ABSTRACT
The 2017/18 winter influenza season in Hong Kong started in early January 2018, predominated by influenza B/Yamagata. We collaborated with private medical practitioners of our sentinel surveillance system to collect respiratory specimens and clinical information from patients with influenza-like illness for estimation of the influenza vaccine effectiveness (VE) using the test-negative case-control design. We found that the overall VE was 59.1% (95%CI 41.1 to 71.8%) against all influenza and 53.5% (95%CI 35.4 to 74.6%) against influenza B. Seasonal influenza vaccine provided moderate to good protection against laboratory-confirmed influenza infection at primary care level in Hong Kong in the 2017/18 winter influenza season.
Introduction
The Government of Hong Kong SAR provides free and subsidised seasonal influenza vaccination through the Government Vaccination Programme (GVP) and the Vaccination Subsidy Scheme (VSS) respectively to target groups such as children aged 6 months to 11 years and elderly aged 65 years or above.Citation1 Members of the public can also receive seasonal influenza vaccine (SIV) in the private medical sector at a cost. According to the Department of Health’s (DH) 2015/16 influenza vaccination coverage survey, the SIV uptake for children aged 6 months to 5 years and elderly aged 65 years or above was 21% and 33% in the 2015/16 season, of which 63% and 82% received SIV under GVP or VSS respectively (primary school children aged 6 to 11 years were not yet included in the subsidy scheme in that season). The overall SIV uptake in the community in 2015/16 was only 12%. Concern of vaccine effectiveness (VE) was one of the major reported reasons for not receiving SIV.Citation2 We estimated the SIV VE against laboratory-confirmed influenza infection at primary care level to generate local evidence for timely monitoring, evaluation and future promotion on the benefit of SIV.
2017/18 influenza vaccination programmes and winter influenza season
In Hong Kong, influenza vaccination for the 2017/18 season started in mid-October 2017.Citation1 By the end of March 2018, about 808,000 doses of SIV were administered under our programmes (GVP and VSS) and 76% were in November and December.Citation3 Although both quadrivalent and trivalent inactivated influenza vaccines (QIV and TIV) are registered for use in Hong Kong, 95% of the SIV used in our programmes and overall imported in Hong Kong were QIV. The northern hemisphere influenza vaccine was used in Hong Kong. The trivalent vaccine consisted of an A/Michigan/45/2015 (H1N1)pdm09-like virus, an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus.Citation4 In addition to the three viruses, the quadrivalent formulation also consisted of a B/Phuket/3073/2013-like virus.
Seasonal influenza in Hong Kong is characterized by a winter peak around the first quarter of a year and another peak in the summer around the third quarter. After a major summer influenza season predominated by influenza A(H3N2) in 2017, influenza activity remained low through September to November. Similar to other countries and areas in the temperate region of the Northern Hemisphere, influenza activity in Hong Kong started to rise in late December 2017, remained high through January to February, and declined to baseline level by end of March 2018.Citation5 According to the influenza virus subtyping conducted by the Public Health Laboratory Service Branch (PHLSB) of the DH, 1641 of 2226 (74%) influenza viruses sub-typed from week 44 of 2017 to week 13 of 2018 were influenza B, among which 91% belonged to the Yamagata lineage.Citation6 The 2017/18 winter season was predominated by influenza B/Yamagata.
Results
From November 2017 to March 2018, our sentinel private medical practitioners (PMP) collected 919 respiratory specimens from influenza-like illness (ILI) patients. Among our ILI patients recruited, 48% were children aged 6 months to 17 years and 45% being adults aged 18 to 64 years (). Only 7% of ILI patients recruited was elderly. The proportion of patients with chronic medical problems was comparable among cases (10%) and controls (12%).
Table 1. Characteristics of influenza cases and non-influenza controls, Centre for Health Protection sentinel surveillance in private medical practitioners, Hong Kong, Nov 2017 to Mar 2018.
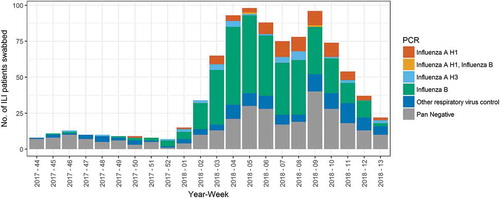
Overall, 490 (54%) were tested positive for influenza A and/or B. Influenza B was the predominant subtype among influenza-positive cases (78%), followed by influenza A(H1) (16%) (). The weekly number of respiratory specimens collected increased from less than 20 from week 44 of 2017 to week 1 of 2018, to over 75 from weeks 4 to 9 of 2018 (). The specimen collection decreased from week 10 and onwards.
