ABSTRACT
Objectives: Little information is available on the characteristics of elderly patients starting TNFα antagonist treatment for rheumatoid arthritis (RA). The objective of this work was to compare prescription patterns in RA patients younger vs. older than 75 years.
Methods: Biologic-naive patients with active RA (DAS28 > 3.2) despite first-line therapy were included between 2007 and 2009 in the prospective, multicentre, longitudinal, observational, population-based CORPUS-RA cohort. TNFα antagonist users were defined as having received at least one TNFα antagonist during the first study year. The groups < 75 years and ≥ 75 years were compared regarding comorbidities, inflammation (CRP and ESR), disease activity (DAS28), disability (HAQ-DI), number of physician visits, and treatment. To verify the impact of the cut off, we also compared patients aged 70 years or more to patients younger than 70 years.
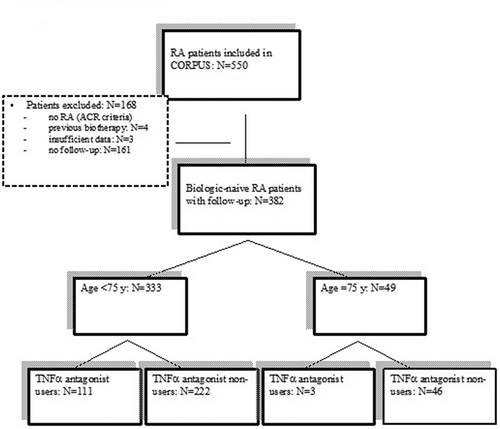
Results: Of 543 RA patients, 382 had complete one-year follow-up data, including 114 TNFα antagonist users, 3 (6%) among the 49 patients aged 75 years or over and 111 (32%) of the 333 patients younger than 75 years (p < 0.01). Disease activity in the two age groups was similar at inclusion and after one year. Comorbidities and a history of auto-immunity were more common in the older group. Compared to their younger counterparts, the older patients received glucocorticoids more often (p = 0.003) and synthetic disease-modifying anti-rheumatic drugs less often (p = 0.01).
Conclusion: TNFα antagonists are used less often and glucocorticoids more often in elderly patients with active RA compared to their younger counterparts. The fact that this study was performed in 2007–9 is a limitation in terms of relevance to today’s patients and further studies should be conducted in new cohorts of active RA.
Introduction
Rheumatoid arthritis (RA) is among the most common chronic inflammatory joint diseases. It manifests as polyarthritis with joint destruction that causes functional disability, thereby adversely affecting quality of life. The current ageing of the general population is mirrored by ageing of the population with RA. Thus, cases of RA diagnosed after 60 years of age are on the rise. Most older patients with RA have an active lifestyle that requires good disease control.
To obtain a remission or low disease activity without further joint destruction, synthetic disease-modifying anti-rheumatic drugs (sDMARDs) should be prescribed as soon as possible after the diagnosis of RA. If these drugs fail, biologics such as TNFα antagonists may provide disease control. TNFα antagonists are licensed for use in older patients. However, the small number of older patients included in randomised controlled trials of TNFα antagonistsCitation1-Citation4 has left gaps in our knowledge of the benefits and risks in this age group. In the few meta-analyses of randomised controlled trials comparing biologics (TNFα antagonists, abatacept, tocilizumab, or anakinra) with or without methotrexate to a placebo in patients with RA, mean age was less than 55 years.Citation5-Citation7 Compared to their younger counterparts, patients older than 75 years had a heavier burden of comorbidities (e.g., kidney dysfunction and heart failure) and more often took multiple chronic medications. These differences suggest a greater risk of adverse drug effects that may lead physicians to limit the aggressiveness of the treatments they prescribe to older patients.Citation8,Citation9 Although driven by concern over patient safety, this attitude may deprive older patients of optimal disease control and quality of life. Several trials comparing patients younger than 65 years and 65 years or over showed similar drug safety profiles and treatment discontinuation rates.Citation10-Citation14 Efficacy also seems unrelated to age,Citation14,Citation15 although questions have been raised about the efficacy of second-line drugs.Citation16,Citation17
In France, although fully reimbursed by the statutory health insurance system, TNFα antagonists may be under-prescribed. In the subgroup of CORPUS cohort patients who had active RA,Citation18 TNFα antagonist initiation was more strongly associated with younger age, longer disease duration, glucocorticoid use, and poorer quality of life than with higher disease activity.
