ABSTRACT
This study aims to describe and characterize incident HR-HPV infections and associated diseases in HIV-infected women. 805 HIV-infected women enrolled in the VALHIDATE Study were screened and followed-up for HPV by co-testing. Social, behavioral and health data were collected. HPV-DNA positive samples were typed using a commercial kit or RFLP analysis. Conventional Pap-smears were evaluated using the 2001 Bethesda System. The participants with abnormal cytological results were referred for colposcopy. 565 HIV-infected women (median age: 43 years) were analysed, 40.9% had >5 lifetime sexual partners, 77.2% contracted HIV through sexual intercourse, 93% were receiving antiretroviral treatment and 77.3% had undetectable HIV-RNA. The women underwent 1254 follow-ups (median follow-up: 33 months) for 1430.6 PersonYear-Follow-Up. 37.4% of baseline HPV-negative women acquired incident HPV-infections, 69.6% of which were HR-HPVs. HPV-53 was the most common HPV type detected (9.3%). 18.2% of women showed incident or progressive cytological abnormalities (7.8% ASC-US, 9.7% LSIL and 0.6% HSIL) and colposcopy revealed CIN2 (N = 2), CIN1 (N = 2) and VIN3 (N = 1). The preventable fraction of incident infections was 11.3%, 16.7%, and 35.2% for the 2v-4v-9v-HPV vaccines respectively (χ2 p < 0.0001). The overall burden of incident lesions attributable to the vaccine types were 9.1% for 2v-, 14.5% for 4v- and 30.9% for 9v-vaccine. High HPV incidence rates and high percentages of multiple HR-HPV infections were observed in a cohort of HIV-infected women receiving effective antiretroviral treatment. Primary prevention strategies based on the new 9v-HPV vaccine may help to prevent incident infections and disease progression in this cohort of women.
Introduction
The lifetime probability of contracting High-Risk Human Papillomavirus (HR-HPV) infections is approximately 70–75% in sexually active womenCitation1 and the risk of infection and progression toward invasive disease is influenced by viral and host factors. The disease is currently controlled through cytological and/or molecular screening and primary vaccine prevention. Three types of HPV vaccines are available: the 2-valent vaccine (Cervarix®, GlaxoSmithKline biologicals), which protects against HR-HPV types 16 and 18; 4-valent vaccine (Gardasil®, Merck, Sanofi Pasteur MSD), which protects against HPV types 6, 11, 16 and 18; and 9-valent vaccine (Gardasil®9, Merck, Sanofi Pasteur MSD), which protects against HPV types 6, 11, 16, 18, 31, 33, 45, 52, 58. Administrating HPV vaccine to females who have not yet been exposed to the targeted types of HPV is the most appropriate and cost-effective strategy for protecting them against HPV infections and related sequelae.Citation2
In the general population, HR-HPV infection is considerably higher in young people and decreases with age,Citation3 yet no decline is evident in women living with HIV/AIDS.Citation4 For these women, the overall risk of HR-HPV infection, the risk of infection with non 16/18 HR-HPV types and of multiple infections and disease progression are significantly higher than for immunocompetent age-matched women.Citation5-Citation7 However, even if the rates of HPV infection are higher for women living with HIV/AIDS, it has been reported that immunity induced by natural infection with HR-HPV is not long-lasting and does not protect against future re-infection with the same type.Citation8
In 2010 we carried out a prospective cohort study (the VALHIDATE Study)Citation9 based on HPV and cytology co-testing aimed at characterizing the molecular epidemiology of HPV infections and associated diseases by comparing women at high-risk of infection with a control group of women belonging to the general population.
The aim of this study is to describe and characterize incident HR-HPV infections in women living with HIV/AIDS enrolled in the VALHIDATE Study and followed up for 5 years and to verify the potential impact of HPV vaccines in these women even if pre-exposed to several HPV infections.
Results
Baseline characteristics of the women enrolled
The median age of the HIV-infected women was 43 years (IQR: 37-47y), most of whom were Italian (82.1%) with a medium/high educational level (51.7%) and 45.3% were smokers. 231 women (40.9%) declared to have had >5 lifetime sexual partners and 71% declared one sexual partner in the previous six months. 53% declared to have contracted at least 1 sexually transmitted infection in their lives. The women mainly contracted HIV infection through sexual intercourse (436/565, 77.2%) and had a median CD4+ count of 569.5 cells/µL. 93% were taking antiretroviral treatment and 77.3% had undetectable HIV-RNA (< 37 copies/mL) ().