Figure 1. RT-PCR results of Centre for Health Protection influenza-like illness sentinel surveillance in private medical practitioners by week of specimen received, Hong Kong, Nov 2017 to Mar 2018.
Note: 5 cases of non-influenza A/B cases with influenza C detection were excluded from this figure.
VE of SIV in the 2017/18 season
After excluding 59 (6%) respiratory specimens (52 with unknown SIV history; five non-influenza A/B cases with influenza C detection and two aged under 6 months), we estimated SIV VE from 467 cases and 393 controls. SIV uptake among cases and controls were 15% and 31% respectively. The overall influenza VE was 59.1% (95%CI 41.1 to 71.8%) against all influenza, 53.5% (95%CI 35.4 to 74.6%) against influenza B and 85.8% (65.9 to 95.2%) against influenza A(H1) (). SIV VE against influenza B was substantially higher in adults [76.8% (95%CI 49.3 to 90.1)] than in children [27.1% (−25.9 to 57.8)]. In contrast, SIV VE against influenza A(H1) was comparable among different age groups (). The overall VE against influenza A(H3) was 40.9% (95%CI −60.3 to 81.6).
Table 2. Seasonal influenza vaccine effectiveness against laboratory-confirmed influenza, Centre for Health Protection sentinel surveillance in private medical practitioners, Hong Kong, Nov 2017 to Mar 2018.
Discussion and conclusions
Our VE estimates showed that SIV offered moderate to good protection against laboratory-confirmed influenza at primary care level in the influenza B/Yamagata predominant 2017/18 winter season in Hong Kong. Our estimates showed that the 2017/18 SIV was effective against both influenza B and influenza A(H1) infections. Due to the small number of influenza A(H3) cases detected during the study period, the VE estimate for this subtype was statistically insignificant, likely due to lack in power. The overall VE estimate of around 50 to 60% (for both all influenza and influenza B only) is comparable to the early season estimates in Canada (55%)Citation7 and Europe (36 to 54% against influenza B)Citation8 but higher than that in the United States (42% against influenza B).Citation9 Hong Kong had a wider use of QIV (with influenza B/Yamagata) as compared to these countries/regions. It should be noted that influenza A(H1) co-circulated with influenza B/Yamagata in Hong Kong whereas influenza A(H3) co-circulated with influenza B/Yamagata in Europe and North America.
In the case-control study design, it is important for controls to be representative of the source population. Our study population was ILI patients who sought medical consultation at PMP. Health-seeking behavior and healthcare workers’ recommendation might affect SIV uptake. According to the 2015/16 influenza vaccination coverage survey, we found that those who sought medical consultation from primary care providers six months before enumeration had higher SIV uptake than those who did not (18.4% vs. 14.7% for children aged 6 months to 17 years and 7.4% vs. 4.7% for adults aged 18 to 64 years). In this TNCC study, the proportion vaccinated among controls in children (34.0%) was higher than our previous estimates in the 2015/16 SIV coverage survey (18.4% for those who sought medical consultation within six months). Although we did not conduct another survey in 2018, the latest administrative statistics from GVP and VSS showed a substantial increase of over 30% in the SIV uptake in the 2017/18 season, and therefore the actual SIV uptake for community children who sought consultation at private medical doctors in the 2017/18 season is likely higher than 18.4% in the 2015/16 season and closer to the SIV uptake among children controls. On the other hand, the proportion vaccinated among adult controls aged 18 to 64 years was 19.2%, which was higher than the survey estimates for the 2015/16 season (7.4%). However, no data on SIV uptake among community adults in the 2017/18 season is available for comparison. The overall vaccination coverage of the control groups was likely comparable to the local population who sought medical consultation at private medical doctors, especially for children. On the other hand, we observed a monthly fluctuation of proportions vaccinated among controls (26%, 16%, 37%, 39% and 31% from November 2017 to March 2018). This may be due to more community members receiving SIV as the influenza season progressed. In addition, the number of controls in Nov (44) and Dec (26) was relatively small and were more prone to random fluctuation.
