ABSTRACT
Hepatitis B virus (HBV) infection is one the most common in the world. Aim of this study is to perform a systematic review on the relationship between HBV vaccination and multiple sclerosis. Research was conducted on Pubmed, ISI Web of Science, and Scopus. Terms “hepatitis b vaccination” and “multiple sclerosis” were used. Meta-analysis and metaregression were performed. 414 papers were found. Seven articles were selected. For the reported crude risk estimates for MS no statistically significant association was observed with pooled OR 1,19 (95%CI: 0,96–1,49). For the adjusted ORs, the pooled odds ratio (OR) was 0, 965 (95%CI: 0,886– 1,051). Meta regression show that year of publication is negatively (β: −0,019; P < 0.001) and NOS score and publishing in Europe are positively associated with O.R. value. Funnel plot showed the presence of publication bias. Results showed that Hepatitis B vaccination is not associated with an increased risk of developing MS.
Introduction
Hepatitis B virus (HBV) infection is one the most common in the world that results in substantial morbidity and mortality, mostly later the develop of chronic infection.Citation1 HBV chronic infection can lead to serious liver damage. The transmission is through exposure to infected blood and body fluids.Citation2 Vaccine against Hepatitis B was developed in 1976 and available since 1982.Citation3 This represent one of the major event in preventive medicine and public health and immunization against hepatitis B is now a priority in all countries. hepatitis B vaccination was added into national immunization programs worldwide in 199.Citation4 In early 90’s in French population developed the rumors about a link by hepatitis B immunization and cases of Multiple Sclerosis or other demyelinating diseases. Cases of multiple sclerosis that developed in few weeks after the administration of the vaccine were reported in Paris.Citation5,Citation6 Based on the results of 2 case-control studies indicating a nonsignificant increase in the risk of multiple sclerosis among individuals who had undergone vaccination,Citation7,Citation8 the French government suspended the school-based program of hepatitis B vaccination. The etiology of MS is not fully understood, and the pathophysiology is said to be immune-mediated.
In literature some systematic review,Citation9-11 two with metanalysisCitation12,Citation13 are present but some of low methodological quality. Some of this take into account a small number of study.Citation13 In recent systematic review and metanalysisCitation12 authors include in the metanalysis for adjusted OR two studies that take into account the same populationCitation14,Citation15 or study in wich not all data are available.Citation8
Aim
So, the aim of the present study is to perform a systematic review on the existing evidence about the relationship between HBV vaccination and multiple sclerosis.
Results
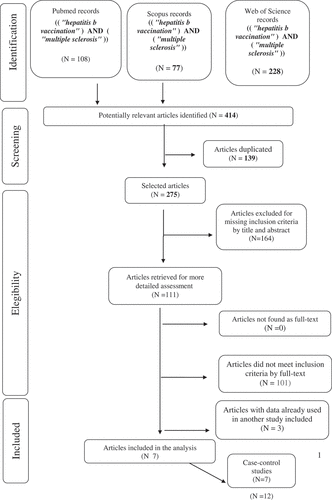
The selection of articles is shown in the flowchart (). Overall 414 papers were found 108 articles through Pubmed, 77 through Scopus and 228 through Web of Science. Successively, 139 duplicates and 164 articles that did not respect the inclusion criteria by title and abstract were excluded. The remaining 110 papers were analyzed, and from these, 104 articles were excluded because were not pertinent, or for inappropriate study design or because there wasn’t outcome of interest. Furthermore, three studiesCitation14–16 were excluded because use data already used in another study included. Seven articles fulfill the inclusion criteria and selected for the meta-analysis.Citation17-23 All work included intended to evaluate the link between HB vaccination and the occurrence of MS. No work was added by references.
presents the main characteristics of the studies retained for the meta-analysis, which included a total of 15,389 cases and 11,856 controls. One was nested case–control studies,Citation17 six were case–control studies. Except for one study,Citation18 the quality of the studies evaluated by the Newcastle Ottawa Scale was good for all papers from six to eight points ().