Table 1. Social, epidemiological and clinical characteristics of women at baseline evaluation.
Baseline evaluation
At baseline evaluation, 485 (85.8%) women were NILM, while ASC-US was diagnosed in 28 (5.0%), LSIL in 43 (7.6%), AGC in 3 (0.5%), ASC-H in 2 (0.3%) and HSIL in 4 (0.7%). 44 histological analyses identified 2 cases of CIN3, 3 cases of CIN2, 12 cases of CIN1 and 1 case of Vaginal Intraepithelial Neoplasia-1 (VAIN-1) ().
Table 2. Cervical abnormalities at baseline evaluation and prevalence rate of HPV infections among HIV-infected women.
Overall, at baseline 153 (27.1%; 95% CI: 23.5–31.0) women were HPV-DNA positive and 127 (22.5%; 95% CI: 19.1–26.2) were HR-HPV infected.
HPV infection and HPV typing at baseline and during the follow-up period
285 HPV infections were identified in 153 HPV infected women at baseline (mean 1.9 HPVs per woman, range 1–8). Single infections were detected in 74 HPV-infected women (48.4%) while multi-type infections (2 to 8 HPVs) in the other 79 HPV-infected women, 146 (51.2%) of which were sustained by HPVs included in group-1, 63 (22.1%) in groups-2A/2B and 76 (26.7%) in group-3, according to the IARC classificationCitation10 ().
Figure 1. HPV types detected at baseline (a), during follow-up (b) and in incident infections (c) grouped according to the IARC classification. The genotypes included in 2v-, 4v-, or 9-v-HPV vaccines are coloured blue and green.
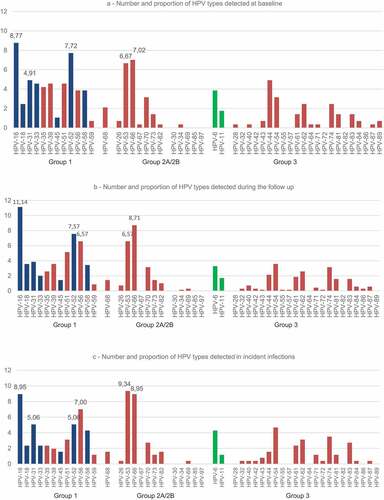
The overall HR-HPV infection rate was 73.3% (209/285). The most common HPV was HPV-16 (8.8%), followed by HPV-52 (7.7%), HPV-66 (7.0%), HPV-53 (6.7%) (). At baseline the percentage of women infected with HPV-16 or HPV-18 alone or combined with other HPV types amounted to 20.3%, while the rate of women with other HR-HPV types was 58.2%.
The research participants underwent 1254 follow-up visits (mean 2.2 follow-ups per woman); median follow-up was 33 months (IQR: 20–40 months) for a total of 1430.6 PY-FU. Overall, 702 HPV infections were detected among the 243 HPV positive women at the follow-up visits (mean 2.9 HPV infections per woman). HPV-16 was the most commonly found (11.1%), followed by HPV-66 (8.7%), HPV-52 (7.6%), HPV-56 (6.6%), HPV-53 (6.6%) (). The overall HR-HPV detection rate remained stable (74.2%).
Incident HPV infections and genital lesions
During follow-up, 154 (37.4%) of the 412 HPV women who tested negative at baseline, developed an incident HPV infection (incidence rate 0.15 cases PY-FU). The distribution of HPV types detected in these new infections are reported in . HPV-53 was the most commonly found type (24 cases, 9.3%), followed by HPV-16 and HPV-66 (23 cases each, 8.9%), HPV-56 (19 cases, 7.0%), HPV-52 and HPV-31 (13 cases each, 5.0%). The overall incident HR-HPV infection rate was 69.6%. HPV-16 or HPV-18 alone or combined with other HPV types were found in 28 women (17.9%), while 90 women (57.7%) had HR-HPV infections other than HPV-16 or 18.
The HPV type distribution between baseline evaluation and that observed in incident infections showed the same figures for the most commonly found HPV types and for the proportion of HR-HPV infections.
Among the women with incident HPV infections, 28 (18.2%) presented an incident or progressive cytological abnormality. Cytology progressed from NILM to ASC-US in 12 (7.8%) cases, while LSIL was found in 15 (9.7%) cases and HSIL in 1 (0.6%) case. The colposcopy-guided cervical biopsies, carried out on 15 evaluable patients detected 2 cases of CIN2, 2 cases of CIN1 and 1 case of Vulvar Intraepithelial Neoplasia-3 (VIN3). The overall incidence rate of any grade of abnormality was 0.03 cases/PY-FU and the incidence rate of HG-SILs was 0.004 cases/PY-FU.
