ABSTRACT
Background/Aim: Pancreatic ductal adenocarcinoma (PDA) remains one of the most aggressive tumors with a dismally poor prognosis. Although surgical resection remains the only potentially curative treatment, most PDAs are not surgically resectable at diagnosis. Therefore, multimodal therapy is urgently needed to improve the long-term survival of PDA patients.
Methods: Six eligible PDA patients underwent multimodal therapy comprising dendritic cells (DCs) pulsed with Wilms’ tumor 1 (WT1) peptide (DC/WT1-I) restricted by the human leukocyte antigen (HLA) class I (A*24:02 or A*02:06) allele, chemotherapy, radiation, and/or surgery. Patient laboratory data, DC/WT1-I-specific delayed-type hypersensitivity (DTH) reactions, and WT1-specific immune responses were analyzed to assess the prognostic markers of multimodal therapy.
Results: Compared to 2-treatment type combinations, multimodal therapy involving 3 to 4 treatment types was significantly associated with longer overall survival (p = 0.0177). Moreover, after 7 DC/WT1-I vaccinations, the progression-free survival (PFS) of PDA patients with a neutrophil to lymphocyte ratio (NLR) or C-reactive protein (CRP) level less than the median was superior to that of PDA patients with values above the median (p = 0.0246). PDA patients with an overall survival (OS)>1000 days had significantly more lymphocytes after one DC/WT1-I vaccination course than did those with an OS<1000 days.
Conclusion: Multimodal therapy involving the DC/WT1-I vaccination may benefit patients with advanced PDA. However, comparing the limited number of PDA patients in terms of survival is difficult because the patients were at different disease stages and received different treatments. Further studies are needed to evaluate the clinical benefits of this multimodal therapy.
Introduction
Pancreatic ductal adenocarcinoma (PDA) is one of the most aggressive tumor entities due to a high rate of metastasis and significant resistance to conventional chemotherapy and radiation.Citation1 Despite many advances in cancer therapy, little substantial progress has been made in improving survival periods.Citation1 Complete surgical resection with negative histological margins is the only curative treatment for PDA, but the five-year survival rate is only 15–25%.Citation2 Disease recurrence after complete surgical resection occurs within 1 year of surgery in a majority of PDA patients, suggesting that most patients may have clinically occult, microscopic, metastatic cells at the time of surgery.Citation3 Therefore, adjuvant chemotherapy, such as with S-1 or gemcitabine (GEM), is the standard treatment for PDA patients following surgery.Citation4 In PDA patients with unresectable disease, chemotherapy regimens such as 5-fluorouracil, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX)Citation5 and GEM plus nab-paclitaxel (nab-PTX)Citation6 have been shown to have greater efficacy than GEM. The exact combinations of chemotherapeutic agents depend primarily on a patient’s performance status because of severe toxicities. For resected PDA patients, compared with GEM, the adjuvant combination therapy (GEM/capecitabineCitation7 or modified FOLFIRINOXCitation8) was safe and provided clinical benefits. Moreover, the benefit of chemotherapy with concurrent stereotactic body radiation therapyCitation9 or proton beam therapyCitation10 following surgical resection of pancreatic cancer has been reported. However, PDA is notoriously resistant to concurrent chemoradiation therapy. As an alternative, cancer vaccine strategies have shown success in PDA.Citation11 Therefore, multimodal therapy involving novel therapies such as cancer vaccines is urgently needed to improve the outcomes of PDA patients.Citation12,Citation13
Dendritic cells (DCs) are specialized antigen-presenting cells capable of presenting tumor-associated antigens (TAAs) to T lymphocytes.Citation14 Therefore, numerous studies have focused on the use of DC-based cancer vaccines to initiate and spread TAA-specific antitumor immune responses and boost TAA-specific cytotoxic T cell lymphocytes (CTLs), which spontaneously exist in cancer patients.Citation15 PDA cells express multiple TAAs, including Wilms’ tumor 1 (WT1), mucin 1 (MUC1), mutated KRAS, p53, survivin, carcinoembryonic antigen (CEA), epidermal growth factor receptor 2 (HER-2), and human telomerase reverse transcriptase (hTERT).Citation16 In particular, WT1 was ranked as the best target antigen for cancer vaccines among 75 TAAs selected by a National Cancer Institute (NCI) prioritization project.Citation17 WT1 is a potent transcriptional regulator that correlates with tumor cell growth, invasion, metastasis, and angiogenesis.Citation18 Moreover, standard chemotherapeutic agents such as GEM and S-1 can enhance WT1 peptide presentation on human PDA cells, thus sensitizing PDA cells to WT1-specific cytotoxic effects.Citation19 All PDA cells express WT1 in the cytoplasm and nucleus.Citation20 Therefore, reactivating the immune system of PDA patients by targeting WT1 may be a powerful therapeutic tool. Moreover, WT1-specific CTLs target not only PDA cells but also tumor vascular endothelial cells and immune suppressive cells such as myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment, thus resulting in good clinical benefits.Citation18,Citation21,Citation22 Indeed, there is considerable clinical interest in chemoimmunotherapy combining WT1-targeted DC vaccines with conventional chemotherapy for PDA patients.Citation18,Citation22 Therefore, the development of surgical, chemotherapeutic and radiation therapy strategies combined with WT1-targeted cancer vaccines might be highly desirable. However, the effects of multimodal therapy on the survival of PDA patients have not been demonstrated.