This study is a post hoc analysis of the data of the French Corpus cohort conducted at the request of the French health authorities to assess TNFα antagonist prescription patterns in patients with active RA. We have chosen this cohort because it is an opportunity to have a longitudinal prospective population-based cohort study of active RA. The primary objective of this work was to compare two age groups, < 75 years and ≥ 75 years, regarding second-line drugs used to treat RA insufficiently controlled by first-line therapy. Secondary objectives were to compare the two age groups among patients started on TNFα antagonist therapy, regarding glucocorticoid dosage and changes in C-reactive protein (CRP) levels, disease activity, and disability over the first study year.
Results
Study population
Patients with RA were recruited by 80 rheumatologists working throughout continental France. Of 550 patients with RA, 382 (69.5%) had complete follow-up data and were naive to biologics, including 333 (87.2%) younger than 75 years and 49 (13%) 75 years or older ().
Data at study inclusion ()
Few characteristics differed significantly between the two age groups. In particular, all patients in both age groups were taking sDMARD therapy, usually methotrexate (74.6%) or leflunomide (18.5%). However, comorbidities and a history of auto-immunity were more common in the older group, which had a higher proportion of patients on glucocorticoid therapy, although the dosage was not different between groups.
Table 1. Patient characteristics at inclusion into the CORPUS cohort.
Data at follow-up ()
At follow-up, the older group had fewer patients receiving TNFα antagonist therapy and sDMARD therapy but more patients receiving glucocorticoids. However, the proportion of older patients on glucocorticoid therapy was lower at follow-up than at inclusion, and the glucocorticoid dosage at follow-up was similar in the two age groups. Despite these treatment differences, the two age groups were not significantly different regarding inflammation severity, disease activity, or disability. The main sDMARDs used were methotrexate (81%) and leflunomide (14%).
Table 2. Characteristics of patient at follow-up.
Older patients receiving TNFα antagonist therapy ()
Of the 49 patients 75 years or older, only 3 were prescribed TNFα antagonist therapy during the study (). None had comorbidities. At the follow up visit, two were taking adalimumab and one infliximab. TNFα antagonist therapy was associated with improvements in the DAS28, HAQ-DI, and morning stiffness duration, whereas the asthenia persisted. No adverse effects were noted during the first year of follow-up.
Table 3. Characteristics of the three patients 75 years or older who received TNFα antagonist therapy.
Comparison of patients < 70 years and 70 years or more of age
At follow-up, 290 patients were younger than 70 years and 43 were 70 to 75 years of age. TNFα antagonist use was significantly more common in the younger patients (102/290 (35.2%) vs. 11/92 (11.9%); p < 0.01).
Continuation of the first-line drug was more common in the younger patients (207/250 (82.8%) vs. 55/79 (69.6%); p = 0.02), whereas glucocorticoid therapy was less common (128/249 vs. 60/79; p < 0.01).
Discussion
This study identified significant differences in the treatment of RA between patients < 75 and ≥ 75 years, during the first year of follow-up. TNFα antagonist therapy was used more often in the younger patients, despite similar disease activity in the two age groups. Glucocorticoid therapy was used more often and sDMARD therapy less often in the older age group.