Vaccine-preventable incident HPV infections
The preventable fraction of all incident infections was 11.3%, 16.7%, and 35.2% for the 2v-4v-9v-vaccines respectively (χCitation2 p < 0.0001).
154 women who tested HPV negative at baseline acquired an HPV infection during the follow-up, thus being incident cases. Of these, 24 (15.6%) cases could have been prevented by administering the 2v-vaccine, 30 (19.5%) by administering the 4v-vaccine and 63 (40.9%) with the 9v-vaccine (χ2 p < 0.0001).
HPV typing carried out on the 28 patients with incident cervical/vulvar abnormalities showed 60 HPV infections (mean 2.1 infections per woman) whose distribution can be seen in ().
Figure 2. Percentage of HPV types detected in 28 incident SILs (blue bars) and in 4 HSILs (red bars).
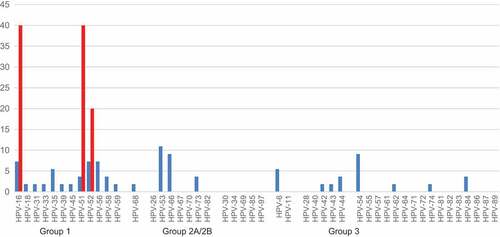
HR-HPVs were responsible for 70.9% of infections in LG-SILs and 100% of infections in HG-SILs. The overall burden of lesions attributable to the vaccine types was 9.1% for the 2v-vaccine, 14.5% for 4v-vaccine and 30.9% for 9v-vaccine. The 9v-vaccine would have prevented 60% of the infections detected in the incident HG-SILs.
Discussion
The baseline HR-HPV infection prevalence (22.5%; 95%CI 19.1–26.2) observed was in line with that reported by Konopnicki et al.Citation11 regarding a cohort of Belgian HIV-infected women (23%; 95%CI 19.3–26.8). In both studies, HIV infection was well controlled by antiretroviral treatments with high baseline CD4 cell counts and with undetectable HIV viremia.
Although well-controlled by antiretroviral treatment, the HR-HPV infection prevalence was significantly higher than that reported for the general population with normal cervical cytology. In a meta-analysis, Bruni et al. reported a global adjusted HPV prevalence of 11.7% (95%CI 11.6–11.7) and an adjusted prevalence of 9.1% (95%CI 9.0–9.2) in women aged 35–44 years.Citation12 It is well known that HIV-induced immune deficiency is associated with increased incidence, long-term persistence of HPV infection and increased risk of progression to precancers/cancers.Citation13-Citation17 However, this study showed that even women receiving effective antiretroviral therapy with high CD4 cells and a well-controlled HIV viremia are at increased risk of infection, thus suggesting that the risk of infection may be due to factors other than immune-depression.
Indeed, although the risk of HR-HPV infections in the general population decreases with age, a significant proportion of incident infections was observed in our cohort of mid-adult women with an infection rate of up to 0.15 cases/year follow-up. These findings may be due to the reported strong association between new sexual partners and HPV incidence in older womenCitation18,Citation19 and by the possible reactivation of latent infections reported in cases of impaired immunity.Citation20 Therefore, when primary vaccine prevention is unadvisable due to the high baseline prevalence of infection, the high incidence rate of new HPV infections associated with the worst outcomes even in presence of immune reconstitution suggests that a vaccine prevention strategy might help to prevent new infections in HIV infected women.
As already reported,Citation15,Citation21 HIV infected women have a different HPV type distribution to that of the general population with a high prevalence of infections and HSILs caused by HR-HPV types other than HPV-16/HPV-18. The large number of multiple-type HPV infections observed prevented us from making a type-specific attribution of the cervical lesions in HIV infected women due to the small number of events observed.
