ABSTRACT
Human papillomavirus (HPV) is a common sexually transmitted infection which is the cause of several cancers, including cervical cancer, and genital warts. Although cervical cancer can be prevented through screening, this cancer persists in the US. More recently, HPV vaccination has the potential to decrease the burden of HPV-related disease among young HPV-unexposed adolescents. Several initiatives aimed to encourage HPV vaccination have been adopted. Unfortunately, uptake of the HPV vaccine remains modest, despite evidence that vaccine-type HPV prevalence is decreasing as a result of HPV vaccination. Further, geographic disparities in vaccination uptake across different US regions and by race/ethnicity may contribute to continuing disparities in HPV-related cancers. More data are needed to evaluate impact of HPV vaccination on HPV prevalence in smaller geographic areas. Further, more information is needed on the impact of individual vaccination programs and policy on population level vaccination and HPV prevalence.
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection,Citation1 with estimates for the probability of infection with the virus exceeding 80% for women and 90% for males across their lifetime.Citation2 Although most HPV infections are asymptomatic and clear naturally, persistent HPV infection is known to cause several types of cancer, genital warts, and respiratory papillomatosis. Approximately 40 types of HPV have been discovered to infect the anogenital skin epithelium or mucous membranes.Citation3 Of these, 12 are considered high-risk types that can cause cancer. Two types, 16 and 18, are responsible for the majority of HPV-related cancers, including cervical, anal, penile, and oropharyngeal cancers.Citation4 Although Papanicolaou (Pap) screening with subsequent treatment for precancerous cervical lesions has reduced the incidence of cervical cancer significantly since it was introduced in the 1950s,Citation5,Citation6 not all women receive screening or follow-up treatment, and cervical cancer remains a significant cause of mortality.Citation7 Further, disparities in HPV infection and cervical cancer rates, particularly among black women and those living in the Southern US, persist.Citation8
Although cervical cancer is preventable through Pap screening and early treatment, vaccination against high-risk HPV types commonly associated with cervical cancer is a promising primary prevention strategy that could reduce cancers, even among women who are not screened according to guidelines. The majority of HPV-related cancers are preventable through HPV vaccination if it is administered before exposure.Citation9,Citation10 Unfortunately, rates of HPV vaccination have been lower than desired by public health advocacy groups to achieve strong herd immunity in the US, particularly among the age groups in which it is recommended (11–12 years old).Citation11–Citation13 Further, vaccination rates vary by geography, and by race/ethnicity, which could contribute to continuing disparities in HPV-related cancers, including oral and cervical cancers.Citation8,Citation14–Citation21 However, there is evidence that HPV prevalence is decreasing in the US since the HPV vaccine was introduced, and limited evidence of herd immunity has also been demonstrated.Citation22–Citation24 Unfortunately, although we can anticipate decreasing HPV-related cancer rates across the US, it is likely that disparities will continue due to uneven vaccination rates across geographies and race/ethnicity. Several factors contribute to uneven HPV vaccination, and careful surveillance is needed to evaluate how variations in HPV vaccination affect cervical cancer incidence and disparities over the next 2 to 3 decades.
HPV-related cancer and economic burden
Cervical cancer is the 4th most common cancer among women worldwide,Citation25 and currently the 14th cause of cancer deaths among women in the US, with close to 13,000 cases each year and more than 4,000 related deaths.Citation26,Citation27 This cancer remains common despite the fact that it is preventable through screening and, more recently, HPV vaccination. It is estimated that close to 26,000 new HPV-related cancers, including cervical cancer, occur each year in the US. The cost of the HPV virus is significant, with estimated costs of diagnosis and treatment of HPV-related cervical abnormalities and anogenital warts approximately $2.9 billion yearly in both males and females.Citation28 Estimates of the economic burden of non-cervical HPV disease are substantial, as well. These conditions include respiratory papillomatosis, anogenital warts, anal cancer, penile cancer, vaginal cancer, vulvar cancer, and oropharyngeal cancer. For four types of HPV (including 6, 11, 16, and 18) alone, which are the cause of the majority of these conditions, the costs are approximately $418 million.Citation29,Citation30
In addition to HPV-related genital cancers, oropharyngeal cancer is a significant source of morbidity and mortality in the US. Oropharyngeal cancer incidence is increasing, and is expected to exceed that of cervical cancer.Citation31 While it is difficult to determine which oropharyngeal cancer cases are HPV-related, the increase is estimated to be mostly attributable to HPV due to decreased population risk factors for other types of oropharyngeal cancers, such as smoking.Citation32 Oropharyngeal cancers associated with HPV are estimated to have a lifetime cost of $38.1 million.Citation29 Currently, there are no accepted screening or other prevention measures that can reduce the burden of these cancers.Citation33 However, HPV vaccination is associated with lower oral prevalence of HPV,Citation34 and will contribute to reduced risk of oropharyngeal cancers in the future, even if only through reduced circulation of cancer-causing HPV types.Citation35 There is clear epidemiological evidence of HPV-associated oropharyngeal cancer as a sexually transmitted disease. Therefore, reductions in genital infection prevalence will lead to a reduction in oral HPV infections that lead to oropharyngeal cancer, even if the HPV vaccine does not provide primary protection against high-risk oral HPV infections.
