ABSTRACT
To evaluate the safety and immunogenicity of a newly 23-valent pneumococcal polysaccharide vaccine (PPV23), a phase Ⅲ clinical trial was conducted in population aged ≥ 2 years. We conducted a randomized, double-blinded, active controlled trial, in which 1760 participants were randomly assigned in a 1:1 ratio to receive one dose of either the test vaccine or the control commercial vaccine. The surveillance period was 28 days. The 2-fold increase rate of anti-pneumococcal for 23 serotypes varied from 49.71% to 90.96% in the treatment group and from 44.52% to 88.24% in the control group. According to −10% non-inferiority margin and 95% confidence intervals of rate difference, all the 23 serotypes of the treatment group were non-inferiority to the control group. The 2-fold increase rate of anti-pneumococcal antibody were significantly higher in the treatment group for 11 serotypes including 1, 2, 3, 4, 10A, 11A, 14, 18C, 20, 22F, and 23F. Serious adverse events occurred in 2 in 879 (0.23%) participants in the treatment group and 2 in 880 (0.23%) participants in the control group, and all the adverse events were unrelated to the vaccination. The overall adverse reaction frequency showed no difference between the treatment (51.19%) and control group (47.95%), and most adverse reactions were mild or moderate in intensity. The newly PPV23 is immunologically non-inferior to the control commercial vaccine and well tolerated in healthy Chinese population aged ≥ 2 years.
Trial registration: ClinicalTrial.gov identifier: NCT02451969.
Introduction
Streptococcus pneumoniae (S. pneumoniae) is a major cause of illness and death in children and adults worldwideCitation1,Citation2, causing invasive pneumococcal diseases (IPDs) including bacteremic pneumonia, meningitis, and septicemia; or noninvasive diseases including non-bacteremic pneumonia, otitis media, and sinusitisCitation2. 91 distinct pneumococcal serotypes have been identified, and infection or vaccination with S. pneumoniae can induce specific protective antibodies that exhibits cross protection against certain serotypesCitation3. Several multivalent vaccines have been developed to reduce the diseases caused by S. pneumoniae. At present, two vaccines are available in the China market, which are 23-valent pneumococcal polysaccharide vaccine (PPV23) and 13-valent pneumococcal conjugate vaccine (PCV13). Covered all the serotypes included in the PCV13 except 6A, the PPV23 contained the serotypes which caused 85%-90% S. pneumoniae infections, including 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33FCitation4.
Currently, in China, PCV13 is approved for use in infants aged from 6 weeks to 15 months with a three-dose primary schedule on 2, 4, and 6 months, and a booster dose on 12 ~ 15 months. The PCV13 application in more extensive population is restricted by the absence of clinical evidence in population aged > 6 months. PPV23 is currently recommended in the population aged ≥ 2 years with a single dose schedule especially in the elderly. As a cost-effective option for the pneumococcal prevention, the PPV23 is of great significance for public health in ChinaCitation5.
American Center for Disease Control and Prevention (CDC) recommended PPV23 vaccination for all adults aged > 65 years and anyone aged 2 ~ 64 years who are at certain high riskCitation6. Besides, a second dose at age 65 years or later was recommended by the Advisory Committee on Immunization Practice (ACIP) for people who receive one dose vaccination before age 65 yearsCitation7. According to the 2012 guideline on application of pneumococcal vaccine in China, PPV23 vaccination was recommended for the adults aged > 60 years and anyone aged 2 ~ 59 years who are at certain high riskCitation8. Use of PPV23 has been limited in infants and children under 2 years, because the T-cell independent response is poorly developed in young childrenCitation9-Citation11. However, as a booster dose following a priming series of PCV, PPV23 can induce higher concentrations of antibodies than a PCV boosterCitation9.