In the present study, the clinical benefits of multimodal therapy involving WT1-targeted chemoimmunotherapy, radiation, or surgery were investigated. Furthermore, we assessed prognostic factors associated with early recurrence in patients with PDA who underwent multimodal therapy.
Results
PDA patient characteristics
Patients with pathologically or cytologically confirmed, measurable, surgically removed, metastatic, or recurrent PDA were enrolled (). PDA patients had UICC stage IIb (n = 1), III (n = 2), or IV (n = 3) disease. The DCs generated from all 6 PDA patients displayed a characteristic phenotype comprising the human leukocyte antigen (HLA)-ABC, HLA-DR, CD40, CD80, CD86, CD83, and CCR7 expression (data not shown). Six PDA patients were administered DCs pulsed with the major histocompatibility complex (MHC) class I-restricted WT1 peptide (DC/WT1-I) vaccine (approximately 1 × 107 cells/dose) at least 7 times every 2 weeks (1 course). One patient (No. 1) received the DC/WT1-I vaccine more than 25 times according to her wishes, while the 5 remaining PDA patients received a total of 7 vaccinations (). As shown in , we identified 3 super-responders (overall survivals [OS] >1000 days, Nos. 1, 2, and 5) and 3 nonsuper-responders (OS ≤1000 days, Nos. 3, 4, and 6). One patient with stage IIb disease (No. 1) underwent surgery followed by GEM plus 7 DC/WT1-I vaccines as adjuvant therapy. However, we identified liver metastases approximately 3 years after the operation. Therefore, the patient was then administered chemotherapy (FOLFIRINOX or GEM/nab-PTX) combined with the DC/WT1-I vaccine. The patient received more than 25 DC/WT1-I vaccinations and remained alive for more than 2747 days. A patient with stage III PDA (No. 2) received GEM combined with X-ray therapy; however, multiple lymph node metastases increased in size. Then, the patient received 7 DC/WT1-I vaccinations alone, resulting in stable lymph node disease and decreased levels of CA19-9 (from 186 to 45.7). The patient remained alive for more than 1571 days, even with advanced inoperable disease. Another super-responder was a patient with stage III disease (No. 5). This patient received conversion surgery after downstaging by GEM/S-1 followed by S-1 plus proton beam therapy. Then, the patient received 7 DC/WT1-I vaccinations and 10 months of S-1 as adjuvant therapy. Although this patient had lung metastases approximately 1 year after the last S-1 treatment, she remained alive for more than 1134 days. The patient is making plans for restarting chemotherapy. On the other hand, 2 non-super-responders (Nos. 3 and 6) had progressive disease (PD) and were treated with proton beam therapy combined with 7 DC/WT1-I vaccinations. These 2 patients died 826 and 846 days after the first treatment. Another non-responder (No. 4) received S-1 combined with 7 DC/WT1-I vaccinations after recurrence subsequent to surgical resection; however, liver metastases rapidly progressed, and this patient died 348 days after the first treatment.
Table 1. Characterization of patients with pancreatic adenocarcinoma.
Table 2. Clinical response by multimodality therapy for PDA patients.
Clinical benefits of multimodal therapy
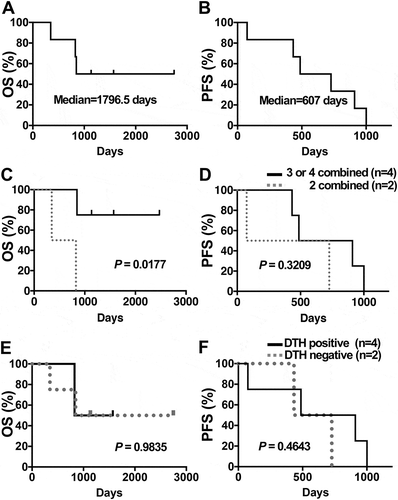
The median OS and progression-free survivals (PFS) of all 6 PDA patients enrolled in this study were 1796.5 and 607 days, respectively ( and ). All 6 patients received at least 7 DC/WT1-I vaccinations. These 6 PDA patients received 3 to 4 or 2 types of therapy. The OS of PDA patients treated with 3 to 4 types of therapy (Nos. 1, 2, 5, and 6) was significantly longer than that of PDA patients treated with 2 types of therapy (Nos. 3 and 4) (p = 0.0117) (). However, there was no significant difference in PFS between PDA patients treated with 3 to 4 types and 2 types of therapy (p = 0.3209) (). Interestingly, 3 (Nos. 1, 2, and 5) of the 4 PDA patients administered 3 to 4 types of treatment, such as DC/WT1-I vaccine, chemotherapy, radiation, and/or surgery, remained alive for more than 1000 days (at least 2747, 1571, and 1134 days).
Figure 1. OS and PFS of PDA patients receiving multimodal therapy including DC/WT1-I vaccines. Kaplan-Meier estimates of the OS and PFS of PDA patients. (A) OS and (B) PFS of all 6 enrolled PDA patients. (C) OS and (D) PFS of PDA patients who received 3 or 4 therapies including DC/WT1-I vaccines (solid line, n = 4) or 2 therapies including DC/WT1-I (dotted line, n = 2). (E) OS or (F) PFS of delayed-type hypersensitivity (DTH)-positive (solid line, n = 4) or DTH-negative (dotted line, n = 2) PDA patients who received multimodal therapy including DC/WT1-I. OS, overall survival; PFS, progression-free survival; PDA, pancreatic ductal adenocarcinoma; DC/WT1-I, dendritic cells pulsed with major histocompatibility complex class I-restricted Wilms’ tumor 1 peptide.