Table 1. Characteristics of included studies
Table 2. Newcastle Ottawa Scale for included studies
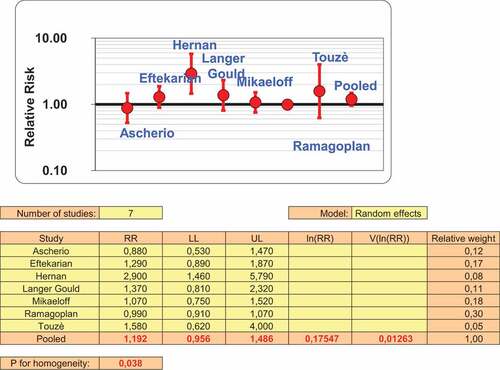
From the seven studies having reported crude risk estimates for MS, using a random effect model, since heterogeneity was found (p = 0.031), no statistically significant association was observed (), the pooled odds ratio (OR) being 1,19 (95%CI: 0,96–1,49).
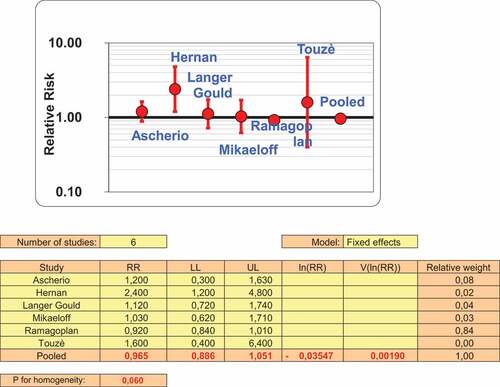
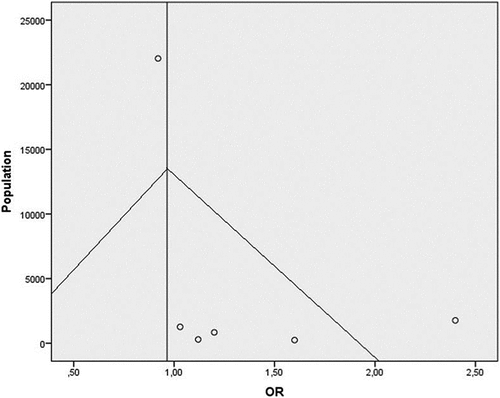
Considering the adjusted ORs, we used the fixed effect model, since no heterogeneity was found (p = 0.060), and the pooled odds ratio (OR) was 0, 965 (95%CI: 0,886– 1,051) (). Because no adjusted O.R. was available it was excluded in this case work by EftekarianCitation18 wich was also of low quality (). A meta regression was performed () and results show that year of publication is negatively associated with O.R. (beta: −0,019; P < 0.001), Newcastle Ottawa Score and Publishing in Europe are positively associated with O.R. value. Publication bias was assessed from funnel plots ()
Table 3. Meta regression analysis
Discussion
In this work was found that for the seven studies included the pooled risk estimates not found statistically significant association between anti-hepatitis B vaccination and the occurrence of multiple sclerosis. The case-control study of AscherioCitation17 showed relative risk of 0.9 (95 percent confidence interval, 0.5 to 1.6) at any time before the onset of the disease. The relative risk associated with hepatitis B vaccination within two years before the onset of the disease was 0.7 (95 percent confidence interval, 0.3 to 1.8). The authors concluded with no association between hepatitis B vaccination and the development of multiple sclerosis.