HPV vaccines
Currently, there are 3 different HPV vaccines that have been, or are currently being used to prevent HPV-related cancers worldwide. The bivalent HPV vaccine (Cervarix, by GlaxoSmithKline) protects against the 2 high-risk types most commonly associated with cervical cancer, types 16 and 18.Citation36 The quadrivalent HPV (4vHPV) vaccine (Gardasil by Merck) was approved by the FDA for use in the US in 2006, and protects against types 16 and 18, as well as 2 types most commonly associated with genital warts, types 6 and 11. The 4vHPV vaccine has recently (2016) been replaced by the nonavalent HPV (9vHPV) vaccine (Gardasil-9 by Merck) which protects against the 4 types in the 4vHPV vaccine plus 5 additional high-risk types, including 31, 33, 45, 52, and 58. Currently, the 9vHPV vaccine is the only one available in the US. When examining the cost-effectiveness of the different vaccines in the 50 US states and DC, it was determined that making the switch to the 9vHPV vaccine was cost effective, even among states that already exhibit high coverage. However, expanding coverage in states with the lowest HPV vaccine coverage would have a stronger effect in reducing national cervical cancer incidence than in states with higher coverage.Citation37 The significant costs of HPV-related cancers and disease, combined with the high effectiveness of the vaccine at preventing HPV, should encourage strong policies that encourage high vaccine uptake consistently across the US. Unfortunately, the rollout of the vaccine where it was recommended only for females, combined with the efforts of anti-vaccine groups, may have encouraged healthcare consumers to perceive the vaccine as being primarily to prevent a sexually transmitted infection. The cancer-prevention qualities of the vaccine were not emphasized well enough, and it is possible that the initial perception of the vaccine as prevention for a sexually transmitted infection, combined with the vaccine’s high costs, led to less than enthusiastic uptake of the vaccine (44.3% of 13–17 year old females in 2009) early after licensure in the US.Citation38
Effects of vaccination on HPV rates in the U.S
Seroprevalence
Before nationwide testing of genital HPV began using the National Health and Nutrition Examination Survey (NHANES) data, seroprevalence of antigens to L1 vaccine-like particles was measured to estimate exposure to HPV. In data collected between 1991 and 1994 by NHANES, it was found that 13% of 12–59 year olds had antibodies for HPV-16, with higher prevalence noted among: women, those who reported more sexual partners, and those who reported younger age at sexual intercourse.Citation39 It was also noted that seroprevalence of these antibodies varied across age groups, with rates peaking among 20–29 year olds at 24.7% before declining to 11% among women 49 years in older.Citation39 Male seroprevalence was demonstrated to peak at 30–39 years of age at 11.5%, but then remained fairly constant through age 59.Citation39 Data from the same time period found that the seroprevalence of antibodies for HPV-11 was 4.7% among both males and females.Citation40 NHANES discontinued measuring seroprevalence of HPV antibodies as HPV vaccination became more common, and seroprevalence became less valid for determining exposure to vaccine-type HPV.
Genital HPV
Despite modest vaccination rates, decreasing HPV prevalence rates have been demonstrated in national studies. A systematic review of HPV prevalence and incidence among women in the US before HPV vaccination was available determined that prevalence rates of genital HPV types 16, or 16 combined with type 18, among US women ranged from 7.2% to 26.7% before HPV vaccination was introduced.Citation41 Pre-vaccination data from the National Health and Nutrition Examination Survey (NHANES), which is a repeated cross sectional survey that is representative of the US population, estimated vaginal prevalence of 37 types of HPV to be 26.8%, with the highest prevalence among women 20–24 years old (44.8%).Citation42 Pre-vaccination vaginal prevalence of the 4 types of HPV that the 4vHPV vaccine protects against (6, 11, 16, 18) was estimated to be 8.8% among 14–59 year olds, with the highest rates found among 20–24 year olds (18.5%).Citation43 Less information about US genital male HPV prevalence before HPV vaccination is known. A systematic review which evaluated the prevalence of HPV among non-immunocompromised men across the globe found that HPV infection was common, exceeded 20% in most populations, and was as high as 73% in some.Citation44 A study of genital HPV prevalence among males from the US, Brazil, and Mexico found that HPV prevalence was 61% of males in the US, with high-risk type prevalence of 23%.Citation45
After the HPV vaccine was introduced, vaccine-type HPV decreased 56% (from 11.5% to 5.1%) during the pre-vaccination period (2003–2006) compared to the post-licensure period (2007–2010) among 14–19 year olds, despite modest vaccination rates.Citation23 Data from the 2009–2012 cycles of NHANES illustrated a decrease of 64% in vaccine-type HPV among 14–19 year old females from the prevaccine period, and a 34% decrease among women 20–24 years of age.Citation46 These decreases among 14–19 year old females were not observed in other types of HPV that were not protected against by the vaccine, indicating that vaccination is the most likely explanation for the decreases in vaccine-type HPV. These decreases in vaccine-type HPV were substantial, but were assessed in a manner that did not evaluate how vaccination rates were affecting HPV in areas where women are at higher risk of cervical cancer, or among groups who are at higher risk. NHANES data are currently the only known source of national data on HPV prevalence in the US.
When comparisons of HPV vaccinated young women were made, it was found that vaccination was significantly associated with reductions in prevalence of vaccine-type HPV, although many would have potentially been exposed before receiving the vaccine.Citation47 In addition to demonstrating the population-level effectiveness of the vaccine among women who received it at older ages, it became clear that vaccination against other high-risk types was desirable. Estimates of the prevalence of 9vHPV types among females 14–59 years of age in the prevaccine era were found to be 16%, with disparities observed by race/ethnicity.Citation48 Black and Hispanic women had a higher prevalence of types 31, 33, 45, 52, 58 compared to white women – types more commonly found in precancerous cervical lesions among Hispanic and black women.Citation48
More recently, gender disparities in the prevalence of genital HPV has been found, even after HPV vaccination was recommended for both males and females. During 2013–2014, overall genital HPV rates were found to be almost 46% among 14–59 year old males, compared to 40% among females in the same age group.Citation49 Males also had elevated prevalence rates of high risk HPV types, although the prevalence of vaccine-type HPV was not described by gender.Citation49 These disparities highlight the importance of HPV vaccination. Recently, it was demonstrated that decreased genital vaccine-type HPV prevalence in 2013–2014 among males had occurred, but was likely a result of general decrease in HPV prevalence among vaccinated females.Citation50
Racial disparities have also been found related to HPV prevalence. Young black women have a higher prevalence of HPV.Citation51 Before vaccination began in the US, HPV prevalence, as detected through a urine sample, among 18–25 year olds was 27%.Citation52 The prevalence of HPV among white women was 25% and 35% among black women.Citation52 Part of the disparity in HPV prevalence may be related to longer persistence time after infection. For example, young black women were found to have significantly longer persistence of high-risk HPV types when compared to young white women.Citation53 Further, black and Hispanic women are less likely to have high-grade cervical lesions positive for HPV types 16 and 18 compared to white women.Citation54–Citation56 Racial differences in distribution of HPV types in cervical cancer have also been noted. In the US, 25% of cervical cancer cases among black women, 24% among Hispanic women, and 18% among white women were positive for oncogenic types other than type 16 or 18.Citation57 Although these differences are not pronounced, even these small differences will contribute to the persistence of racial/ethnic disparities in HPV-related cancers. However, the 9-valent vaccine, which includes types more prevalent in high-grade lesions found among black and Hispanic women, is expected to decrease disparities in HPV-related disease after it has been administered to a significant portion of the population for up to a decade. Thus, understanding of how the new vaccine impacts HPV infection in racial/ethnic minorities and among males is important to be able to better predict how vaccination will impact disparities in the future.