Assessment of DC/WT1-i-specific immune responses in vivo
After vaccination with DC/WT1-I, 4 (Nos. 1, 4, 5, and 6) of the 6 PDA patients showed DC/WT1-I-specific delayed-type hypersensitivity (DTH) positivity (), while DTH positivity was undetected in the 2 patients with PDA (Nos. 2 and 3). The median survival time (MST) of the DTH-positive and DTH-negative PDA patients was 1797 and 1199 days, respectively. Moreover, the median PFS of the DTH-positive and DTH-negative PDA patients was 698.5 and 580 days, respectively. However, the MST and PFS of the DTH-positive PDA patients tended to be longer than those of the DTH-negative PDA patients, but these differences were not significant (p = 0.9835 and p = 0.4643, respectively; and ).
WT1-specific CTLs in peripheral blood mononuclear cells (PBMCs)
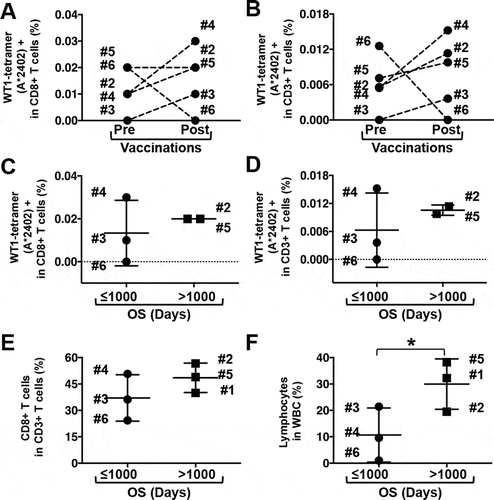
All 5 PDA patients with HLA-A*24:02 were assessed for the induction of HLA-A*24:02-restricted WT1-CTLs by the DC/WT1-I vaccine, as adequate HLA-A*02:06 tetramers were not available. In 4 (Nos. 2, 3, 4, and 5) out of 5 PDA patients, HLA-A*24:02-restricted WT1-CTLs in CD8 + CD3 + T cells () or total CD3 + T cells () were increased after 1 course of DC/WT1-I vaccination. However, no significant difference was observed in the percentages of HLA-A*24:02-restricted WT1-CTLs in the CD8 + CD3 + T cells or total CD3 + T cells pre- and postvaccination ( and ). Moreover, the percentages of HLA-A*24:02-restricted WT1-CTLs in the CD8 + T cells () and total CD3 + T cells () in super-responders (OS>1000 days, Nos. 1, 2, and 5) increased after one DC/WT1-I vaccination course but did not significantly differ from those in the nonsuper-responders (OS≤1000 days, Nos. 3, 4, 6) (p = 0.1369). Next, we compared the percentage of CD8 + T cells in CD3 + T cells; this percentage increased in super-responders after one vaccination course, but the difference between super-responders and nonsuper-responders was not significant (). However, the percentage of lymphocytes in the white blood cells (WBCs) after one vaccination course was significantly higher in super-responders than in nonsuper-responders (p = 0.0377) ().
Figure 2. Frequency of immune-related cells in PDA patients receiving multimodal therapy including DC/WT1-I vaccines. Five patients (Nos. 2, 3, 4, 5, and 6) with HLA-A*2402 were analyzed for WT1-specific CTLs using WT1 HLA-A*2402 tetramers. The percentage of WT1-tetramer (HLA-A*2402) + CD8 + T cells in CD8 + T cells (A) and in total CD3 + T cells (B) before and after 1 vaccination course was evaluated. After 1 vaccination course, the percentage of WT1-tetramer (HLA-A*2402) + CD8 + T cells in CD8 + T cells (C) and in total CD3 + T cells (D) in nonsuper-responders (OS ≤1000 days, Nos. 3, 4, and 6) and super-responders (OS>1000 days, Nos. 2 and 5) was compared. After 1 course of vaccination, the percentages of CD8 + T cells in CD3 + T cells (E) and of lymphocytes in whole WBCs (F) in non-super-responders (OS≤1000 days, Nos. 3, 4, and 6) and super-responders (OS>1000 days, No. 1, 2 and 5) were compared. The black horizontal bar shows the mean ± SD. * Significant differences. OS, overall survival; PDA, pancreatic ductal adenocarcinoma; WBC, white blood cell; DC/WT1-I, dendritic cells pulsed with major histocompatibility complex class I-restricted Wilms’ tumor 1 peptide.
Prognostic markers of multimodal therapy as indicated by laboratory data
The numbers of lymphocytes, neutrophils, and platelets and the levels of hemoglobin (Hb), lactate dehydrogenase (LDH), and albumin (Alb) were obtained by analyzing the samples before and after one DC/WT1-I vaccination course and assessed for prognostic impact in PDA patients following multimodal therapy. The median neutrophil to lymphocyte ratio (NLR) (), C-reactive protein (CRP) (), and the prognostic nutritional index (PNI) in 6 PDA patients were calculated, and associations with clinical benefit were assessed. Laboratory data, including NLR ( and ) and CRP ( and ) before vaccination were not associated with OS or PFS. However, after 1 course of DC/WT1-I vaccination, below-median NLR and CRP values were not associated with improved OS ( and ) but were significantly associated with PFS (p = 0.0246 and p = 0.0246, respectively) ( and ). In addition, there was no association of an above-median PNI with improved OS or PFS during DC/WT1-I vaccination (data not shown).