Langer Gould et al in 2014Citation20 did not found an association of new-onset MS or in the 1 to 3 years after vaccination either adjusted or unadjusted models. The base population study of MikaeloffCitation21 conducted in French general population of children and adolescents younger than 16 years between January 1994 and December 2003 found no increase in the risk of a first episode of MS in childhood after vaccination against HB .Eftekharian et al (2018) reported that hepatitis B vaccination did not increase the risk of MS significantly (p > 0.05), although found trend toward more vaccination rate in the patients. RamagoplanCitation17 found no significant differences between cases and controls for the frequency of hepatitis B (p = 0.69). The multicentre case-control study of TouzèCitation23 found that the risk of a first CNS demyelinating event was not significantly increased within the 2 months after HB vaccination but considered “not implausible” an association between HB vaccine and MS. The only study that found an association between MS and Hepatitis B vaccination was that of Hernan et al.Citation19 In this study after adjustment for smoking sex, age calendar time, clinical course of the disease and type of first symptoms (eye symptoms, sensory symptoms, other) The OR (95% CI) of MS for vaccination vs no vaccination was 2.4 (crude OR was 3.1. The review by MouchetCitation12 use as crude O.R a value equal to 2.9 instead of 3.1
The investigation by Sturkenboom et al.Citation8 included by Mouchet was excluded because of few details of this study are available, since it was published only as an abstract, but the results appear compatible with a lack of association. To our knowledge few systematic review on argument exist in literature.Citation9-12,Citation24 The systematic review with metanalysisCitation13 conducted in 2011 found not association between Ep. B vaccination and an increased risk of developing MS but this work was affected by a poor quality. An Umbrella review in 2015Citation25 wich aim was to summarize the environmental risk factors that have been studied in relation to onset of multiple confirms no association between the Hepatitis B vaccine and multiple sclerosis onset.
This meta-analysis has some strengths. It includes studies of good quality except for one. The scores based on the Newcastle Ottawa Scale raging from 6 point and over. It was limited the heterogeneity.
This review found results that failed to find conclusive evidence of the association between administration of HBV and MS.
Materials and methods
Identification of relevant studies
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.Citation26 The research was conducted on the electronic databases Pubmed, ISI Web of Science, and Scopus to February 2018. The combination of terms “hepatitis b vaccination” and “multiple sclerosis” was used to retrieve studies. No restriction about language or time period was applied. Furthermore, the references of review, letters, comments, editorials and case reports, identified by the search strategy, were checked for retrieving further relevant literature.
Study selection
The first selection was performed filtering duplicate articles by JabRef 4.10 program and Mendeley for Windows. The articles identified were selected initially by title and abstract, independently by two researchers, and then each investigator evaluated the inclusion criteria by full-text. Disagreements between the two reviewers were resolved by a third one. Articles that take into account relationship between Hepatitis B vaccination and multiple sclerosis were included in the systematic review. Primary study case-control and cohort studies were included.
Data extraction and quality assessment
Data extraction was carried out with the same strategy of the selection of the studies: two researchers collected the data, and the disagreement was resolved by a third researcher.
A quality assessment was performed according to the Newcastle-Ottawa Scale (NOS) for cohort and case control study. The following characteristics were collected: first author, study design (cohort, case-control), year of publication, country of the first author, quality score, age of patients, O.R., sample size, main results.
Statistical analysis
The meta-analysis was realized in the case of the data were homogenous and available. The pooled prevalence with relative 95% confidence interval (95% CI) was calculated. The level of significance was set p < 0.05. The effect size heterogeneity was considered significant when heterogeneity probability values p < 0.05.
Analyses were performed using “Episheet,” an Excel spreadsheet-based analytical package for meta-analysesCitation27 and IBM SPSS v.25 . For meta-analysis, was estimated the summary effect size and its 95% CI with both fixed-effects and random effects models. A multivariate analysis was performed with a metaregression. Funnel plot was constructed to evaluate publication bias. Statistical review of the study was performed by a biomedical statistician.
Conclusion
The present systematic review identified seven studies. Pooled estimates demonstrate a lack of association between multiple sclerosis and Hepatitis B vaccination. There is suggestive evidence that Hepatitis B immunization is not associated with an increased risk of developing MS.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2009;30(12):2212–2219. doi:10.1016/j.vaccine.2011.12.116.