However, it is well known that the HPV-16 and HPV-18 prevalence rate increases with the severity of cervical abnormalities and that these two types account for approximately 70% of all cervical cancers in the general population worldwide.Citation7 Although Clifford et al.Citation7 found that HPV-16 is the most carcinogenic type in HIV infected women, the association between HPV-16/18 and cancer is less evident than that reported for the general population. There is evidence that HPV-16 is less affected by HIV immune-depression due to its innate ability to elude immune control while other oncogenic HPVs are more affected by impaired immunity. However, the decrease in the percentages of pre-cancers and cancers associated with HPV-16 is limited: HPV-16 is however the most carcinogenic HPV type worldwide even in HIV infected women with an associated increased burden of non-HPV-16 types resulting in a shift of the HSIL towards other oncogenic HPV types.Citation21
These observations may affect the screening and prevention strategies used for HIV infected women. Keller et al.Citation22 suggested that HIV-infected women may benefit from cervical cancer co-testing using Pap and HR-HPV assays since HIV infected women with negative cytology who test HPV-16 positive have a similar pre-cancer risk to those with LSIL cytology and therefore warrant immediate colposcopy; repeat screening in 1 year is recommended for other HR-HPV infections.
HPV vaccines have proved to be highly effective for preventing cancer in the general population. Identifying the appropriate vaccination strategy in middle-aged HIV-infected individuals already exposed to multiple HPV infections seems to be crucial. The high rates of newly-acquired HR-HPV infections, different HPV type distributions and progressive genital lesions in HIV infected women underline how a 2v- or 4v-vaccine directed against the two main carcinogenic HPV types could result in shortfalls for these women. In fact, the incident infections caused by HPV-16 and/or HPV-18 accounted for 11.3% of all the new HPV infections acquired during the follow-up period. By including the infections sustained by LR HPV-6/-11, the preventable fraction of infections with 4v-vaccine rose to 16.7% thus covering a limited fraction of the HPV types affecting HIV-infected women. More recently, the 9v-vaccine has included coverage against five additional HR-HPVs.Citation23 In this cohort, the preventable fraction of incident infections with a 9v-vaccine rose to 35.2%. These observations suggest that the cost/effectiveness of 9v-vaccine, which has already been evaluated for the general populationCitation24,Citation25 should be even more effective for HIV-infected women. Although there is positive anticipation, the limitation of this study is that the effectiveness of the 9v-vaccine is only hypothetic, since it is based on the distribution of HPV types in incident infections and lesions in this middle-aged and partially-immunosuppressed population. Further research is required to obtain evidence of vaccine effectiveness in order to identify the best primary prevention strategy for HPV-related cancers in adults living with HIV/AIDS.
Material and methods
Study design
Among the 805 HIV-infected women enrolled in the VALHIDATE Study, 565 (70.2%) were considered eligible for the study as they had attended at least one follow-up visit. The women were followed up for 5 years and were evaluated for incident HR-HPV infections and HPV type characterization. Social, behavioural and health data, as well as immune-virological and antiretroviral treatment data observed at baseline evaluation and follow-up visits were collected from the eCRF (electronic Case Report Form) of the study protocol. Written informed consent was required from all of the eligible female participants enrolled in the VALHIDATE Study. The study was approved by the L. Sacco Hospital ethical committees (R.n°174/2010, 9 March 2010).
Pap cytology and HPV tests
As already reported in the Study protocol,Citation9 the Pap-smear samples were obtained using a cervical brush (Cytobrush Plus MedscandW Medical AB, Sweden). The pap-smears were evaluated by cytopathologists from the participating centres in accordance with the 2001 Bethesda System terminology.Citation26 According to cytology at baseline, the cases were classified as NILM, Negative for Intraepithelial Lesion or Malignancy; ASC-US, Abnormal Squamous Cells of Undetermined Significance; ASC-H, Abnormal Squamous Cells cannot exclude High-grade squamous intraepithelial lesion; AGC, Atypical Glandular Cells; LSIL, Low-grade Squamous Intraepithelial Lesion; HSIL, High-grade Squamous Intraepithelial Lesion. Women with abnormal cytological results underwent colposcopy and biopsy if required and were followed up in accordance with Italian guidelines.Citation27 Abnormal biopsy results were classified as Cervical Intraepithelial Neoplasia (CIN) grade 1–3, adenocarcinoma in situ or cancer.
Cytological ASC-H and HSIL and histological lesions grade 2 or more were referred as HG-SILs (High Grade Squamous Intraepithelial Lesions). Cytological results of ASC-US, LSIL, AGC and histological results of CIN1 were referred as LG-SILs (Low Grade Squamous Intraepithelial Lesions).
The cervical brushes used for cytology were then immersed in a PreservCyt® solution (ThinPrep® Pap Test, Hologic Italia Srl) and tested for HPV-DNA with an in-house PCR.Citation28 The HPV-DNA positive samples underwent HPV typing carried out with the reverse hybridization Inno-LiPA® HPV Genotyping Extra (Fujirebio, Belgium) test, which is able to identify 28 HPVs (HPV: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68, 69, 70, 71, 73, 74, 82).