Figure 3. Kaplan-Meier estimates of the OS and PFS of PDA patients receiving multimodal therapy including DC/WT1-I vaccines. (A) OS and (B) PFS of all 6 enrolled PDA patients with an NLR greater than (dotted line) or less than (solid line) the median prior to vaccination. (C) OS and (D) PFS of all 6 enrolled PDA patients with an NLR greater than (dotted line) or less than (solid line) the median after 7 vaccinations. E: Prior to vaccination, the NLR of PDA patients with super-OS (OS>1000 days, Nos. 1, 2 and 5) and non-super-OS (PFS≤1000, Nos. 3, 4, and 6) were compared. Moreover, the NLR of PDA patients with super-PFS (OS>700 days, Nos. 1, 3 and 5) and non-super-PFS (PFS≤700, Nos. 2, 4, and 6) were compared. F: After 7 vaccinations, the NLR of PDA patients with super-OS (OS>1000 days, Nos. 1, 2 and 5) and non-super-OS (OS≤1000, Nos. 3, 4, and 6) was compared. Moreover, the NLR of PDA patients with super-PFS (PFS>700 days, Nos. 1, 3 and 5) and non-super-PFS (PFS≤700, Nos. 2, 4, and 6) was compared. NLR, neutrophil to lymphocyte ratio; OS, overall survival; PFS, progression-free survival; PDA, pancreatic ductal adenocarcinoma.
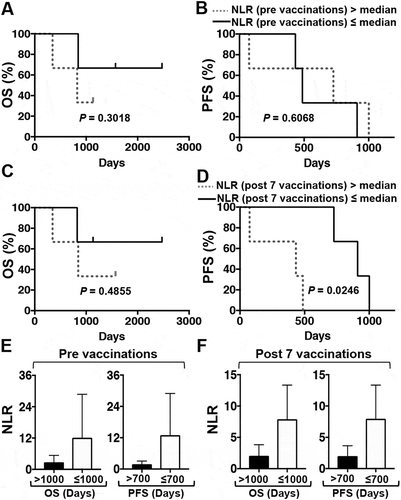
Figure 4. Kaplan-Meier estimates of the OS and PFS of PDA patients receiving multimodal therapy including DC/WT1-I vaccines. (A) OS and (B) PFS of all 6 enrolled PDA patients with CRP levels above (dotted line) or below (solid line) the median prior to vaccination. (C) OS and (D) PFS of all 6 enrolled PDA patients with CRP levels above (dotted line) or below (solid line) the median after 7 vaccinations. E: Prior to vaccination, the CRP levels of PDA patients with super-OS (OS>1000 days, Nos. 1, 2 and 5) and non-super-OS (OS≤1000, Nos. 3, 4, and 6) were compared. Moreover, the CRP levels of PDA patients with super-PFS (PFS>700 days, Nos. 1, 3 and 5) and non-super-PFS (PFS≤700, Nos. 2, 4, and 6) were compared. F: After 7 vaccinations, the CRP levels of PDA patients with super-OS (OS>1000 days, Nos. 1, 2 and 5) and non-super-OS (OS≤1000, Nos. 3, 4, and 6) were compared. Moreover, the CRP levels of PDA patients with super-PFS (PFS>700 days, Nos. 1, 3 and 5) and non-super-PFS (PFS≤700, Nos. 2, 4, and 6) were compared. CRP, C-reactive protein; OS, overall survival; PFS, progression-free survival; PDA, pancreatic ductal adenocarcinoma; DC/WT1-I, dendritic cells pulsed with major histocompatibility complex class I-restricted Wilms’ tumor 1 peptide.
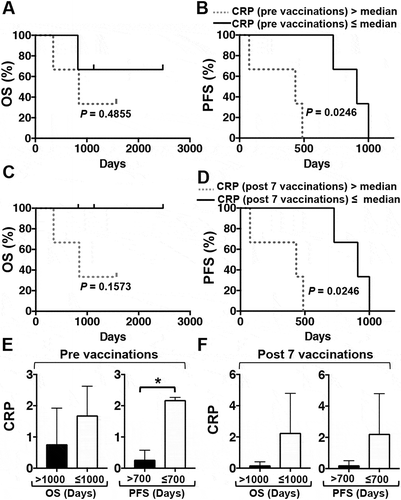
In this study, the PDA patients were divided into two groups based on OS: OS>1000 days (n = 3; Nos. 1, 2, and 5) and OS≤1000 days (n = 3; Nos. 3, 4, and 6). Moreover, the PDA patients were divided into two groups based on PFS: PFS>700 days (n = 3; Nos. 1, 3, and 5) and ≤700 days (n = 3; Nos. 2, 4, and 6). Then, we assessed the association of laboratory data (NLR, CRP, or PNI) with clinical benefit (super-OS>1000 days or super-PFS>700 days). NLR values tended to be lower in the super-OS and super-PFS groups than in the non-super-OS and non-super-PFS groups before and after the 7 vaccinations, but this difference was not significant ( and ). On the other hand, before the 7 vaccinations, the CRP level in the super-PFS group was significantly lower than that in the non-super-PFS group (p = 0.0004) (). After 7 vaccinations, the CRP level in the super-PFS group tended to decrease compared with that in the non-super-PFS group, but this difference was not significant (). In addition, PNI; the numbers of lymphocytes, neutrophils, and platelets; and the levels of Hb, LDH, and Alb pre- and postvaccination were unassociated with clinical benefits (OS or PFS) in this study (data not shown).
