cncr.29111.
- Chesson HW, Markowitz LE, Hariri S, Ekwueme DU, Saraiya M. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: estimates from a simplified transmission model. Hum Vaccin Immunother. 2016;12(6):1363–1372. doi:10.1080/21645515.2016.1140288.
- Haeussler K, Marcellusi A, Mennini FS, Favato G, Picardo M, Garganese G, Bononi M, Costa S, Scambia G, Zweifel P, et al. Cost-effectiveness analysis of universal human papillomavirus vaccination using a dynamic bayesian methodology: the BEST II study. Value Health. 2015;18(8):956–968. doi:10.1016/j.jval.2015.08.010.
- Boiron L, Joura E, Largeron N, Prager B, Uhart M. 2016. Estimating the cost-effectiveness profile of a universal vaccination programme with a nine-valent HPV vaccine in Austria. BMC Infect Dis. 16:153. doi:10.1186/s12879-016-1987-z.
- Sharma M, Sy S, Kim JJ. The value of male human papillomavirus vaccination in preventing cervical cancer and genital warts in a low-resource setting. Bjog. 2016;123(6):917–926. doi:10.1111/1471-0528.13503.
- Largeron N, Petry KU, Jacob J, Bianic F, Anger D, Uhart M. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. JMIR Res Protoc. 2017; 17(1): 85–98.
- Kotsopoulos N, Connolly MP, Remy V. Quantifying the broader economic consequences of quadrivalent human papillomavirus (HPV) vaccination in Germany applying a government perspective framework. Health Econ Rev. 2015;5(1):54. doi:10.1186/s13561-015-0054-6.
- Bresse X, Goergen C, Prager B, Joura E. Universal vaccination with the quadrivalent HPV vaccine in Austria: impact on virus circulation, public health and cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):269–281. doi:10.1586/14737167.2014.881253.
- Burger EA, Stephen S, Nygard M, Kristiansen IS, Lim JJ. Prevention of HPV-related cancers in Norway: cost-effectiveness of expanding the HPV vaccination program to include pre-adolescent boys. PLoS One. 2014;9(3):e89974. doi:10.1371/journal.pone.0089974.
- Olsen J, Jørgensen TR. 2015. Revisiting the cost-effectiveness of universal HPV-vaccination in Denmark accounting for all potentially vaccine preventable HPV-related diseases in males and females. Cost Eff Resour Alloc. 13:4. doi:10.1186/s12962-015-0029-9.
- Deshmukh AA, Jagpreet Chhatwal EY, Chiao AG, Nyitray PD, Cantor SB. Long-term outcomes of adding HPV vaccine to the anal intraepithelial neoplasia treatment regimen in HIV-positive men who have sex with men. Clin Infect Dis. 2015;61(10):1527–1535. doi:10.1093/cid/civ628.
- Deshmukh AA, Chiao EY, Dasc P, Cantord SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32(51):6941–6947. doi:10.1016/j.vaccine.2014.10.052.
- Lin A, Ong KJ, Hobbelen P, King E, Mesher D, Edmunds WJ, Sonnenberg P, Gilson R, Bains I, Choi YH, et al. Impact and cost-effectiveness of selective human papillomavirus vaccination of men who have sex with men. Clin Infect Dis. 2016. doi:10.1093/cid/ciw845.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10(12):845–852. doi:10.1016/S1473-3099(10)70219-X.
- Fesenfelda M, Hutubessya R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: a systematic review. Vaccine. 2013;31(37):3786–3804. doi:10.1016/j.vaccine.2013.06.060.
- Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, McNamee K, Garefalakis M, Phillip S, Cummins E, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14(10):958–966. doi:10.1016/S1473-3099(14)70841-2.
- Fairley CK, Zou H, Zhang L, Chow EPF. Human papillomavirus vaccination in men who have sex with men - what will be required by 2020 for the same dramatic changes seen in heterosexuals. Sex Health. 2017;14(1):123–125. doi:10.1071/SH16067.
- Markowitz LE. 2017. Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination-updated recommendations of the advisory committee on immunization practices. American Journal Of Transplantation : Official Journal Of The American Society Of Transplantation and The American Society Of Transplant Surgeons. 17(3): 834–837.
- World Health Organization. Weekly epidemiological record. 2014 http://www.who.int/wer/2014/wer8921.pdf.
- Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, Garland SM, Herrero R, David M-P, Wheeler CM, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. Lancet Oncol. 2015;16(7):775–786. doi:10.1016/S1470-2045(15)00047-9.
- Dasbach EJ, Insinga RP, Elbasha EH. The epidemiological and economic impact of a quadrivalent human papillomavirus vaccine (6/11/16/18) in the UK. Bjog. 2008;115(8):947–956. doi:10.1111/j.1471-0528.2008.01743.x.
- World Health Organization. WHO price report: vaccine product, price and procurement. Geneva, Switzerland: World Health Organization, 2016.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi:10.1016/j.ijsu.2010.02.007.
- HPV Information Center. HPV and cervical cancer prevention statistics. 2016 http://www.hpvcentre.net/references.php.
- National Cancer Institute. Cancer statistics. 2016 https://seer.cancer.gov/.
- International Agency for Research on Cancer. Data visualization tools that present current national estimates of cancer incience, mortality, and prevalence. 2016 http://gco.iarc.fr/today/home.
- Deshpande SN, van Asselt AD, Tomini F, Armstrong N, Allen A, Noake C, Khan K, Severens JL, Kleijnen J, Westwood ME, Rapid fetal fibronectin testing to predict preterm birth in women with symptoms of premature labour: a systematic review and cost analysis. Health Technology Assessment 2013 accessed May 5. https://www.ncbi.nlm.nih.gov/books/NBK261009/. doi:10.3310/hta17400.