HPV vaccination has directly contributed to decreases in HPV prevalence in the US. However, there are indications that herd immunity effects may already be occurring despite modest vaccination rates. Both local and national data found evidence of herd immunity, with an approximate 50% decrease in vaccine-type HPV among unvaccinated young women observed between the prevaccine period and the post-licensure period.Citation58,Citation59 Evidence of herd immunity among unvaccinated women has been observed in countries other than the US, as well.Citation60,Citation61
Oral HPV
Rising incidence rates of oral squamous cell carcinomas (OSCC) have been noted between the 1970s and early 2000s. Although smoking is a commonly known cause of oral cancers, decreasing trends in this behavior did not explain increasing rates of OSCC. HPV and changing sexual behaviors was postulated to be the source of this increasing trend.Citation62 There is also evidence of disparities in OSCC incidence. Incidence rates of carcinomas occurring at the base of the tongue have been found to be highest among black men than other racial/ethnic groups.Citation63 Reasons for these observed differences may have been due to increased exposure to high-risk HPV infection or other known confounders, such as smoking or alcohol.Citation63 HPV-related OSCC has been found to be lower among women (27.4% of cases) compared to men (72.6% of cases), although the reason for this is not yet fully understood.Citation62,Citation63
Currently, HPV vaccination is not approved to prevent HPV-related oral cancers. However, there is evidence that HPV vaccination produces an immune response that could protect the oral cavity from vaccine-type HPV infection.Citation64 Protection against oral vaccine-type HPV has been demonstrated in post hoc analyses of a randomized controlled trial among vaccinated young women.Citation65 Population-level protection from vaccine-type oral HPV among vaccinated young men and women have also been found in the US.Citation34,Citation66 The vaccine’s efficacy at preventing HPV-related oral cancer, however, is yet to be determined due to the long period between infection and development of cancer.
HPV vaccination disparities
Although the vaccine has demonstrated high efficacy in preventing HPV infection and precancerous lesions that progress into cancer, it has had modest levels of uptake and a low rate of series completion in the US. In 2016, 60% of all 13–17 year old adolescents had received 1 dose of the vaccine series (65% of females and 56% of males) and 37% had received 3 doses, well below the Healthy People 2020 goal of 80% complete vaccination of 13–15 year olds.Citation12,Citation13 Most states in the US do not require HPV vaccination for school enrollment, which has resulted in several barriers for this vaccine that do not exist for other vaccines which are mandated for school entry. One barrier highly associated with HPV vaccination in the literature is the lack of a strong health provider recommendation. Parents who did not receive a provider recommendation were 2 times more likely to report that they did not intend to vaccinate their child in a national survey.Citation67 Further, health providers have been found to provide recommendations that were not strong or consistent to their patients, and were more likely to be made to females and older adolescents.Citation68
In order to understand vaccination rates and its effect on HPV prevalence in the United States, it is important to understand the history of vaccination policy and the ensuing barriers. Early recommendations for the vaccine were gender-based, and have become more complicated over time.Citation69 Parents were concerned that the vaccine could increase high-risk sexual behaviors,Citation70 a concern which may have contributed to uneven vaccination patterns in the US. In addition, the vaccine has not been mandated by most states for school entry. Although mandates are effective with other vaccines, states with HPV vaccine mandates appear to have similar rates of HPV vaccination compared to those without mandates.Citation71 Mandates in these states had little support by constituents, which resulted in legislation that worked more to promote education about the vaccine rather than ensuring high uptake.Citation71 Parents easily opt out in those states, which likely resulted in vaccination rates that varied little from other states without mandates.Citation71 Strong mandates that are supported through education campaigns and support from communities are needed in order to effectively increase uptake of the HPV vaccine. Other barriers, especially early after the vaccine’s introduction, included: cost to both patients and providers, parents’ lack of awareness, and missed opportunities for vaccination.Citation72–Citation74 Some of these barriers were likely caused directly or indirectly by lack of state mandate, rapidly changing guidelines, gender-based guidelines, and poor tracking of HPV vaccination.
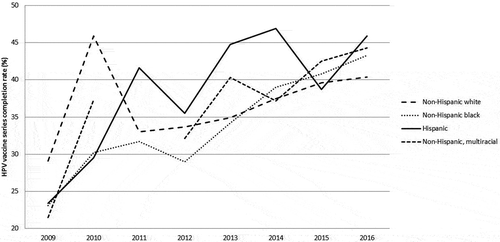
Early after the vaccine’s introduction, disparities in vaccination uptake were observed. In particular, low vaccination occurred among young women who were eligible for catch-up vaccination (unvaccinated 13–26 year olds), with particularly low rates among young black women and those using public health insurance 19–26 years old.Citation75 In a study of Medicaid enrollees from 2008, black adolescents had low initiation and completion rates for the HPV vaccine series.Citation76 Since then, efforts to increase vaccination in these groups appear to have been successful, with national data indicating that vaccine initiation rates no longer differed by race/ethnicity, but completion of the series remained modest in those groups.Citation17 Using HPV vaccination rates among 13–17 year old females captured by the nationally representative National Immunization Survey-Teen (NIS-Teen) data between 2009 and 2016, figures were developed to graphically illustrate vaccination rates by race/ethnicity across time.Citation12,Citation38,Citation77–Citation82 No disparities in HPV vaccine series initiation are noted in the early years after HPV vaccine licensure (). However, Hispanics had higher increases in uptake of the vaccine over time, with black adolescents catching up in 2014. While white female adolescents had steadily increasing rates of HPV vaccination, HPV vaccine initiation rates did not increase as dramatically as other racial/ethnic groups. Disparities in HPV vaccine completion among adolescent females in the US were not as clear, although national data shows less disparity in the most recent data compared to initiation rates for the HPV vaccine ().