In China, as a category B vaccine which people volunteer to be vaccinated at their own expense, the coverage of PPV23 is far from that of immunization program vaccines and that in developed countries. Although nationwide survey data are unavailable still now, some regional investigations revealed a low coverage of PPV23 in the elder adults, which is the priority target population of PPV23 vaccination. For instance, an investigation in Chaoyang district in Beijing city reported a coverage rate of 2.1% in the adults aged ≥ 60 years in 2010Citation2,Citation12. Another investigation in Qingpu district in Shanghai city in 2014 reported the PPV23 coverage rate in males and females aged ≥ 65 years was 1.8% and 2.0% respectivelyCitation13. A similar situation was reported in Taiwan before 2007, when the coverage rate was < 1%. However, that rate among adults aged ≥ 75 years reached 12% in 2007 and 41% in 2008, attributed to the implementation of PPV23-free policy in this population, but thereafter dropped to 29.5% with unclear reasons. In contrast, Martinelli et al estimated the PPV23 coverage rate among individuals ≥ 65 years during the years of 2002–2007 was 46.6% in ItalyCitation14. While Zhou et al estimated the PPV23 coverage rate among adults ≥ 65 years in Quebec province of Canada increased from 48% in 2006 to 57% in 2014Citation15. According Yu et al’s estimation, if the coverage rate of PPV23 for the elderly and of PCV13 for the children both reach 50%, the economic burden due to pneumococcal disease will be acceptableCitation16. Thus, endeavors should be made to promote the PPV23 vaccination in adults especially in elder adults for the disease burden alleviation in China.
A newly PPV23 have been developed by Sinovac Biotech Co., Ltd., which has several features compared with the commercial PPV23. First, chromatography steps are developed in the purification process, which is not in other available PPV23. Second, the absence of phenol extraction steps in the purification process make the process more environmentally friendly and healthy. Last, the final products do not contain preservative like phenol, which makes them safer. In this study, we examined the safety and immunogenicity of the Sinovac PPV23 in healthy Chinese people aged ≥ 2 years.
Results
Study population
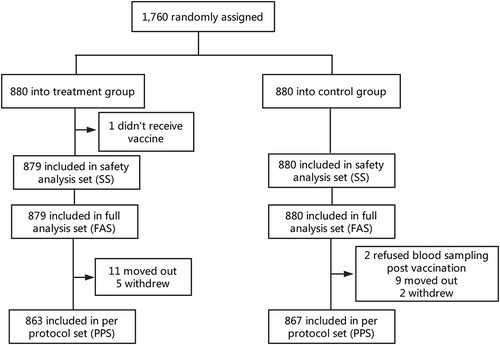
1760 participants aged ≥ 2 years were enrolled in total, with 880 participants per group. Of these, 1 did not received vaccine and was excluded from the analysis. As a result, 879 (99.89%) participants in the treatment group and 880 (100%) participants in the control group were included in the safety analysis set. Furthermore, in the treatment group, 11 participants moved out and 5 withdrew. In the control group, 2 participants refused blood sampling post vaccination, 9 moved out, and 2 withdrew. Finally, 863 (98.07%) participants in the treatment group and 867 (98.52%) participants in the control group were included in the per-protocol set (). The demographic characteristics of the participants were balanced in the two groups ().
Table 1. Baseline characteristics of participants.
Immunogenicity post vaccination
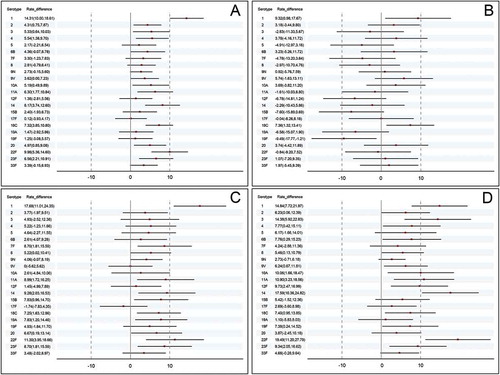
Pre-immunization antibody levels in two groups were well balanced. () For all 23 serotypes, the 2-fold increase rate of anti-pneumococcal antibody varied from 49.71% to 90.96% in the treatment group and from 44.52% to 88.24% in the control group. For both groups, increase rate of serotype 9N was the highest and serotype 10A was the lowest. According to −10% non-inferiority margin and 2-side 95% confidence intervals (95%CI) of 2-fold increase rate difference, the treatment group was non-inferior to the control group for all 23 serotypes. Moreover, for the 11 serotypes including 1, 2, 3, 4, 10A, 11A, 14, 18C, 20, 22F, and 23F, the 2-fold increase rate was significantly higher in the treatment group compared to the control group (76.13% vs. 61.82% for serotype1, 84.82% vs. 80.51% for serotype 2, 55.74% vs 50.40% for serotype 3, 76.01% vs 70.47% for serotype 4, 49.71% vs. 44.52% for serotype 10A, 66.28% vs. 59.98% for serotype 11A, 70.57% vs. 62.40% for serotype 14, 87.25% vs. 79.93% for serotype 18C, 76.71% vs.71.74% for serotype 20, 63.62% vs. 53.63% for serotype 22F, 72.19% vs. 65.63% for serotype 23F). (; Table S1)
Table 2. Immune response to pneumococcal vaccine in the per protocol population.