Discussion
Only 15 to 20% of PDA patients are candidates for surgical resection at diagnosis. However, the majority of PDA patients develop local recurrence or metastases after surgical resection. PDA tissue is dense and highly resistant to many traditional chemoradiation therapeutics, making it difficult to improve the OS of patients with this disease. The purpose of the current study was to evaluate the clinical benefits of treating PDA patients with multimodal therapy involving WT1-targeted DC-based cancer vaccines. In this study, multimodal therapy including 3 to 4 types of conventional treatments and DC/WT1-I vaccines was significantly associated with longer OS.
In this study, six eligible PDA patients were treated with DC/WT1-I vaccines, chemotherapy, radiation, and/or surgery. The median OS and PFS of PDA patients were 1796.5 and 607 days, respectively. Two PDA patients (Nos. 1 and 5) underwent surgery. One patient (No. 1) with stage IIb disease underwent surgery followed by DC/WT1-I vaccines combined with GEM as adjuvant therapy. Another patient (No. 5) had inoperable locally advanced PDA; however, this patient could undergo conversion surgery after GEM/S-1 downstaging followed by S-1 plus proton beam therapy. Then, the patient was treated with DC/WT1-I vaccines and S-1 as adjuvant therapy. Although adjuvant chemotherapy is now the standard after surgical resection, it has had no real impact on the long-term survival of PDA patients. Indeed, patients with resectable PDA have an average survival of 18 to 20 months Citation2. After surgical resection, 2 patients (Nos. 1 and 5) presented with metastases, and the administration of multimodal therapy may have resulted in the prolonged OS (more than 2747 and 1134 days). Improved outcomes in terms of decreased perioperative morbidity and mortality suggest the importance of multimodal therapy. However, the management of patients with PDA remains poorly defined. In this study, the administration of 3 to 4 types of therapy, including DC/WT1-I vaccines, significantly prolonged the OS compared to 2 types of therapy, and 3 of the 6 patients remained alive for more than 1000 days (at least 2747, 1571, and 1134 days). Therefore, at least 3 types of combination therapy may help improve the prognosis of PDA patients. Moreover, DC/WT1-I vaccines may be associated with prolonged survival when combined with multimodal therapy.
To induce WT1-specific antitumor immunity in this study, DCs pulsed with an MHC class I (HLA-A*02:06 or HLA-A*24:02)-restricted WT1 peptide were injected as a cancer vaccine at least 7 times into all 6 PDA patients, depending on the patient’s HLA type. A positive DTH response against the DC/WT1-I vaccine was detected in 4 of the 6 PDA patients. DTH is an inflammatory reaction mediated by antigen-specific CD4+and CD8 + T cells that infiltrate the DC/WT1-I injection site.Citation23 The significant correlation between favorable clinical outcomes and a positive vaccine-related DTH response is mediated by CD4+and CD8 + T cells.Citation23 In this small number of PDA patients, there was no significant correlation between clinical benefit (OS and/or PFS) and DC/WT1-I-specific DTH reactions. However, 2 (Nos. 1 and 5) of the 4 DTH-positive PDA patients experienced long-term survival (more than 2747 and 1134 days) and remained alive. One (No. 3) of the 2 DTH-negative PDA patients died 826 days after treatment, and the other DTH-negative patient (No. 2), who underwent 3 types of combination therapy, remained alive for more than 1671 days. The long-term survival (OS>1000 days) of PDA patient No. 2 may not be primarily correlated with the DC/WT1-I vaccination but may be correlated with chemoradiation therapy. Vaccination of PDA patients with DCs pulsed with WT1 peptides for both MHC classes I and II (DC/WT1-I/II) may more effectively induce long-term survival.Citation24 One (No. 4) of the 4 DTH-positive PDA patients died 348 days (less than 1 year) after the 1st treatment. This patient received 2 types of therapy: the chemotherapeutic agent S-1 and DC/WT1-I vaccination. Chemoimmunotherapy consisting of DC/WT1-I/II, not DC/WT1-I, is effective.Citation25 Only WT1-specific CD8 + T reactions generated following vaccination may be functionally impaired.Citation26 Functional antitumor immunity targeting WT1 antigens may be essential to stimulate both WT1-specific CD8+CTLs and CD4+helper T cells, resulting in long-lived WT1-specific functional antitumor responses.Citation25,Citation26 Indeed, the simultaneous activation of antigen-specific CD4+helper T cells augments the induction, proliferation, and maintenance of functional antigen-specific CD8+CTLs, resulting in clinical benefits.Citation25,Citation27
It is important to identify the predictive and prognostic markers of multimodal therapy. Previous studies have reported that laboratory data, serum Alb levels,Citation28 systemic inflammatory response markersCitation29 and CRP levelsCitation28 can serve as prognostic factors in various cancers. Recently, the predictive value of NLR has also been identified in PDA patients.Citation29 A high NLR has been associated with the poor prognosis of PDA patients treated with chemotherapy or surgical resection.Citation29 In this study, DC/WT1-I-vaccinated PDA patients who had a below-median NLR exhibited a significantly prolonged PFS compared to those with an above-median NLR. However, before vaccination, there was no association between below-median NLR and clinical benefit (OS or PFS). A series of 7 vaccinations with DC/WT1-I may influence systemic inflammation in PDA patients with a prolonged PFS. As the NLR denotes the balance between the systemic inflammatory and antitumor immune responses,Citation30 DC/WT1-I vaccines may be, at least in part, associated with an improvement of the immune balance in PDA patients. Decreased neutrophil and increased lymphocyte numbers in peripheral blood result in the activation of antitumor immunity.Citation31 Therefore, vaccination with DC/WT1-I may create a hospitable microenvironment in which antitumor immunity can be induced, thereby prolonging PFS. Meanwhile, CRP is a nonspecific marker of the systemic inflammatory response.Citation32 Before and after vaccination, poor PFS was observed in PDA patients with CRP levels above the median. Therefore, high CRP in PDA patients was significantly related to a poor PFS. In this study, there was no significant difference in long-term OS between PDA patients with high systemic inflammation (high NLR or CRP) and those with low systemic inflammation. All 6 enrolled PDA patients permitted continuous multimodal therapy consisting of 2 to 4 treatments. These results suggest the possibility of a crossover effect in combination therapy.