All HPV-DNA positive cervical samples found to be non-typeable by the Inno-LiPA® test were subjected to the Restriction Fragment Length Polymorphism (RFLP) analysis which identifies all types of the High-Risk-clade (HR-clade) and Low-Risk (LR) types of the alpha genus according to the 2011 IARC classification.Citation10 The oncogenic potential of HPV types depends on their association with disease malignancy. The HR-clade includes HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, classified in group-1 as “human carcinogens” and groups-2A (HPV 68) and −2B (HPV types 26, 30 34, 53, 66, 67, 69, 70, 73, 82, 83, 97) which include types that are “probably carcinogenic to humans” and “possibly carcinogenic to humans” respectively. The LR types in group-3 are not classifiable as human carcinogens.
Follow-up
Regardless of the Pap result, a 6-month screening interval was established for HIV-positive women with a CD4 cell counts < 200 cells/µL and a yearly screening interval for those with CD4 cell counts >200 cell/µL.
Statistical analysis and end-points
The end-points of the analysis were the incident HPV infections from HR-HPV types in women with baseline HPV negative results as well as incident abnormal cytological results.
Descriptive statistics [mean±Standard Deviation (SD) or median and InterQuartile Range (IQR)] were used to describe demographic, behavioural, epidemiological and clinical/immunological characteristics of the study population.
We evaluated the type distribution of incident infections, the role of specific risk factors for incident infections and cytological abnormalities; the incidence rate was expressed as Person-Years-of-Follow-Up (PY-FU). PY rates were calculated using all participants who were not positive for that specific type at baseline as denominator.
Univariate logistic regression analyses were performed to calculate the Odds Ratios (OR) and 95% Confidence Interval (95%CI) of the association between the selected variables and outcomes. The dependent variables were the incident HPV infections and cytological abnormalities. All of the reported P-values are two-sided; the data were analysed using GraphPad Prism v5.00 (GraphPad Software, San Diego California USA, www.graphpad.com).
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, Juliar BE, Breen T, Fortenberry JD. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191(2):182–192. doi:10.1086/426867.
- Harper DM, DeMars LR. HPV vaccines – A review of the first decade. Gynecol Oncol. 2017;146(1):196–204. doi:10.1016/j.ygyno.2017.04.004.
- Lewis RM, Markowitz LE, Gargano JV, Steinau M, Unger ER. Prevalence of genital human papillomavirus among sexually experienced males and females aged 14-59 years, United States, 2013-2014. J Infect Dis. 2018;204(4):566–573. doi:10.1093/infdis/jix655.
- Tartaglia E, Falasca K, Vecchiet J, Sabusco GP, Picciano G, Di Marco R, Ucciferri C. Prevalence of HPV infection among HIV-positive and HIV-negative women in Central/Eastern Italy: strategies of prevention. Oncol Lett. 2017;14(6):7629–7635. doi:10.3892/ol.2017.7140.
- Ellerbrock TV, Chiasson MA, Bush TJ, Sun XW, Sawo D, Brudney K, Wright TC jr. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA. 2000;283(8):1031–1037. doi:10.1001/jama.283.8.1031.
- Gilles C, Manigart Y, Konopnicki D, Barlow P, Rozenberg S. Management and outcome of cervical intraepithelial neoplasia lesions: a study of matched cases according to HIV status. Gynecol Oncol. 2005;96(1):112–118. doi:10.1016/j.ygyno.2004.10.003.
- Clifford GM, Tully S, Franceschi S. Carcinogenicity of Human Papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV Infection to Cervical Cancer. Clin Infect Dis. 2017;64(9):1228–1235. doi:10.1093/cid/cix135.
- Viscidi RP, Schiffman M, Hildesheim A, Herrero R, Castle PE, Bratti MC, Rodriguez AC, Sherman ME, Wang S, Clayman B, et al. Seroreactivity to Human Papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in costa rica. Cancer Epidemiol Biomarkers Prev. 2004;13(2): 324–327.doi:10.1158/1055-9965.
- Orlando G, Tanzi E, Chatenoud L, Gramegna M, Rizzardini G; VALHIDATE Study Group. Rationale and design of a multicenter prospective cohort study for the eVALuation and monitoring of HPV infections and relATEd cervical diseases in high-risk women (VALHIDATE study). BMC Cancer. 2012;12:204. doi:10.1186/1471-2407-12-204.
- IARC. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2011;100B:261–319.