Figure 1. Prevalence of HPV vaccine series initiation (≥ 1 dose) by race/ethnicity across time among 13–17 year old females, NIS-Teen 2009–2016.
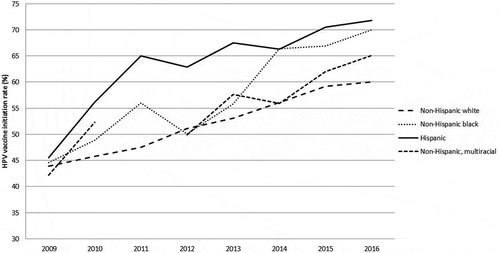
Figure 2. Prevalence of HPV vaccine series completion (≥ 3 dose) by race/ethnicity across time among 13–17 year old females, NIS-Teen 2009–2016.
Geographic disparities in vaccination have also been observed. The Southern states, in particular, have had lower HPV vaccination rates compared to other regions of the US.Citation14 In 2008–2010, adolescents in the South and Southwestern states were less likely to initiate the HPV vaccine series compared to states in the rest of the US.Citation14,Citation15 More recent data of 13–17 year olds showed that adolescents in the Western US states were more likely to have been vaccinated than those in the South.Citation83 Unfortunately, many Southern states in the US also have high rates of cervical cancer. Other geographic disparities in HPV vaccination have been observed in smaller areas. In the city of Minneapolis, Minnesota, HPV vaccination prevalence were found to vary according to ZIP code.Citation84 Vaccination rates in Texas counties have been found to vary, with a range between 1.9% and 23.8% reporting to have received at least 1 dose in 2008.Citation85 These disparities in HPV vaccination indicate that there is a need for additional data to evaluate how vaccination affects HPV prevalence in areas with high vaccination rates compared to areas with lower vaccination rates. This is particularly important, as areas with high cervical cancer rates may have less access to medical care due to being in medically underserved areas, and thus have lower access to HPV vaccines.
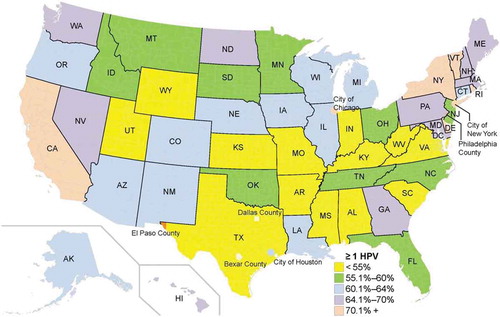
NIS-Teen data were used to illustrate state-level distribution of HPV vaccination by state and select cities and counties in 2009 () and in 2016 () among females.Citation12,Citation38 These data confirm low vaccination rates among 13–17 year old adolescent females in much of the South and Midwest. Rates remained low in the South and Midwestern regions compared to Western and Northeastern states (), with modest to strong increases in vaccination among adolescent females in Southern and Midwestern regions between 2009 and 2016 ().Citation12,Citation38 Although many Southern and Midwestern states showed these increases in HPV vaccination rates, baseline rates were quite low in 2009, so 2016 vaccine initiation rates continued to be lower than rates observed in the West and Northeast for many Southern and Midwestern states. In 2016, several Southern and Midwestern states also appeared to lag behind HPV vaccination rates in Western and Northeastern states among combined adolescent females and males (). Despite having excellent data on HPV vaccination rates by state, there is little information about HPV prevalence within states or by region, or how changing vaccination rates affect HPV prevalence directly in each region.
Figure 3. Changes in HPV vaccine initiation (≥ 1 dose) in the United States among 13–17 year old females, NIS-Teen 2009–2016. a. HPV vaccination rate by state, NIS-Teen 2009.Citation38 b. HPV vaccination rate by state, NIS-Teen 2016.Citation12 c. Percent change in HPV vaccination rate by state between 2009 and 2016, NIS-Teen 2009 & 2016.Citation12,Citation38
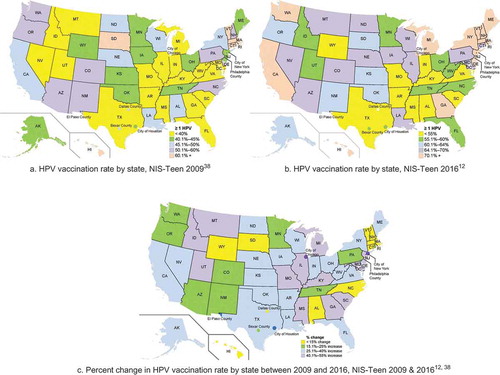
Figure 4. HPV vaccination rate among 13–17 year old males and females in the United States, NIS-Teen 2016.12
Reasons for geographic vaccination disparities are currently not well understood. Lower access to HPV vaccines between regions is not likely to be driven by affordability among children under 18 years of age, as the Vaccines for Children program covers the cost. In addition, there is very little information about how vaccination is affecting HPV prevalence in rural areas. These areas may be medically underserved, have high rates of cervical cancer, and low rates of HPV vaccination. These areas may continue to be a source of future geographical disparities in cervical cancer incidence as a result.