This study has several limitations. First, date of vaccination was unavailable for exclusion of those receiving SIV within 14 days before onset of symptoms. These patients might not fully develop immunity against influenza, thus including them as vaccinated cases might under-estimate the VE. Second, the number of SIV received by children aged below 9 years was unknown. Thus those having received only the first dose of SIV would be classified as vaccinated. This may also under-estimate the VE. Our 2015/16 influenza vaccination coverage survey showed that 87% of vaccinated children aged under 9 years were fully vaccinated. These two potential limitations might have contributed to the lower VE estimate (39% overall in our study) when compared to another local estimate in hospitalized children by Chiu et al (66% overall),Citation10 who regarded cases receiving SIV within two weeks before hospitalization and those SIV-naïve children receiving only one dose as unvaccinated. It should be noted that, however, SIV VE estimates were generally higher against severe outcomes.Citation11 Third, we do not have details of chronic medical problems to allow further stratification. Forth, we do not have previous vaccination history to study any potential immunological effect of prior vaccinations.Citation12 Fifth, we did not collect information on whether patients had received antiviral therapy before specimen collection. Patients on antiviral therapy should preferably be excluded from the study to avoid biasing the VE estimation. We do not have information on the prevalence of antiviral prescription in ILI patients attending primary care providers in Hong Kong, although a study in hospitalized patients showed that it is much more common among older adults.Citation13 Finally, although we aimed at recruiting all ILI patients attending our partner PMPs, some refused to be swabbed and their vaccination uptake might differ from those who consented. PMP might also be aware of patients’ vaccination status, especially for those who received SIV in their clinics. We tried to limit this potential bias by reminding PMP to swab all patients meeting the clinical criteria, irrespective of vaccination status.
Our study aimed at estimating SIV VE at primary care level. However, we were only able to recruit patients attending PMP in the 2017/18 season. Locally, private Western medicine practitioners are the most common source of medical consultation (50%), as compared to public Western medicine practitioners (29%), private Chinese medicine practitioners (18%) and the Emergency Departments of public hospitals (2%).Citation14 To make our VE estimates more representative, we aim at recruiting different primary care providers in the upcoming seasons.
In conclusion, our estimates showed that SIV offered moderate to good protection against laboratory-confirmed influenza infection in the 2017/18 winter season at primary care level in Hong Kong. We plan to expand our PMP network and recruit different primary care providers to increase the potential sample size to generate more reliable and representative VE estimates in the early season. This would allow timely dissemination of influenza vaccine effectiveness to the public for promotion of vaccination campaign. An early and reliable VE estimate would also inform public health officials to consider alternative prevention strategies in case VE is found to be low.
Patients and methods
Study designs and population
The Centre for Health Protection (CHP) under the DH has established a sentinel surveillance system for ILI and other syndromes. This sentinel surveillance system consists of several networks in the community, including the PMP, general out-patient clinics under the public sector, preschools, residential care homes for the elderly and Chinese Medicine Practitioners. Our PMP network consists of about 50 doctors. As part of the influenza surveillance, PMP reports the number of patients presenting with ILI. In addition, PMP invited all ILI patients within 3 days of onset, irrespective of influenza vaccination status, to collect respiratory specimens for testing in the PHLSB of the CHP. Starting in October 2017, the CHP enhanced the ILI surveillance among PMP for VE estimation as PMP are encouraged to increase the collection of respiratory specimens from ILI patients. They also started to collect information on influenza vaccination status in the current season and chronic medical problemsCitation15 when submitting respiratory specimens for RT-PCR test for respiratory viruses (including influenza) by PHLSB. Influenza virus subtyping was not routinely conducted for these specimens.
Our study population included ILI patients swabbed in one of our partnered PMP from November 2017 to March 2018. ILI cases were patients who presented with fever > 38°C and cough or patients who presented with fever > 38°C and sore throat. We excluded cases who met any one of the following criteria: (i) patients aged under six months, (ii) those with missing information on age and SIV history, and (iii) non-influenza A/B cases with influenza C detection.
Data analysis
We estimated the SIV VE in the 2017/18 season by using the test-negative case-control (TNCC) design.Citation16 We defined cases as ILI patients who tested positive for influenza, and controls as those who tested negative for influenza. We computed the odds ratio (OR) of vaccination among cases and controls using logistic regression with adjustment for covariates including age, sex, chronic illness status and week of specimen delivered. VE was estimated as ((1 – OR)*100). We stratified VE estimation by age group (children aged 6 months to 17 years, adults aged 18 to 64 years, and all ages) and influenza subtypes (influenza B, influenza A(H1), influenza A(H3) and all influenza).
Disclosure of potential conflicts of interest
The authors reported no potential conflict of interest.
Authors’ contribution
All authors contributed to design of the study. Desmond Chan and Miu-ling Wong analysed the data and wrote the first draft. All authors revised the draft manuscript and approved the final version.
Acknowledgments
We thank the PHLSB for their works on laboratory testing and collation of laboratory and clinical information. We thank all participating PMP for their contributions in specimen and data collection. We thank all colleagues in the CHP who work on the sentinel surveillance system of PMP. We appreciate the helpful discussion from colleagues of EpiConcept on an earlier version of this manuscript. We thank the reviewers for their comments which help improved this manuscript.