Figure 2. Rate difference of 2-fold increase after vaccination. (a) the entire population (b) the subgroup aged 2 ~ 17 years (c) the subgroup aged 18 ~ 60 years (d) the subgroup aged > 60 years.
At day 28, for all 23 serotypes, the geometric mean concentrations (GMCs) varied from 32.89 to 130.06 in the treatment group and from 30.35 to 124.54 in the control group. The GMCs were significantly higher in the treatment group compared to the control group for 10 serotypes including 1, 2, 3, 4, 5, 8, 11A, 14, 18C, and 20. For other serotypes, no significant difference was found in two groups. ()
At day 28, for all 23 serotypes, the geometric mean fold increases (GMFIs) varied from 2.33 to 7.44 in the treatment group and from 2.17 to 6.39 in the control group. The GMFIs were significantly higher in the treatment group compared to the control group for 14 serotypes including 1, 2, 4, 6B, 8, 9N, 9V, 10A, 14, 15B, 18C, 19F, 20, 22F. ()
Subgroup analysis
Based on the age strata of the participants in the study design and randomization, we further analyzed the primary endpoint for the subgroups aged 2 ~ 17 years, 18 ~ 60 years and > 60 years respectively.
In the subgroup aged 2 ~ 17 years, the treatment group did not reach non-inferiority for 10 serotypes including 3, 5, 7F, 8, 11A, 12F, 14, 15B, 19A, 19F. Whereas, the 2-fold increase rates were significantly higher in the treatment group for serotype 1 and 18C. (; Table S2)
In the subgroup aged 18 ~ 60 years, the treatment group was non-inferiority to the control group for all 23 serotypes. Furthermore, the 2-fold increase rates were significantly higher for 11 serotypes including 1, 7F, 8, 11A, 14, 15B, 18C, 19A, 20, 22F, 23F. (; Table S3)
In the subgroup aged > 60 years, the treatment group was non-inferiority to the control group for all 23 serotypes. Furthermore, the 2-fold increase rates were significantly higher for 15 serotypes including 1, 2, 3, 4, 6B, 8, 9V, 10A, 11A, 12F, 14,18C, 19F, 22F, 23F. (; Table S4)
Within the treatment group, differences of 2-fold increase rate in three age subgroups showed statistically significance for all serotypes except for 2 and 18C. The 2-fold increase rate showed an increasing trend with the ascending age for most serotypes. The control group showed a similar pattern in immunogenicity. (Table S5, Table S6)
Adverse reactions
During the study period, 4 serious adverse events (SAEs) were reported: 2 in treatment group (1 case of hemorrhoids and 1case of acute appendicitis) and 2 in control group (1 case of femoral neck fracture and 1 case of acute exacerbation of chronic bronchitis). All the SAEs were considered unrelated to the vaccination. No deaths occurred during the trial.
The overall frequency of adverse reactions was 51.19% in the treatment group and 47.95% in the control group (P = 0.1819). In any age subgroups, no significant difference in adverse reaction frequency was found between the treatment and control group. The most common injection-site and systemic adverse reaction was pain and fever respectively. 19 participants (2.16%) per group had grade 3 adverse reactions within 28 days after vaccination ().
Table 3. Adverse events in the safety analysis population.
Discussion
This study showed the Sinovac PPV23 has similar immunogenicity and safety with the control commercial vaccine.
Kong et al reported, for all the 23 serotypes, the 2 fold increase rate post Walvax PPV23 varied from 62.47% to 97.01%, and that post Merk PPV23 varied from 51.49% to 95.77%, which are both similar to the immunogenicity level (from 49.71% to 90.96%) induced by Sinovac PPV23Citation17.
The 2-fold increase rate for serotype 9N was the highest, followed by 18C, 2, 33F. The high immunogenicity for these serotypes were also have been reported in other phase Ⅲ clinical trials of PPV23Citation17,Citation18. An overview for year 2006 ~ 2016 indicated the dominant S. pneumoniae serotypes in China were 19F, 19A, 23F, 14, and 6B, for which the Sinovac PPV23 induced a 2-fold increase rate at about 70%.