HPV vaccine policy
Most states in the US do not mandate the HPV vaccine for school entry, which makes the recommendations issued by the ACIP important for the success of this vaccine. The perception of the vaccine as a “sex” vaccine has caused controversy, and it is possible that initial recommendations of the vaccine for females only perpetuated this issue. The 4vHPV vaccine was initially licensed by the FDA for use in females 9–26 years of age in 2006. Recommendations by the ACIP in 2007 were gender based, and were for 3 doses, timed at 0, 2, and 6 months for girls 11–12 years of age, with catch-up vaccination recommended for females 13 to 26 years of age, and permissible to be administered as young as 9 years old.Citation86
The ACIP did not issue similar recommendations for males when the FDA licensed the vaccine for them in 2009. Instead, in 2010, the ACIP stated that the vaccine “may be given to males aged 9 through 26 years to reduce their likelihood of acquiring genital warts” and did not recommend it for routine use among males until 2011.Citation87,Citation88 Unfortunately, the recommendation for catch-up vaccinations among males differed from those recommended for females, with catch-up only including males 13–21 years old.Citation87 Although the ACIP also stated that males 22–26 years of age may be vaccinated, particularly those considered to be at high risk, lack of strong recommendation similar to those provided to females may contribute to lower uptake and completion in this age group. The reasons for the differences in recommendation for males stemmed from doubt that the vaccine would be cost-effective in the general male population who were not at increased risk of HPV-related anal cancer or genital warts, including those with immune deficiencies or men who have sex with men.Citation87,Citation89
The 9vHPV vaccine was recommended by the ACIP in 2015. Gendered age recommendations, unfortunately, remained the same as those issued in 2011.Citation90 In late 2016, the FDA approved the use of 2-dose vaccination among males and females between 9 and 14 years of age. Based on mounting evidence that 2 doses spaced 6 months apart elicited non-inferior immunogenicity among young adolescents as compared to 3 doses in older adolescents and young adults,Citation91 the ACIP recommended the 2-dose schedule for the same age group later in 2016, but kept recommendations for the 3-dose schedule for those 15–26 years of age.Citation92 Although this change will likely provide a small boost to estimates of HPV vaccine completion, it is possible that these recommendations may also contribute to some confusion among parents of children in both age ranges who are recommended to receive only 2 doses for a younger child and 3 doses for an older child.
Conclusion
HPV vaccination has reduced the prevalence of vaccine-type HPV in the US. However, uneven vaccination rates by gender, age, in different regions, and among different sub-populations of the US could result in continuing disparities in HPV-related cancers.Citation93 Further, states with higher rates of cervical cancer mortality and lower rates of Pap test screening have had lower levels of HPV vaccination, a disparity that could put those regions at continued risk for continued higher rates of HPV-related cancers and diseases.Citation94 Unfortunately, the data that are currently available may be inadequate for measuring some of these disparities in the US. Information about how strong HPV vaccination programs influence not only HPV vaccination rates, but also oral and genital HPV prevalence in the populations which they serve are needed in order to better show the positive effects of the HPV vaccine. These types of studies can help to better predict where increased screening efforts will be needed in the coming decades when children with low levels of vaccination reach the recommended screening age, as well as inform about the effectiveness of HPV vaccination programs and policy. Although currently available data provide strong national information about the impact of HPV vaccination on HPV prevalence nationally, it is more limited in its ability to assess smaller geographic areas and the impact of individual vaccination programs and policy on local HPV prevalence.
Expert opinion
Currently, even with modest HPV vaccination rates, HPV vaccination has been found to be associated with decreased vaccine-type HPV prevalence in the US using population-based methods among both females and males. The current results highlight the success of the vaccine in reducing the prevalence of these cancer-causing viruses, and should encourage greater uptake and completion of the vaccine series. However, there is much room for improvement in HPV vaccination. Strong policy backed by the community can help to effectively increase uptake and completion of the HPV vaccine series.
Now that the 9vHPV vaccine is available, it is particularly important to improve HPV vaccination rates among higher risk populations and in regions where HPV-related cancer rates are high. In order to better understand where disparities remain, or even develop in the next 2 decades, HPV infection surveillance needs to continue and be improved along with increased advocacy for HPV vaccination in local communities. Current information about the vaccine’s effectiveness should be more widely disseminated to different audiences, including within communities as well as to health providers and legislators. Focusing only on providing information to patients through their clinicians may not achieve desired high population vaccination rates in all regions of the US. Due to the fact that the vaccine is not mandated in most states, providers may forget to inform their patients about the vaccine. Further, patients are often unaware of the vaccine or may not remember to ask about it until their healthcare provider discusses it with them. Demonstrating the positive effects of HPV vaccination to communities and policy advocates will promote greater understanding, improve community and individual awareness, and increase support of effective policy to promote HPV vaccination.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author.
References
- Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MCB, Su J, Xu F, Weinstock H. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193. doi:10.1097/OLQ.0b013e318286bb53.
- Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660–664. doi:10.1097/OLQ.0000000000000193.
- De Villiers E-M, Fauquet C, Broker TR, Bernard H-U, Zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi:10.1016/j.virol.2004.03.033.
- Lowy DR, Schiller JT. Reducing HPV-associated cancer globally. Cancer Prev Res. 2012;5(1):18–23. doi:10.1158/1940-6207.CAPR-11-0542.
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Women’s Health. 2012;21(10):1031–1037. doi:10.1089/jwh.2011.3385.
- Simard EP, Naishadham D, Saslow D, Jemal A. Age-specific trends in black-white disparities in cervical cancer incidence in the United States: 1975-2009. Gynecol Oncol. 2012;127(3):611–615. doi:10.1016/j.ygyno.2012.08.021.
- Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, Stubbs RW, Bertozzi-Villa A, Morozoff C, Charara R, Allen C, Naghavi M, Murray CJL. Trends and patterns of disparities in cancer mortality among us counties, 1980-2014. JAMA. 2017;317(4):388–406. doi:10.1001/jama.2016.20324.
- Yoo W, Kim S, Huh WK, Dilley S, Coughlin SS, Partridge EE, Chung Y, Dicks V, Lee J-K, Bae S, et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PLoS ONE. 2017;12(2):e0172548. doi:10.1371/journal.pone.0172548.
- Villa LL, Costa RLR, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–278. doi:10.1016/S1470-2045(05)70101-7.
- Harper DM, Franco EL, Wheeler CM, Moscicki A-B, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4·5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. The Lancet. 2006;367(9518):1247–1255. doi:10.1016/S0140-6736(06)68439-0.
- Lin X, Rodgers L, Zhu L, Stokley S, Meites E, Markowitz LE. Human papillomavirus vaccination coverage using two-dose or three-dose schedule criteria. Vaccine. 2017;35(43):5759–5761. doi:10.1016/j.vaccine.2017.08.090.
- Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents Aged 13–17 Years — united States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874–882. doi:10.15585/mmwr.mm6633a2.