The PNI is used for predicting the nutritional status and clinical outcome of patients with advanced biliary tract cancer administered DC-based cancer vaccines.Citation33 Therefore, we estimated the significance of the PNI for the prognosis of PDA patients treated with multimodal therapy including DC/WT1-I vaccines. There was no significant association between the PNI and clinical benefit (OS or PFS). During vaccination, five of the 6 eligible PDA patients had a low PNI (≤49.5), which is a risk factor for a poor prognosis, especially in patients with resectable and metastatic disease.Citation34 Although 5 of the 6 eligible PDA patients had a low PNI, the administration of 3 to 4 types of therapy in combination resulted in a prolonged OS. One PDA patient with a high PNI (=51.9) during vaccination remained alive for more than 2747 days. It was difficult to assess the significance of the PNI due to the small sample size. In particular, the systemic inflammatory response, which is common in cancer patients, has been shown to be associated with poor prognosis in patients with various types of cancer.Citation35
In summary, the present study demonstrated that multimodal therapy consisting of DC/WT1-I vaccines, chemotherapy, radiation, and/or surgery may benefit patients with advanced PDA. Three PDA patients (Nos. 1, 2, and 5) were super-responders based on their OS>1000 days, as they had nonmetastatic disease; however, patients 3, 4, and 6 were worse off because they had metastatic disease. Therefore, comparing the limited number of PDA patients in terms of survival is difficult because they were at different disease stages and received different treatments. Moreover, a limitation of the present study is that it included relatively few PDA patients. Further studies are needed to evaluate the clinical benefits of multimodal therapy including DC/WT1-I vaccines in a large sample of PDA patients.
Materials and methods
Study design
The ethics committee of the Institutional Review Board at National Hospital Organization Kagoshima Medical Center (No. 23–05) reviewed and approved this study. Informed written consent was obtained from all patients before administering treatment per the Declaration of Helsinki, and sample collection was approved by the Institutional Review Board. All 6 patients with PDA underwent multimodal therapy involving WT1-targeted DC vaccinations, chemotherapy, radiation, and/or surgery.
Multimodal therapy
A PBMC-rich fraction was obtained by leukapheresis. Autologous PBMCs were isolated using Ficoll-Paque Premium density gradient solution (GE Healthcare Bio-Sciences, Piscataway, NJ). PBMCs were cultured for 30 min in 100-mm petri dishes, and nonadherent cells were removed. Adherent PBMCs were further cultured for 5 days in AIM-V medium (Gibco Life Technologies, NY) supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF) (50 ng/mL, Primmune Corp., Kobe, Japan) and interleukin-4 (IL-4) (50 ng/mL, R&D Systems, Minneapolis, MN) to generate immature DCs. The immature DCs were stimulated with penicillin-killed and lyophilized preparations of a low-virulence strain (Su) of Streptococcus pyogenes (OK-432; 10 μg/mL, Chugai Pharmaceutical Co, Ltd., Tokyo, Japan) and prostaglandin E2 (PGE2; 50 ng/mL, Daiichi Fine Chemical Co, Ltd., Toyama, Japan) for an additional 24 h.
The mature DCs were cryopreserved until the day of vaccination. After thawing, each cell aliquot’s viability for vaccination was confirmed to be greater than 90% using Trypan blue exclusion analysis. The mature DCs were pulsed with WT1 peptide restricted for MHC class I (HLA-A*02:06 [126–134: RMFPNAPYL] or HLA-A*24:02 [235–243: CYTWNQMNL]), depending on the patient’s HLA type. A total of 1 × 107 DC/WT1-I were intradermally and bilaterally administered to PDA patients near the axillary region and groin regardless of the standard chemotherapy regimen. However, most vaccines overlapped with standard chemotherapy. The initial treatment protocol was planned as 1 course (seven vaccinations every 2 weeks). OK-432 (0.1–0.2 mg) was also administered if no drug allergy occurred. The PDA patients could receive additional DC/WT1-I vaccinations until they withdrew consent. To assess their phenotype, the mature DCs were stained with the following monoclonal antibodies (mAbs): fluorescein isothiocyanate (FITC)-conjugated anti-human CD14 (61D3, eBioscience), HLA-ABC (W6/32), CD80 (2D10), CD40 (5C3), phycoerythrin (PE)-conjugated anti-human CCR7 (150,503, R&D Systems), CD11c (3.9), HLA-DR (L243), CD83 (HB 15e), and CD86 (IT2.2; BioLegend, San Diego, CA). Cellular expression was analyzed using FlowJo analysis software (Tree Star, OR). The mature DC preparations were tested for endotoxins, bioburden, and mycoplasma at BMS, Inc. (Osaka, Japan).