Short-term glucocorticoid therapy is often prescribed to patients with RA, to control flares and while waiting for DMARD therapy to take effect. Long-term glucocorticoid therapy, in contrast, is not recommended, as its risk/benefit ratio remains unclear. In everyday practice, however, 50% to 60% of patients with RA take glucocorticoid therapy.Citation19,Citation20 A systematic literature review showed that long-term low-dose glucocorticoid therapy combined with DMARD therapy was generally effective but that glucocorticoid-related adverse events, although dose-related, occurred even with low dosesCitation21 and were of greatest concern in older patients with multiple comorbidities. Of six studies that assessed major cardiovascular events, four found an increase associated with low-dose glucocorticoid therapy.Citation21 In a study of mortality in RA, taking more than 5 mg/d of glucocorticoids was associated with a significantly higher risk of death.Citation22 Glucocorticoid therapy is also associated with a higher frequency of serious infections.Citation21-Citation23
The differences in first-line therapy between our two age groups are consistent with earlier reports. In a registry study reported in 2006, onset of RA after 60 years of age was associated with significantly less use of biologics (25% vs. 33.1% for onset < 60 years) and DMARDs (30.9% vs. 40.5%) despite similar disease duration, activity, and severity.Citation8 A cross-sectional study done in 2008 found that treatment was less aggressive in older patients with RA compared to their younger counterparts; in particular, initial methotrexate therapy was significantly less commonly prescribed (47% vs. 57% of patients) and methotrexate dosages were lower (5.46 ± 1.66 mg/week vs. 5.96 ± 1.77 mg/week).Citation9
In general, the risk of adverse drug events increases with advancing age, due to the gradual accumulation of comorbidities such as heart failure and kidney dysfunction, as well as to the age-associated decline in immune function. Also, drugs are often less effective in older patients. In a review of four randomised and five open-label studies of etanercept, treatment efficacy was independent from age.Citation15 Similarly, an analysis of data from two randomised trials showed no age-related differences in the efficacy of methotrexate alone or of TNFα antagonist therapy with or without methotrexate.Citation14 In four randomised trials and two long-term extensions, etanercept used to treat RA was slightly less effective in patients older than 65 years than in younger patients, but the difference was small and the frequency of adverse events was similar in the two groups.Citation16
These data suggest that older patients with RA should be treated aggressively if needed to achieve good disease control and quality of life. A prospective study evaluated a treat-to-target strategy in 151 patients with elderly-onset RA (mean age, 74.9 years).Citation24 Adherence to the strategy was 83.4% after 6 months and 75.5% after 1 year. Importantly, after 1 year, nearly 50% of patients were in structural remission and 63% were in functional remission. Similarly, Cho et al shown the retention rate of TNF alpha was comparable in the elderly and younger patients although the major cause of discontinuation was AEs in the elderly patients, while it was drug ineffectiveness in younger patients.Citation25
A 2005 review article indicated that sDMARDs and TNFα antagonists were similarly tolerated by patients younger than 65 years and those aged 65 years or over.Citation11 Nevertheless, older patients were at higher risk for non-serious infections and tuberculosis reactivation. The risk of cardiac events is higher in patients with NYHA class II, II, or IV heart failure treated with TNFα antagonists, and the risk of lymphoma increased with age although there is no proven association with biologics. In patients with RA aged 65 years or over, the higher risk of infections associated with etanercept therapy seemed related to age-related comorbidities rather than to the drug itself.Citation14
The first strength of this study is its nationwide, longitudinal, prospective design. The second strength is that it included patients based on disease activity and not on prescription criteria. This point allowed us to assess the rates and reasons of prescribing or not TNFα antagonist therapy.
This study had two main limitations. One is the small number of patients with RA older than 75 years and treated by TNFα antagonists. The other is the moderate relevance of this study performed in 2007–9, to today’s patients as new biologics agents are now available. Nevertheless, adalimumab and etanercept remain the most frequently used anti TNF in France.
In conclusion, older patients (≥ 75 years or ≥ 70 years) with active RA receive sDMARDs and TNFα antagonists less often, and glucocorticoids more often, than do their younger counterparts. A personalised evaluation of the risk/benefit ratio of various drugs is mandatory, with a special regard to comorbidities in older patients with RA. Further studies should be conducted in new cohorts of active RA.
Materials and methods
Study design and population
CORPUS is a French, prospective, observational, multicentre, longitudinal, population-based cohort of biologics-naive patients with inflammatory joint disease (RA, spondyloarthritis, or juvenile idiopathic arthritis) recruited in private practices and hospitals between 2007 and 2009 by 102 rheumatologists, internists, and paediatricians.Citation18 This cohort was established at the request of French health authorities to assess TNFα antagonist use in patients with these disorders. The patients were monitored prospectively for at least one year.
Before inclusion, all patients gave their written consent to participate in the CORPUS study. The study was approved by the Ethics Committee of Nancy, France.
This study was conducted in the subset of CORPUS patientsCitation18 older than 18 years who met American College of Rheumatology (ACR) criteria for RA,Citation26 had active disease (DAS28 > 3.2) at inclusion despite first-line therapy, had never taken biologics, and underwent at least one follow-up evaluation 3 to 24 months after inclusion. All treatments were at the discretion of the managing rheumatologists. TNFα antagonist users were defined as patients who received at least one TNFα antagonist injection between inclusion and 3 months before the follow-up visit. Between 2007 and 2009, the TNFα antagonists available in France were etanercept, adalimumab, and infliximab.
Data collection
The study data were collected on standardized forms by the rheumatologists who recruited the patients to the cohort. Each patient was evaluated twice, at inclusion and 12 months later. Each evaluation consisted of a standardised interview, general physical examination, laboratory tests, and self-administered questionnaires.