According to the subgroup analysis, in both adult and elderly subgroup, the test vaccine was non-inferior to the control vaccine for all the 23 serotypes. Whereas, in the children subgroup, the test vaccine was non-inferior to the control vaccine for 13 serotypes, and not achieved the non-inferior criteria for the other 10 serotypes. With regard to this finding, there were two possible reasons: First, the sample size in this study was estimated and determined based on the entire population, making the assigned sample size in subgroups not enough to detect the non-inferior results. Second, different production conditions and processes of these two vaccines may lead to the distinctions in immune response patterns.
A general trend that the immune efficacy increasing with the ascending age was found in treatment group as well as control group. Yang et alCitation19 reported, one month after a single dose PPV23 manufactured by Chengdu Institute of Biological Products Co., Ltd., the mean 2-fold increase rate of 23 serotypes was 79.3% in population aged 6 ~ 14 years, 92.7% in population aged 15 ~ 45 years, and 87.5% in population aged 46 ~ 55 years, indicating the immune response of PPV23 was better in adults than children, which is similar with our study finding. However, the profiles of immune response in elderly were not available in Yang et al’s study. Meanwhile, there were WHO position paper and previous studies indicated efficacy and effectiveness of PPV23 were generally lower in elderly persons than in younger adultsCitation9,Citation10,Citation20–Citation22, which seems to be conflict with our findings. In fact, the above conclusions were achieved within the population aged > 60 years. For example, Musher et al found the immune response of PPV23 in population aged < 65 years were stronger than that in populations aged 65 ~ 75 years and > 75 yearsCitation22,Citation23. Regrettably, elderly aged > 70 years were not included in this study. Thus, further studies in population of extensive age range may be needed to strengthen the current immunogenicity evidence of the test vaccine in the elderly.
The most common injection-site and systemic adverse reaction was pain and fever respectively, which is in accordance with the former studiesCitation18,Citation19,Citation24. However, frequency of local and systemic AEs differed widely in historical studies in which patient diaries were usedCitation18,Citation19,Citation21,Citation23–Citation26. For example, fever frequency ranged from 0 ~ 9% after the primary doseCitation19,Citation21,Citation23 in historical studies, compared to which the fever frequency in the treatment group in this study was slightly higher. The injection-site pain widely ranged from 16%~ 88% in existing studiesCitation18,Citation19,Citation21,Citation23–Citation25. The symptom frequency variation can be largely attributed to the differences in definition of fever, sample size, targeted population et al. In our study, most adverse events were mild and moderate, and disappeared shortly, suggesting the newly PPV23 was safe and well tolerated.
Conclusions
The newly Sinovac PPV23 is immunologically non-inferior to the control commercial vaccine and well tolerated in healthy Chinese population aged ≥ 2 years. The antibody persistence and efficacy studies are needed to further provide guidance for the vaccine application.
Methods
Study design
This study is a double-blinded randomized, active-controlled non-inferiority clinical trial designed by Henan CDC and performed at Kaifeng city. This trial was registered at clinicaltrials.gov (NCT02451969).
Participants
Healthy participants aged ≥ 2 years with proven legal identity were enrolled into the study. The exclusion criteria included (1) Axillary temperature > Celsius 37 degrees (2) acute disease (3) prior vaccination with pneumococcal vaccine (4) history of bacterial pneumonia within 3 years prior to this study (5) pregnant, breast feeding, or women expected to conceive within 60 days after the vaccination (6) allergy (7) any known immunodeficiency and so on. All enrolled participants were stratified by age (2 ~ 17, 18 ~ 60, > 60 years old), and randomized to receive one dose of test or control vaccine by injection into the deltoid muscle.
Vaccine
The test pneumococcal vaccine, developed by Sinovac Biotech Co., Ltd., is a sterile, liquid vaccine for intramuscular injection. This vaccine contained a mixture of highly purified capsular polysaccharides from the 23 serotypes of S. pneumococcal (serotype 1, 2, 3, 4, 5, 6B,7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F). The control pneumococcal vaccine is a commercial PPV23 manufactured by Chengdu Institute of Biological Products Co., Ltd.
Immunogenicity assessment
Blood was drawn immediately before injection and day 28 after the injection. Serum IgG serotype specific pneumococcal antibodies to 23 serotypes contained in the vaccine were quantified by enzyme-linked immunospot assay (ELISA) at the Chinese National Institute for Food and Drug Control in blindness.