Standard chemotherapeutic regimens, including GEM alone, S-1 alone, GEM/S-1, GEM/nab-PTX, and FOLFIRINOX, were administered at the patient’s request. PDA patients who refused standard chemotherapy could receive other treatments, including DC/WT1-I vaccines. Moreover, surgery and/or radiation (X-ray therapy or proton beam therapy) were also allowed per the patient’s request.
DC/WT1-I-specific immune responses in vivo
DC/WT1-I-specific immune responses were assessed during each DC/WT1-I vaccination course. The maximum diameter of erythema at the DC/WT1-I injection site was measured 48 h after injection. Analysis of DC/WT1-I-specific DTH is an in vivo method for ascertaining WT1-specific immune function mediated by DC/WT1-I vaccinesCitation24. We defined erythema and induration as DC/WT1-I-specific reactions.
WT1-specific CTLs in PBMCs
The cryopreserved PBMCs were thawed after 1 vaccination course, and WT1-specific CTLs were assessed using the PE-conjugated tetramer for WT1/HLA-A*24:02 (MBL, Nagoya, Japan), FITC-conjugated anti-human CD8 mAb (BioLegend), and APC-Cy7-conjugated anti-human CD3 mAb (BioLegend). The population of HLA-A*24:02-restricted WT1-specific CD8 + T cells (WT1-CTLs) among CD8+ CD3+or CD3 + T cells is shown as the percentage of the double-positive population (WT1/HLA-A*24:02 tetramer-positive cells+) in the total CD8+ CD3+or CD3 + T cell population. Background cells were excluded by gating to identify less than 0.01% WT1-specific CD8 + T cells in the CD8+ CD3+ population. The PE-conjugated tetramer for the human immunodeficiency virus (HIVenv)/HLA-A*24:02 (RYLRDQQLL) was used as a negative control.
Laboratory data
All laboratory data, including the numbers of lymphocytes, neutrophils, WBCs, and platelets and the levels of Hb, CRP, LDH, and Alb, were obtained from PDA patients prior to and after one DC/WT1-I vaccination course. Moreover, the NLR was analyzed prior to and during treatment. The NLR was defined as the ratio of the number of neutrophils to lymphocytes in the blood. We also calculated PNI as 10 × serum Alb (g/dL) + 0.005 × total lymphocyte count (cells/mm3) Citation33. All data were used to assess the prognostic significance of administering DC/WT1-I vaccines combined with conventional therapy for PDA patients.
Statistical analysis
The laboratory data were assessed to determine their association with OS and PFS. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The statistical analyses of prognostic factors for OS and PFS were performed using the Kaplan-Meier method and the log-rank test. The prognostic value of systemic parameters (CRP, NLR, or PNI) and the percentage of immune-related cells (CD8 + T cells among CD3 + T cells or lymphocytes among WBCs) in PDA patients with OS>1000 days or ≤1000 days were evaluated using a t-test. Moreover, the prognostic parameters (CRP, NLR, or PNI) for PDA patients with PFS>700 days and PFS ≤ 700 days were evaluated using a t-test. P < 0.05 indicated statistical significance.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
References
- Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–348. doi:10.1038/s41575-018-0005-x.
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi:10.1056/NEJMra0901557.
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–1481. doi:10.1001/jama.2013.279201.
- Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–257. doi:10.1016/S0140-6736(16)30583-9.
- Kim R. FOLFIRINOX: a new standard treatment for advanced pancreatic cancer? Lancet Oncol. 2011;12:8–9. doi:10.1016/S1470-2045(10)70237-0.
- Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107: dju413.
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;11:1011–1024. doi:10.1016/S0140-6736(16)32409-6.
- Conroy T, Hammel P, Hebba Mr, Abdelghani MB, Wei AC, Raoul JL, Chone L, Artru EFP, Biagi JJ, Lecomte T, et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J Clin Oncol. 2018;36(18_suppl):LBA4001–LBA4001. doi:10.1200/JCO.2018.36.18_suppl.LBA4001.
- Hsu CC, Herman JM, Corsini MM, Winter JM, Callister MD, Haddock MG, Cameron JL, Pawlik TM, Schulick RD, Wolfgang CL, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990. doi:10.1245/s10434-009-0743-7.
- Boimel PJ, Berman AT, Li J, Apisarnthanarax S, Both S, Lelionis K, Larson GL, Teitelbaum U, Lukens JN, Ben-Josef E, et al. Proton beam reirradiation for locally recurrent pancreatic adenocarcinoma. J Gastrointest Oncol. 2017;8:665–674. doi:10.21037/jgo.2017.03.04.
- Amedei A, Niccolai E, Prisco D. Pancreatic cancer: role of the immune system in cancer progression and vaccine-based immunotherapy. Hum Vaccin Immunother. 2014;10:3354–3368. doi:10.4161/hv.34392.
- Weinberg BA, Yabar CS, Brody JR, Pishvaian MJ. Current standards and novel treatment options for metastatic pancreatic adenocarcinoma. Oncology (Williston Park, NY). 2015;29(809–20):86.
- Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, Javle MM, Eads JR, Allen P, Ko AH, et al. Locally advanced, unresectable pancreatic cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34:2654–2668. doi:10.1200/JCO.2016.67.5561.