The following data were recorded at inclusion: age; sex; disease duration; anti-citrullinated peptide antibody (ACPA) and rheumatoid factor (RF) status; ACR criteria for RA; history of treatment with sDMARDs, biologics, and glucocorticoids; extra-articular signs of RA; and medical history and comorbidities. Data recorded at both visits were the tender and swollen joint counts, plasma CRP level, erythrocyte sedimentation rate (ESR), patient visual analogue scale (VAS) score for global disease activity, DAS28 (calculated with ESR if available and CRP otherwise),Citation27 Health Assessment Questionnaire Disability Index (HAQ-DI),Citation28 radiographic erosions, and treatments (sDMARDs, biologics, and glucocorticoids) with their start and stop dates and dosages.
Statistical analyses
The data collected at inclusion and at follow-up were described as mean± SD or median (range) for quantitative data and n (%) for qualitative data (missing data were excluded when computing percentages).
The groups < 75 years and ≥ 75 years were compared regarding comorbidities, inflammation (CRP and ESR), disease activity (DAS28), disability (HAQ-DI), number of physician visits and admissions, and first-line treatment. TNFα antagonist use was compared in the two age groups. Univariate analyses were performed to identify variables associated with TNFα antagonist use in each age group, using the chi-square test or Fisher’s exact test, as appropriate, for qualitative variables and the Mann-Whitney test for quantitative variables.
To verify the impact of the cut off to separate old and young patients, we also compared patients aged 70 years or more to patients younger than 70 years.
All tests were two-sided and p values ≤ 0.05 were considered significant. Statistical analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA).
Abbreviations
| CRP | = | plasma C-reactive protein level |
| ACPA | = | anti-citrullinated peptide antibodies |
| RF | = | rheumatoid factors |
| ESR | = | erythrocyte sedimentation rate |
| DAS28 | = | Disease Activity Index on 28 joints |
| HAQ-DI | = | Health Assessment Questionnaire Disability Index |
| sDMARD | = | synthetic disease-modifying anti-rheumatic drug |
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, et al. Infliximab (chimeric anti-tumour necrosis factor a monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet. 354;1999:1932–1939.
- Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H, et al Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor. Lancet. 1994;344:8930.
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human antitumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi:10.1002/art.10697.
- Klareskog L, Van Der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, Martín Mola E, Pavelka K, Sany J, Settas L, et al. Therapeutic effect of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double blind randomized controlled trial. Lancet. 2004;363:675–681. doi:10.1016/S0140-6736(04)15640-7.
- Pincus T, Richardson B, Strand V, Bergman MJ. Relative efficiencies of the 7 rheumatoid arthritis Core Data Set measures to distinguish active from control treatments in 9 comparisons from clinical trials of 5 agents. Clin Exp Rheumatol. 2014;32(5 Suppl 85):S-47–S-54.
- Michaud TL, Rho YH, Shamliyan T, Kuntz KM, Choi HK. The comparative safety of tumor necrosis factor inhibitors in rheumatoid arthritis: a meta-analysis update of 44 trials. Am J Med. 2014;127:1208–1232. doi:10.1016/j.amjmed.2014.06.012.
- Jansen JP, Buckley F, Dejonckheere F, Ogale S. Comparative efficacy of biologics as monotherapy and in combination with methotrexate on patient reported outcomes (PROs) in rheumatoid arthritis patients with inadequate response to conventional DMARDs—a systematic review and network meta-analysis. Health Qual Life Outcomes. 2014;12:102. doi:10.1186/1477-7525-12-102.
- Tutuncu Z, Reed G, Kremer J, Kavanaugh A. Do patients with older-onset rheumatoid arthritis receive less aggressive treatment? Ann Rheum Dis. 2006;65:1226–1229. doi:10.1136/ard.2005.051144.
- Ogasawara M, Tamura N, Onuma S, Kusaoi M, Sekiya F, Matsudaira R, Kempe K, Yamaji K, Takasaki Y. Observational cross-sectional study revealing less aggressive treatment in Japanese elderly than nonelderly patients with rheumatoid arthritis. J Clin Rheumatol. 2010;16:370–374. doi:10.1097/RHU.0b013e3181fe8b37.
- Alivernini S, Mazzotta D, Zoli A, Ferraccioli G. Leflunomide treatment in elderly patients with rheumatoid or psoriatic arthritis; retrospective analysis of safety and adherence to treatment. Drugs Aging. 26;2009:395–402.