- Healthy People 2020. Immunization and infectious diseases, 2015. 2015; https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives.
- Hirth JM, Rahman M, Smith JS, Berenson AB. Regional variations in HPV vaccination among 9–17 year old adolescent females from the BRFSS, 2008–2010. Hum Vaccin Immunother. 2014;10(12):3475–3483. doi:10.4161/21645515.2014.980202.
- Rahman M, McGrath CJ, Berenson AB. Geographic variation in human papillomavirus vaccination uptake among 13–17 year old adolescent girls in the United States. Vaccine. 2014;32(21):2394–2398. doi:10.1016/j.vaccine.2014.02.097.
- Jeudin P, Liveright E, Del Carmen MG, Perkins RB. Race, ethnicity and income as factors for HPV vaccine acceptance and use. Hum Vaccin Immunother. 2013;9(7):1413–1420. doi:10.4161/hv.24422.
- Agénor M, Pérez AE, Peitzmeier SM, Borrero S. Racial/ethnic disparities in human papillomavirus vaccination initiation and completion among U.S. women in the post-Affordable Care Act era. Ethn Health. 2018:1–15. doi:10.1080/13557858.2018.1427703.
- Zhan FB, Lin Y. Racial/ethnic, socioeconomic, and geographic disparities of cervical cancer advanced-stage diagnosis in Texas. Women’s Health Issues. 2014;24(5):519–527. doi:10.1016/j.whi.2014.06.009.
- Niccolai LM, Julian PJ, Bilinski A, Mehta NR, Meek JI, Zelterman D, Hadler JL, Sosa L. Geographic poverty and racial/ethnic disparities in cervical cancer precursor rates in Connecticut, 2008–2009. Am J Public Health. 2013;103(1):156–163. doi:10.2105/AJPH.2011.300447.
- D’Souza G, Westra WH, Wang SJ, Van Zante A, Wentz A, Kluz N, Rettig E, Ryan WR, Ha PK, Kang H, et al. Differences in the prevalence of human papillomavirus (hpv) in head and neck squamous cell cancers by sex, race, anatomic tumor site, and hpv detection method. JAMA Oncol. 2017;3(2):169–177. doi:10.1001/jamaoncol.2016.3067.
- Auluck A, Walker BB, Hislop G, Lear SA, Schuurman N, Rosin M. Socio-economic deprivation: a significant determinant affecting stage of oral cancer diagnosis and survival. BMC Cancer. 2016;16:569. doi:10.1186/s12885-016-2579-4.
- Berenson AB, Laz TH, Rahman M. Reduction in vaccine-type human papillomavirus prevalence among women in the United States, 2009–2012. J Infect Dis. 2016;214(12):1961–1964. doi:10.1093/infdis/jiw515.
- Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, Unger ER. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208(3):385–393. doi:10.1093/infdis/jit192.
- Oliver SE, Unger ER, Lewis R, McDaniel D, Gargano JW, Steinau M, Markowitz LE. Prevalence of human papillomavirus among females after vaccine introduction—national Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216(5):594–603. doi:10.1093/infdis/jix244.
- World Health Organisation. GLOBOCAN 2012 cancer incidence and mortality worldwide. Lyon (France): International Agency for Research on Cancer; 2016.
- American Cancer Society. Key Statistics for Cervical Cancer. 2018; https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. Accessed January 8, 2018.
- American Cancer Society. Cancer facts & figures 2017. Atlanta, GA: Guttmacher Institute; 2017.
- Chesson HW, Blandford JM, Gift TL, Tao G, Irwin KL. The estimated direct medical cost of sexually transmitted diseases among American younth, 2000. Perspect Sex Reprod Health. 2004;36(1):11–19. doi:10.1363/psrh.36.11.04.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198(5):500.e501–500.e507. doi:10.1016/j.ajog.2008.03.064.
- Omland T, Lie KA, Akre H, Sandlie LE, Jebsen P, Sandvik L, Nymoen DA, Bzhalava D, Dillner J, Brøndbo K, et al. Recurrent respiratory papillomatosis: HPV genotypes and risk of high-grade laryngeal neoplasia. PLoS ONE. 2014;9(6):e99114. doi:10.1371/journal.pone.0099114.
- Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012;6(Suppl 1):16–24. doi:10.1007/s12105-011-0304-9.
- Simard P, Ward M, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62(2):118–128. doi:10.3322/caac.20141.
- Kreimer AR, Chaturvedi AK. HPV-associated oropharyngeal cancers—are they preventable? Cancer Prev Res. 2011;4(9):1346–1349. doi:10.1158/1940-6207.CAPR-11-0379.
- Hirth JM, Chang M, Resto VA. Prevalence of oral human papillomavirus by vaccination status among young adults (18–30years old). Vaccine. 2017;35(27):3446–3451. doi:10.1016/j.vaccine.2017.05.025.
- Kreimer AR. Prospects for prevention of HPV-driven oropharynx cancer. Oral Oncol. 2014;50(6):555–559. doi:10.1016/j.oraloncology.2013.06.007.
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter DL, Kitchener HC, Castellsague X, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. The Lancet. 2007;369(9580):2161–2170. doi:10.1016/S0140-6736(07)60946-5.
- Durham DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci. 2016;113(18):5107–5112. doi:10.1073/pnas.1515528113.
- Dorell C, Stokley S, Yankey D, Cohn A, Markowitz LE. National, state, and local area vaccination coverage among adolescents aged 13–17 Years — united States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(32):1018–1023.
- Stone KM, Karem KL, Sternberg MR, McQuillan GM, Poon AD, Unger ER, Reeves WC. Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis. 2002;186(10):1396–1402. doi:10.1086/344354.
- Hariri S, Dunne EF, Sternberg M, Unger ER, Meadows KS, Karem KL, Markowitz LE. Seroepidemiology of human papillomavirus type 11 in the United States: results from the Third National Health and Nutrition Examination Survey, 1991–1994. Sex Transm Dis. 2008;35(3):298–303. doi:10.1097/OLQ.0b013e31815abaef.
- Revzina NV, DiClemente RJ. Prevalence and incidence of human papillomavirus infection in women in the USA: a systematic review. Int J STD AIDS. 2005;16(8):528–537. doi:10.1258/0956462054679214.
- Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813. doi:10.1001/jama.297.8.813.
- Dunne EF, Sternberg M, Markowitz LE, McQuillan G, Swan D, Patel S, Unger ER. Human papillomavirus (HPV) 6, 11, 16, and 18 prevalence among females in the United States—national Health and Nutrition Examination Survey, 2003–2006: opportunity to measure HPV vaccine impact? J Infect Dis. 2011;204(4):562–565. doi:10.1093/infdis/jir342.
- Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: a systematic review of the literature. J Infect Dis. 2006;194(8):1044–1057. doi:10.1086/507432.
- Giuliano AR, Lazcano-Ponce E, Villa LL, Flores R, Salmeron J, Lee J-H, Papenfuss MR, Abrahamsen M, Jolles E, Nielson CM, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17(8):2036–2043. doi:10.1158/1055-9965.EPI-08-0151.
- Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3):e20151968. doi:10.1542/peds.2015-1968.
- Guo F, Hirth JM, Berenson AB. Comparison of HPV prevalence between HPV-vaccinated and non-vaccinated young adult women (20–26 years). Hum Vaccin Immunother. 2015;11(10):2337–2344. doi:10.1080/21645515.2015.1066948.
- Liu G, Unger ER, Hariri S, Steinau M, Markowitz LE. Prevalence of 9-valent human papillomavirus types by race/ethnicity in the Prevaccine Era, United States, 2003–2006. Sex Transm Dis. 2016;43(10):633–636. doi:10.1097/OLQ.0000000000000492.
- Lewis RM, Markowitz LE, Gargano JW, Steinau M, Unger ER. Prevalence of genital human papillomavirus among sexually experienced males and females aged 14–59 Years, United States, 2013–2014. J Infect Dis. 2018;217(6):869–877. doi:10.1093/infdis/jix655.
- Gargano JW, Unger ER, Liu G, Steinau M, Meites E, Dunne E, Markowitz LE. Prevalence of genital human papillomavirus in Males, United States, 2013–2014. J Infect Dis. 2017;215(7):1070–1079. doi:10.1093/infdis/jix057.
- Bansal M, Zhao C. Prevalence of high-risk human papillomavirus in women with abnormal and normal vaginal ThinPrep papanicolaou cytology. J Low Genit Tract Dis. 2011;15(2):105–109. doi:10.1097/LGT.0b013e3181f41ee4.
- Manhart LE, Holmes KK, Koutsky LA, Wood TR, Kenney DL, Feng Q, Kiviat NB. Human papillomavirus infection among sexually active young women in the United States: implications for developing a vaccination strategy. Sex Transm Dis. 2006;33(8):502–508. doi:10.1097/01.olq.0000204545.89516.0a.
- Banister CE, Messersmith AR, Cai B, Spiryda LB, Glover SH, Pirisi L, Creek KE. disparity in the persistence of high-risk human papillomavirus genotypes between African American and European American women of college age. J Infect Dis. 2015;211(1):100–108. doi:10.1093/infdis/jiu394.
- Niccolai LM, Russ C, Julian PJ, Hariri S, Sinard J, Meek JI, McBride V, Markowitz LE, Unger ER, Hadler JL, et al. Individual and geographic disparities in human papillomavirus types 16/18 in high‐grade cervical lesions. Cancer. 2013;119(16):3052–3058. doi:10.1002/cncr.28038.
- Vidal AC, Smith JS, Valea F, Bentley R, Gradison M, Yarnall KSH, Ford A, Overcash F, Grant K, Murphy SK, et al. HPV genotypes and cervical intraepithelial neoplasia in a multiethnic cohort in the southeastern USA. Cancer Causes Control. 2014;25(8):1055–1062. doi:10.1007/s10552-014-0406-2.
- Hariri S, Unger ER, Powell SE, Bauer HM, Bennett NM, Bloch KC, Niccolai LM, Schafer S, Steinau M, Markowitz LE, et al. Human papillomavirus genotypes in high grade cervical lesions in the United States. J Infect Dis. 2012;206: 1878–1886.
- Hopenhayn C, Christian A, Christian WJ, Watson M, Unger ER, Lynch CF, Peters ES, Wilkinson EJ, Huang Y, Copeland G, et al. Prevalence of human papillomavirus types in invasive cervical cancers from seven US cancer registries prior to vaccine introduction. J Low Genit Tract Dis. 2014;18(2):182–189. doi:10.1097/LGT.0b013e3182a577c7.
- Kahn JA, Brown DR, Ding L, Widdice LE, Shew ML, Glynn S, Bernstein DI. Vaccine-type human papillomavirus and evidence of herd protection after vaccine introduction. Pediatrics. 2012;130(2):e249–e256. doi:10.1542/peds.2011-3587.
- Berenson AB, Hirth JM, Chang M. Change in human papillomavirus prevalence among U.S. Women aged 18–59 years, 2009–2014. Obstetrics Gynecol. 2017;130(4):693–701. doi:10.1097/AOG.0000000000002193.
- Cameron RL, Kavanagh K, Pan J, Love J, Cuschieri K, Robertson C, Ahmed S, Palmer T, Pollock KGJ. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg Infect Dis. 2016;22(1):56–64. doi:10.3201/eid2201.150736.
- Tabrizi SN, Brotherton JML, Kaldor JM, Skinner SR, Liu B, Bateson D, McNamee K, Garefalakis M, Phillips S, Cummins E, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14(10):958–966. doi:10.1016/S1473-3099(14)70841-2.
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus–related and –unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi:10.1200/JCO.2007.14.1713.
- Ryerson AB, Peters ES, Coughlin SS, Chen VW, Gillison ML, Reichman ME, Wu X, Chaturvedi AK, Kawaoka K. Burden of potentially human papillomavirus‐associated cancers of the oropharynx and oral cavity in the US, 1998–2003. Cancer. 2008;113(10):2901–2909. doi:10.1002/cncr.23745.