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi:10.1038/nature06175.
- Garg AD, Vara Perez M, Schaaf M, Agostinis P, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: dendritic cell-based anticancer immunotherapy. Oncoimmunology. 2017;6:e1328341. doi:10.1080/2162402X.2017.1328341.
- Kajihara M, Takakura K, Kanai T, Ito Z, Matsumoto Y, Shimodaira S, Okamoto M, Ohkusa T, Koido S. Advances in inducing adaptive immunity using cell-based cancer vaccines: clinical applications in pancreatic cancer. World J Gastroenterol. 2016;22:4446–4458. doi:10.3748/wjg.v22.i18.4446.
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi:10.1158/1078-0432.CCR-09-0737.
- Koido S, Okamoto M, Kobayashi M, Shimodaira S, Sugiyama H. Significance of Wilms’ tumor 1 antigen as a cancer vaccine for pancreatic cancer. Discov Med. 2017;24:41–49.
- Takahara A, Koido S, Ito M, Nagasaki E, Sagawa Y, Iwamoto T, Komita H, Ochi T, Fujiwara H, Yasukawa M, et al. Gemcitabine enhances Wilms’ tumor gene WT1 expression and sensitizes human pancreatic cancer cells with WT1-specific T-cell-mediated antitumor immune response. Cancer Immunol Immunother. 2011;60:1289–1297. doi:10.1007/s00262-011-1033-3.
- Kanai T, Ito Z, Oji Y, Suka M, Nishida S, Takakura K, Kajihara M, Saruta M, Fujioka S, Misawa T, et al. Prognostic significance of Wilms’ tumor 1 expression in patients with pancreatic ductal adenocarcinoma. Oncol Lett. 2018;16:2682–2692. doi:10.3892/ol.2018.8961.
- Wagner KD, Cherfils-Vicini J, Hosen N, Hohenstein P, Gilson E, Hastie ND, Michiels JF, Wagner N. The Wilms’ tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat Commun. 2014;5:5852. doi:10.1038/ncomms5972.
- Koido S, Okamoto M, Shimodaira S, Sugiyama H. Wilms’ tumor 1 (WT1)-targeted cancer vaccines to extend survival for patients with pancreatic cancer. Immunotherapy. 2016;8:1309–1320. doi:10.2217/imt-2016-0031.
- Kalish RS, Askenase PW. Molecular mechanisms of CD8+ T cell-mediated delayed hypersensitivity: implications for allergies, asthma, and autoimmunity. J Allergy Clin Immunol. 1999;103:192–199.
- Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor gene 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clinical Cancer Res. 2014. doi:10.1158/1078-0432.CCR-14-0314.
- Koido S, Homma S, Okamoto M, Takakura K, Gong J, Sugiyama H, Ohkusa T, Tajiri H. Chemoimmunotherapy targeting Wilms’ tumor 1 (WT1)-specific cytotoxic T lymphocyte and helper T cell responses for patients with pancreatic cancer. Oncoimmunology. 2014;3:e958950. doi:10.4161/21624011.2014.958950.
- Uttenthal B, Martinez-Davila I, Ivey A, Craddock C, Chen F, Virchis A, Kottaridis P, Grimwade D, Khwaja A, Stauss H, et al. Wilms’ Tumour 1 (WT1) peptide vaccination in patients with acute myeloid leukaemia induces short-lived WT1-specific immune responses. Br J Haematol. 2014;164:366–375. doi:10.1111/bjh.12637.
- Tanaka Y, Koido S, Ohana M, Liu C, Gong J. Induction of impaired antitumor immunity by fusion of MHC class II-deficient dendritic cells with tumor cells. J Immunol (Baltimore, Md: 1950). 2005;174:1274–1280.
- Zhang P, Zou M, Wen X, Gu F, Li J, Liu G, Dong J, Deng X, Gao J, Li X, et al. Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer Journal International Du Cancer. 2014;134:2646–2655. doi:10.1002/ijc.28584.
- Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol. 2015;21:2807–2815. doi:10.3748/wjg.v21.i9.2807.
- Tang H, Li B, Zhang A, Lu W, Xiang C, Dong J. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Colorectal Liver Metastasis: A Systematic Review and Meta-Analysis. PloS one. 2016;11:e0159447. doi:10.1371/journal.pone.0159447.
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi:10.1016/j.ccr.2009.06.017.
- Szkandera J, Gerger A, Liegl-Atzwanger B, Absenger G, Stotz M, Samonigg H, Maurer-Ertl W, Stojakovic T, Ploner F, Leithner A, et al. Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. Br J Cancer. 2013;109:2316–2322. doi:10.1038/bjc.2013.595.
- Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, et al. Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. J Gastrointest Surg. 2013;17:1609–1617. doi:10.1007/s11605-013-2286-2.
- Lee SH, Chung MJ, Kim B, Lee HS, Lee HJ, Heo JY, Kim YJ, Park JY, Bang S, Park SW, et al. The significance of the prognostic nutritional index for all stages of pancreatic cancer. Nutr Cancer. 2017;69:512–519. doi:10.1080/01635581.2016.1250921.
- Proctor MJ, Talwar D, Balmar SM, O’Reilly DS, Foulis AK, Horgan PG, Morrison DS, McMillan DC. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the glasgow inflammation outcome study. Br J Cancer. 2010;103:870–876. doi:10.1038/sj.bjc.6605855.