- Ranganath VK, Furst DE. Disease-modifying antirheumatic drug use in the elderly rheumatoid arthritis patient. Clin Geriatr Med. 2005;21:649–669. doi:10.1016/j.cger.2005.02.010.
- Radovits BJ, Kievit W, Laan RF. Tumour necrosis factor-alpha antagonists in the management of rheumatoid arthritis in the elderly: a review of their efficacy and safety. Drugs Aging. 2009;26:647–664. doi:10.2165/11316460-000000000-00000.
- Soubrier M, Tatar Z, Couderc M, Mathieu S, Dubost JJ. Rheumatoid arthritis in the elderly in the era of tight control rheumatoid arthritis in the elderly in the era of tight control. Drugs Aging. 2013;30:863–869. doi:10.1007/s40266-013-0122-8.
- Bathon JM, Fleischmann RM, Van der Heijde D, Tesser JR, Peloso PM, Chon Y, White B. Safety and efficacy of etanercept treatment in elderly subjects with rheumatoid arthritis. J Rheumatol. 33;2006:234–243.
- Köller MD, Aletaha D, Funovits J, Pangan A, Baker D, Smolen JS. Response of elderly patients with rheumatoid arthritis to methotrexate or TNF inhibitors compared with younger patients. Rheumatology (Oxford). 2009;48:1575–1580. doi:10.1093/rheumatology/kep291.
- Fleischmann RM, Baumgartner SW, Tindall EA, Weaver AL, Moreland LW, Schiff MH, Martin RW, Spencer-Green GT. Response to etanercept (Enbrel) in elderly patients with rheumatoid arthritis: a retrospective analysis of clincal trial results. J Rheumatol. 30;2003:691–696.
- Lahaye C, Tatar Z, Dubost JJ, Soubrier M. Overview of biologic treatments in the elderly. Joint Bone Spine. 2015;82:154–160. doi:10.1016/j.jbspin.2014.10.012.
- Saraux A, Benichou J, Guillevin L, Idbrik L, Job-Deslandre C, Sibilia J, Soudant M, Wendling D, Guillemin F. Which patients with rheumatoid arthritis, spondyloarthritis, or juvenile idiopathic arthritis receive TNF-α antagonists in France? The CORPUS cohort study. Clin Exp Rheumatol. 33;2015:602–610.
- Sokka T, Kautiainen H, Toloza S, Mäkinen H, Verstappen SMM, Lund Hetland M, Naranjo A, Baecklund E, Herborn G, Rau R, et al. QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann Rheum Dis. 2007;66:1491–1496. doi:10.1136/ard.2006.069252.
- Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, Skakic V, Badsha H, Peets T, Baranauskaite A, et al Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11:R7. doi:10.1186/ar2684.
- Kavanaugh A, Wells AF. Benefits and risks of low-dose glucocorticoid treatment in the patient with rheumatoid arthritis. Rheumatol (Oxford). 2014;53:1742–1751. doi:10.1093/rheumatology/keu135.
- Ruyssen-Witrand A, Fautrel B, Saraux A, Le Loët X, Pham T. Cardiovascular risk induced by low-dose corticosteroids in rheumatoid arthritis: a systematic literature review. Joint Bone Spine. 2011;78:23–30. doi:10.1016/j.jbspin.2010.02.040.
- Listing J, Kekow J, Manger B, Burmester G-R, Pattloch D, Zink A, Strangfeld A. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann Rheum Dis. 2015;74:415–421. doi:10.1136/annrheumdis-2013-204021.
- Sugihara T, Ishizaki T, Hosoya T, Iga S, Yokoyama W, Hirano F, Miyasaka N, Harigai M. Structural and functional outcomes of a therapeutic strategy targeting low disease activity in patients with elderly-onset rheumatoid arthritis: a prospective cohort study (CRANE). Rheumatology (Oxford). 2015;54:798–807. doi:10.1093/rheumatology/keu395.
- Cho SK, Sung YK, Kim D, Won S, Choi CB, Kim TH, Jun JB, Yoo DH, Bae SC. Drug retention and safety of TNF inhibitors in elderly patients with rheumatoid arthritis. BMC Musculoskelet Disord. 2016;17:333. doi:10.1186/s12891-016-1134-4.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31;1988:315–324.
- Prevoo ML, van ‘T Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 38;1995:44–48.
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 23;1980:137–145.