- Pinto LA, Kemp TJ, Torres BN, Isaacs-Soriano K, Ingles D, Abrahamsen M, Pan Y, Lazcano-Ponce E, Salmeron J, Giuliano AR. Quadrivalent human papillomavirus (HPV) vaccine induces HPV-specific antibodies in the oral cavity: results from the mid-adult male vaccine trial. J Infect Dis. 2016;214(8):1276–1283. doi:10.1093/infdis/jiw359.
- Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC, Solomon D, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS ONE. 2013;8(7):e68329. doi:10.1371/journal.pone.0068329.
- Chaturvedi AK, Graubard BI, Broutian T, Pickard RKL, Tong Z-Y, Xiao W, Kahle L, Gillison ML. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36(3):262–267. doi:10.1200/JCO.2017.75.0141.
- Cheruvu VK, Bhatta MP, Drinkard LN. Factors associated with parental reasons for “no-intent” to vaccinate female adolescents with human papillomavirus vaccine: national Immunization Survey - Teen 2008–2012. BMC Pediatr. 2017;17:52. doi:10.1186/s12887-017-0969-7.
- Rosen BL, Shepard A, Kahn JA, Health Care US. Clinicians’ knowledge, attitudes, and practices regarding human papillomavirus vaccination: a qualitative systematic review. Acad Pediatr. 2018;18(2, Supplement):S53–S65. doi:10.1016/j.acap.2017.10.007.
- Daley EM, Vamos CA, Thompson EL, Zimet GD, Rosberger Z, Merrell L, Kline NS. The feminization of HPV: how science, politics, economics and gender norms shaped U.S. HPV vaccine implementation. Papillomavirus Res. 2017;3:142–148. doi:10.1016/j.pvr.2017.04.004.
- Ratanasiripong NT. Human papillomavirus vaccine increases high-risk sexual behaviors: a myth or valid concern. J Sch Nurs. 2014;30(6):456–463. doi:10.1177/1059840513520042.
- Perkins RB, Lin M, Wallington SF, Hanchate AD. Impact of school-entry and education mandates by states on HPV vaccination coverage: analysis of the 2009–2013 National Immunization Survey-Teen. Hum Vaccin Immunother. 2016;12(6):1615–1622. doi:10.1080/21645515.2016.1150394.
- Conroy K, Rosenthal SL, Zimet GD, Jin Y, Bernstein DI, Glynn S, Kahn JA. Human papillomavirus vaccine uptake, predictors of vaccination, and self-reported barriers to vaccination. J Women’s Health. 2009;18(10):1679–1686. doi:10.1089/jwh.2008.1329.
- Keating KM, Brewer NT, Gottlieb SL, Liddon N, Ludema C, Smith JS. Potential barriers to HPV vaccine provision among medical practices in an area with high rates of cervical cancer. J Adolesc Health. 2008;43(4):S61–S67. doi:10.1016/j.jadohealth.2008.06.015.
- Berenson AB. An update on barriers to adolescent human papillomavirus vaccination in the USA. Expert Rev Vaccines. 2015;14(10):1377–1384. doi:10.1586/14760584.2015.1078240.
- Dempsey A, Cohn L, Dalton V, Ruffin M. Worsening disparities in HPV vaccine utilization among 19–26 year old women. Vaccine. 2011;29(3):528–534. doi:10.1016/j.vaccine.2010.10.051.
- Cook RL, Zhang J, Mullins J, Kauf T, Brumback B, Steingraber H, Mallison C. Factors associated with initiation and completion of human papillomavirus vaccine series among young women enrolled in Medicaid. J Adolesc Health. 2010;47(6):596–599. doi:10.1016/j.jadohealth.2010.09.015.
- Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Curtis CR, MacNeil J, Markowitz LE, Singleton JA. National and state vaccination coverage among adolescents aged 13 through 17 years — united States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850–858. doi:10.15585/mmwr.mm6533a4.
- Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Singleton JA, Curtis CR, MacNeil J, Markowitz LE, Stokley S. National and state vaccination coverage among adolescents aged 13 through 17 years — united States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–792.
- Elam-Evans LD, Yankey D, Jeyarajah J, Singleton JA, Curtis RC, MacNeil J, Hariri S. National and state vaccination coverage among adolescents aged 13 through 17 years — united States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(29):625–633.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years — united States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(34):685–693.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years — united States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(34):671–677.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years — united States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(33):1117–1123.
- Choi Y, Eworuke E, Segal R. What explains the different rates of human papillomavirus vaccination among adolescent males and females in the United States? Papillomavirus Res. 2016;2:46–51. doi:10.1016/j.pvr.2016.02.001.
- Nelson EJ, Hughes J, Oakes JM, Pankow JS, Kulasingam SL. Geospatial patterns of human papillomavirus vaccine uptake in Minnesota. BMJ Open. 2015;5(8):e008617. doi:10.1136/bmjopen-2015-008617.
- Eberth JM, Zhang X, Hossain M, Tiro JA, Holt JB, Vernon SW. County-level estimates of human papillomavirus vaccine coverage among young adult women in Texas, 2008. Texas Public Health J. 2013;65(1):37–40.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson HW, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56(RR02):1–24.
- Advisory Committee on Immunization Practices. Recommendations on the use of quadrivalent human papillomavirus vaccine in males — advisory committee on immunization practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–1708.
- Advisory Committee on Immunization Practices. FDA Licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59(20):630–632.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis. 2010;10(12):845–852. doi:10.1016/S1473-3099(10)70219-X.
- Petrosky E, Bocchini JA, Hariri S, Chesson H, Curtis CR, Saraiya M, Unger ER, Markowitz LE. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304.
- Dobson SM, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. Immunogenicity of 2 doses of hpv vaccine in younger adolescents vs 3 doses in young women: A randomized clinical trial. JAMA. 2013;309(17):1793–1802. doi:10.1001/jama.2013.1625.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination — updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–1408. doi:10.15585/mmwr.mm6549a5.
- William WW, Lu P-J, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. Surveillance of vaccination coverage among adult populations — united States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(11):1–28. doi:10.15585/mmwr.mm6601a1.
- Moss JL, Reiter PL, Brewer NT. Correlates of human papillomavirus (HPV) vaccine coverage: A state-level analysis. Sex Transm Dis. 2015;42(2):71–75. doi:10.1097/OLQ.0000000000000225.