- Konopnicki D, Manigart Y, Gilles C, Barlow P, De Marchin J, Feoli F, Delforge M, Clumeck N, De Wit S. High-risk human papillomavirus genotypes distribution in a cohort of HIV-positive women living in Europe: epidemiological implication for vaccination against human papillomavirus. Aids. 2016;30(3):425–433. doi:10.1097/QAD.0000000000000929.
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–1799. doi:10.1086/657321.
- Rowhani-Rahbar A, Hawes SE, Sow PS, Toure P, Feng Q, Dem A, Dembele B, Critchlow CW, N’Doye I, Kiviat NB. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis. 2007;196(6):887–894. doi:10.1086/520883.
- Massad LS, Xie X, D’Souza G, Darragh TM, Minkoff H, Wright R, Colie C, Sanchez-Keeland L, Strickler HD. Incidence of cervical precancers among HIV-seropositive women. Am J Obstet Gynecol. 2015;212(5):606.e1–8. doi:10.1016/j.ajog.2014.12.003.
- Clifford GM, Franceschi S, Keiser O, Schoni-Affolter F, Lise M, Dehler S, Levi F, Mousavi M, Bouchardy C, Wolfensberger A, et al. Immunodeficiency and the risk of cervical intraepithelial neoplasia 2/3 and cervical cancer: a nested case-control study in the Swiss HIV cohort study. Int J Cancer. 2016;138(7): 1732–1740.doi:10.1002/ijc.29913.
- Abraham AG, Strickler HD, D’Souza G. Invasive cervical cancer risk among HIVinfected women is a function of CD4 count and screening. J Acquir Immune Defic Syndr. 2013;63(5):e163. doi:10.1097/QAI.0b013e31829cb7c3.
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370(9581):59–67. doi:10.1016/S0140-6736(07)61050-2.
- Munoz N, Mendez F, Posso H, Molano M, van Den Brule AJ, Ronderos M, Meijer C, Munoz A; Instituto Nacional de Cancerologia HPV Study Group. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190(12):2077–2087. doi:10.1086/425907.
- Trottier H, Ferreira S, Thomann P, Costa MC, Sobrinho JS, Prado JC, Rohan TE, Villa LL, Franco EL. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res. 2010;70(21):8569–8577. doi:10.1158/0008-5472.
- Maglennon GA, McIntosh PB, Doorbar J. Immunosuppression facilitates the reactivation of latent papillomavirus infections. J Virol. 2014;88(1):710–716. doi:10.1128/JVI.02589-13.
- Massad LS, Xie X, Burk RD, D’Souza G, Darragh TM, Minkoff H, Colie C, Burian P, Palefsky J, Atrio J, et al. Association of cervical precancer with human papillomavirus types other than 16 among HIV co-infected women. Am J Obstet Gynecol. 2016;214:354. e1-6. doi:10.1016/j.ajog.2015.09.086
- Keller MJ, Burk RD, Massad LS, Eltoum IE, Hessol NA, Castle PE, Anastos K, Xie X, Minkoff K, Xue X, et al. Cervical precancer risk in HIV-infected women who test positive for oncogenic human papillomavirus despite a normal Pap test. Clin Infect Dis. 2015;61(10): 1573–1581.doi:10.1093/cid/civ569.
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8): 711–723.doi:10.1056/NEJMoa1405044.
- Chesson HW, Markowitz LE, Hariri S, Ekwueme DU, Saraiya M. The impact and cost-effectiveness of nonavalent HPV vaccination in the United States: estimates from a simplified transmission model. Hum Vaccin Immunother. 2016;12(6):1363–1372. doi:10.1080/21645515.2016.1140288.
- Durham DP, Ndeffo-Mbah ML, Skrip LA, Jones FK, Bauch CT, Galvani AP. National- and state-level impact and cost-effectiveness of nonavalent HPV vaccination in the United States. Proc Natl Acad Sci U S A. 2016;113(18):5107–5112. doi:10.1073/pnas.1515528113.
- Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T jr, et al. The 2001 bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002;287(16): 2114–2119.doi:10.1001/jama.287.16.2114.
- Gestione della paziente con Pap test anormale. Linee guida Edizione 2006. www.colposcopiaitaliana.it/pdf07/linee-guida-2006.pdf
- Tanzi E, Bianchi S, Fasolo MM, Frati ER, Mazza F, Martinelli M, Colzani D, Beretta R, Zappa A, Orlando G. High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J Med Virol. 2013;85(1):91–98. doi:10.1002/jmv.23434.