References
- Centre for Health Protection, Department of Health, Hong Kong SAR. Vaccination schemes at a glance 2017 [accessed 2017 Sep 14]. http://www.chp.gov.hk/en/features/18870.html.
- Chan DYW. Seasonal influenza vaccination coverage survey for the 2015/16 season. Commun Dis Watch. 2016;13(27):4 https://www.chp.gov.hk/en/resources/29/112.html.
- Centre for Health Protection, Department of Health, Hong Kong SAR. Statistics on 2017/18 vaccination programmes/schemes [ accessed 2018 Mar 9]. https://www.chp.gov.hk/en/features/26734.html.
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2017-2018 northern hemisphere influenza season. [ accessed 2018 Jul 15]. http://www.who.int/influenza/vaccines/virus/recommendations/2017_18_north/en/.
- Centre for Health Protection, Department of Health, Hong Kong SAR. Flu express. [ accessed 2018 Mar 9]. https://www.chp.gov.hk/en/resources/29/304.html.
- Centre for Health Protection, Department of Health, Hong Kong SAR. Influenza virus subtyping. [ accessed 2018 Mar 9]. https://www.chp.gov.hk/en/statistics/data/10/641/643/6781.html.
- Skowronski DM, Chambers C, De Serres G, Dickinson JA, Winter AL, Hickman R, Chan T, Jassem AN, Drews SJ, Charest H, et al. Early season co-circulation of influenza A(H3N2) and B(Yamagata): interim estimates of 2017/18 vaccine effectiveness, Canada, January 2018. Euro Surveill. 2018;23. doi:10.2807/1560-7917.ES.2018.23.5.18-00035.
- Rondy M, Kissling E, Emborg HD, Gherasim A, Pebody R, Trebbien R, Pozo F, Larrauri A, McMenamin J, Valenciano M. Interim 2017/18 influenza seasonal vaccine effectiveness: combined results from five European studies. Euro Surveill. 2018;23. doi:10.2807/1560-7917.ES.2018.23.9.18-00086.
- Flannery B, Chung JR, Belongia EA, McLean HQ, Gaglani M, Murthy K, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness - United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180–185. doi:10.15585/mmwr.mm6706a2.
- Chiu SS, Kwan MYW, Feng S, Wong JSC, Leung CW, Chan ELY, Peiris JSM, Cowling BJ. Interim estimate of influenza vaccine effectiveness in hospitalised children, Hong Kong, 2017/18. Euro Surveill. 2018;23. doi:10.2807/1560-7917.ES.2018.23.8.18-00062.
- Restivo V, Costantino C, Bono S, Maniglia M, Marchese V, Ventura G, Casuccio A, Tramuto F, Vitale F. Influenza vaccine effectiveness among high-risk groups: A systematic literature review and meta-analysis of case-control and cohort studies. Hum Vaccin Immunother. 2018;14:724–735. doi:10.1080/21645515.2017.1321722.
- Saito N, Komori K, Suzuki M, Kishikawa T, Yasaka T, Ariyoshi K. Dose-dependent negative effects of prior multiple vaccinations against Influenza A and Influenza B among school children: a study of Kamigoto Island in Japan during the 2011/12, 2012/13 and 2013/14 influenza seasons. Clin Infect Dis. 2018. doi:10.1093/cid/ciy202.
- Cowling BJ, Chui CSL, Lim WW, Wu P, Hui CKM, Peiris JSM, Chan EW, Manicassamy B. Use of influenza antivirals in patients hospitalized in Hong Kong, 2000-2015. PLoS One. 2018;13:e0190306. doi:10.1371/journal.pone.0190306.
- Census & Statistics Department, Hong Kong SAR. Thematic household survey report no. 63 [ accessed 2018 Jul 15]. https://www.censtatd.gov.hk/hkstat/sub/sp140.jsp?productCode=B1130201.
- Centre for Health Protection, Department of Health, Hong Kong SAR. Scientific committee on vaccine preventable diseases - recommendations on seasonal influenza vaccination for the 2017/18 season. [ acceseed 2018 Mar 9]. https://www.chp.gov.hk/files/pdf/short_version_of_recommendations_on_seasonal_influenza_vaccination_for_the_2017_18.pdf.
- Fukushima W, Hirota Y. Basic principles of test-negative design in evaluating influenza vaccine effectiveness. Vaccine. 2017;35:4796–4800. doi:10.1016/j.vaccine.2017.07.003.