The primary endpoint was the 2-fold increase rate of anti-pneumococcal antibody for all included 23 serotypes.
The secondary endpoints were GMCs and GMFIs of IgG post vaccination.
Safety assessment
For the first 7 days, participants (or guardians) who received vaccination were required to record the injection-site adverse events and systemic adverse events on the diary cards. From day 8 to day 28, the participants reported the adverse events spontaneously. Data on SAEs were collected throughout the trial. The grade and relationship of the adverse event with the vaccination were decided by the investigators in blindness.
Statistical analysis
Non-inferiority test was carried out on the 2-fold increase rate of 23-valent antibody. On the assumption of the 2-fold increase rate of 70% for each type and the non-inferiority margin of −10%, 791 participants per group were required to achieve the overall power of 80% (power for each serotype: 99.13%) with the one-sided significance level of 2.5%. Furthermore, on the assumption of a dropout rate no more than 10%, 880 participants per group were finally determined as the final sample size.
The 95% confidence interval (95% CI) of the 2-fold increase rate difference between the treatment and control group was calculated, and non-inferior was concluded if the lower bound of the 95%CI was larger than −10%. The Student’s t-test was used for the analysis of log-transformed antibody concentration and fold increase. The Chi-square test or Fisher’s exact test was used for the analysis of dichotomous outcomes. Hypothesis testing was 2-sided with an α value of 0.05. All analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
The safety analysis was performed on the safety analysis set (SS) containing all the participants who received either the test or control vaccine and had the safety information. The immunogenicity analysis was performed on the per protocol set (PPS), which is a subset of the full analysis set (FAS). The FAS contains the intention-to-treat (ITT) participants who met all the inclusion criteria and didn’t met the exclusion criteria, received either test or control vaccine randomly and had the effective pre-vaccination serum results. The PPS contains the ITT participants who did not deviate from the trial protocol, received the vaccination and blood sampling within the protocol-required time window and had the effective pre/post-vaccination results of serum antibody assay.
Ethical statements
This trial was approved by the ethics committee of the Henan CDC and conducted according to Good Clinical Practices and the Declaration of Helsinki. Written informed consent was obtained from each subject/subject’s guardian before the enrollment.
Disclosure of potential conflicts of interest
Yuansheng Hu, Weixiao Han, Zhen Han and Jing Li are employees of Sinovac Biotech Co., LTD. All other authors: no conflicts.
Supplemental Material
Download MS Word (41.9 KB)Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, Damaske B, Stefonek K, Barnes B, Patterson J. Epidemiology of invasive streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. Jama. 2001;285(13):1729–1735. doi:10.1001/jama.285.13.1729.
- Yao KH, Yang YH. Streptococcus pneumoniae diseases in Chinese children: past, present and future. Vaccine. 2008;26(35):4425–4433. doi:10.1016/j.vaccine.2008.06.052.
- Huang S, Liu X, Lao W, Zeng S, Liang H, Zhong R, Dai X, Wu X, Li H, Yao Y. Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolates collected at a Chinese hospital from 2011 to 2013. Bmc Infect Dis. 2015;15(1):312–321. doi:10.1186/s12879-015-1042-5.
- Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations Rep. MMWR-Morbid Mortal W. 2010;59(RR–11):1–18. PMID:21150868.
- Mo X, Tobe RG, Liu X, Mori R. Cost-effectiveness and health benefits of pediatric 23-valent pneumococcal polysaccharide vaccine, 7-valent and forecasting 13-valent pneumococcal conjugate vaccines in China. Pediatr Infect Dis J. 2016;35(11):e353–e361. doi:10.1097/INF.0000000000001288.
- CDC. Pneumococcal polysaccharide vaccine, what you need to know. US Department of Health and Human Services, CDC. 2015 http://www.immunize.org/vis/ppsv.pdf.
- CDC. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23)-Advisory Committee on Immunization Practices (ACIP). MMWR-Morbid Mortal W. 2010;59:1102–1106. PMID:20814406.
- Chinese Prevention Medicine Association. Technical guidline on application of pneumococcal vaccine in China (2012). Chin J Epidemiol. 2012;33(11):1101–1110. PMID: 23290891.
- O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7(9):597–606. doi:10.1016/S1473-3099(07)70210-4.
- Aliberti S, Mantero M, Mirsaeidi M, Blasi F. The role of vaccination in preventing pneumococcal disease in adults. Clin Microbiol Infec. 2014;20(Supplement s5):52–58. doi:10.1111/1469-0691.12518.
- Liang Q, Li GF, Zhu FC. Vaccine profile of PPV23: beijing Minhai Biotech 23-valent pneumococcal vaccine. Expert Rev Vaccines. 2016;15(11):1351–1359. doi:10.1080/14760584.2016.1239536.
- Zhang GH, Zheng DY, Shi NM, Ai X, Bai YH. Pneumonia vaccination coverage and influencing factors among part of community elderly in Chaoyang District, Beijing City, China. Chin J Biol Prod. 2013;26(1):93–95. (In Chinese). doi:10.13200/j.cjb.2013.01.98.zhanggh.009.
- You ZJ, Lin B, Gao Z. Pneumonia vaccination coverage and influencing factors among elderly in Qingpu Distric, Shanghai City, China. Med Heal Care. 2015;3:89. (In Chinese).
- Martinelli D, Tafuri S, Caputi G, Fortunato F, Reggio P, Germinario C, Prato R. Eight years of active proposal of pneumococcal 23-valent polysaccharide vaccine: survey on coverage rate among elderly and chronic patients. Ajic Am J Infec Contr. 2010;38(4):e8–e15. doi:10.1016/j.ajic.2009.09.019.
- Zhou Z, Deceuninck G, Lefebvre B, De WP. Forecasting trends in invasive pneumococcal disease among elderly adults in Quebec. Can J Infect Dis Med Microbiol. 2017;2017(37):4347206. doi:10.1155/2017/4347206.
- Wen YW, Wu H, Chang CJ. Using dynamic transmission modeling to determine vaccination coverage rate based on 5-year economic burden of infectious disease: an example of pneumococcal vaccine. Value Heal Reg Issues. 2015;6(7):46–52. doi:10.1016/j.vhri.2015.03.014.
- Kong Y, Zhang W, Jiang Z, Wang L, Li C, Li Y, Xia J. Immunogenicity and safety of a 23-valent pneumococcal polysaccharide vaccine in Chinese healthy population aged >2 years: A randomized, double-blinded, active control, phase III trial. Hum Vacc Immunother. 2015;11(10):2425–2433. doi:10.1080/21645515.2015.1055429.
- Guifan L, Qi L, Jichun S, Yuemei H, Hong L, Wenjin W, Fengcai Z, Qiang Y. Safety and immunogenicity of 23-valent pneumococcal polysaccharide vaccine in 2 to 70 year old healthy people in China: A phase III double blind, randomized clinical trial. Hum Vacc Immunother. 2015;11(3):699–703. doi:10.1080/21645515.2015.1011015.
- Yang Y, Su KX, Song SZ, Zhang Y, Jiang S, Xie QH, Lan F, Huang J, Li YD, Li FX, et al. Clinical Effect of 23-valent Pneumococcal Polysaccharide Vaccine. J Prev Med Infor. 2007;4:390–391.
- World Health Organization. 23-valent pneumococcal polysaccharide vaccine. WHO Position Pap. 2008;83(42):373–384. http://www.who.int/iris/handle/10665/241221.
- Jackson LA, Gurtman A, Van CM, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594–3602. doi:10.1016/j.vaccine.2013.04.084.
- Sankilampi U, Honkanen PO, Bloigu A, Leinonen M. Persistence of antibodies to pneumococcal capsular polysaccharide vaccine in the elderly. J Infect Dis. 1997;176(4):1100–1104. doi:10.1086/516521.
- Musher DM, Manoff SB, Mcfetridge RD, Liss CL, Marchese RD, Raab J, Rueda AM, Walker ML, Hoover PA. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum Vacc Immunother. 2011;7(9):919–928. doi:10.4161/hv.7.9.15996.
- Musher DM, Manoff SB, Liss C, Mcfetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. 2010;201(4):516–524. doi:10.1086/649839.
- Hammitt LL, Bulkow LR, Singleton RJ, Nuorti JP, Hummel KB, Miernyk KM, Zanis C, Whaley M, Romero-Steiner S, Butler JC. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55–74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine. 2011;29(12):2287–2295. doi:10.1016/j.vaccine.2011.01.029.
- Grabenstein JD, Manoff SB. Pneumococcal polysaccharide 23-valent vaccine: long-term persistence of circulating antibody and immunogenicity and safety after revaccination in adults. Vaccine. 2012;30(30):4435–4444. doi:10.1016/j.vaccine.2012.04.052.