- A S L, McMahon BJ. Chronic Hepatitis B. Hepatology. 2007;45(25):507–539. doi:10.1002/hep.21513.
- Zuckerman JN. Protective efficacy, immunotherapeutic potential, and safety of hepatitis B vaccines. J Med Virol. 2006;78(2):169–177. doi:10.1002/jmv.20524.
- Buynak EB, Roehm RR, Tytell AA, Bertland AU, Lampson GP, Hilleman MR. Development and chimpanzee testing of a vaccine against human hepatitis B. Proc Soc Exp Biol Med. 1976;151(4):694–700.
- Gout O, Lyon-Caen O. Multiple sclerosis and hepatitis B vaccination Sclerose en plaques et vaccination contre le virus de l’hepatite B. Rev Neurol. 1998;154(3):205–207.
- Tourbah A, Gout O, Liblau R, Lyon-Caen O, Bougniot C, Iba-Zizen MT, Cabanis EA. Encephalitis after hepatitis B vaccination: recurrent disseminated encephalitis or MS? Neurology. 1999;53(2):396–401. doi:10.1212/WNL.53.2.396.
- Fourrier A, Touzé E, Alpérovitch A, Bégaud B. Association between hepatitis B vaccine and multiple sclerosis. Pharmacoepidemiol Drug Safety. 1999;8(Suppl):S140–S141.
- Sturkenboom M, Wolfson C, Roullet E, Heinzlef O, Abenhaim L. Demyelination, multiple sclerosis, and hepatitis B vaccination: A population-based study in the UK. Neurology. 2000;54(7):A166.
- Rouge-Maillart CI, Guillaume N, Jousset N, Penneau M. Recognition by French courts of compensation for post-vaccination multiple sclerosis: the consequences with regard to expert practice. Med Sci Law. 2007;47(3):185–190. doi:10.1258/rsmmsl.47.3.185.
- Demicheli V, Rivetti A, Pietrantonj CD, Clements C, Jefferson T. Hepatitis B vaccination and multiple sclerosis: evidence from a systematic review. J Viral Hepat. 2003;10(5):343–344. doi:10.1046/j.1365-2893.2003.00423.x.
- Martínez-Sernández V, Figueiras A. Central nervous system demyelinating diseases and recombinant hepatitis B vaccination: a critical systematic review of scientific production. J Neurol. 2013;260(8):1951–1959. doi:10.1007/s00415-012-6683-3.
- Mouchet J, Salvo F, Raschi E, Poluzzi E, Antonazzo IC, De Ponti F, Bégaud B. Hepatitis B vaccination and the putative risk of central demyelinating diseases–A systematic review and meta-analysis. Vaccine. 2018;36(12):1548–1555. doi:10.1016/j.vaccine.2018.02.036.
- Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J Neurol. 2011;258(7):1197–1206. doi:10.1007/s00415-011-5984-2.
- Hocine MN, Farrington CP, Touzé E, Whitaker HJ, Fourrier A, Moreau T. Tubert-Bitter P Hepatitis B vaccination and first central nervous system demyelinating events: reanalysis of a case–control study using the self-controlled case series method. Vaccine. 2007;25(31):5938–5943. doi:10.1016/j.vaccine.2007.05.032.
- Mikaeloff Y, Caridade G, Suissa S, Tardieu M. Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood. Neurology. 2009;72(10):873–880. doi:10.1212/WNL.0b013e3181a9fad1.
- Touze E, Gout O, Verdier-Taillefer MH, Lyon-Caen O, Alperovitch A. First central nervous system demyelination and hepatitis B vaccination: a pilot case control study. Rev Neurol. 2000;156(3):242–246.
- Ascherio A, Zhang SM, Hernan MA, Olek MJ, Coplan PM, Brodovicz K, Walker AM. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med. 2001;344(5):327–332. doi:10.1056/NEJM200102013440502.
- Eftekharian MM, Mousavi M, Hormoz MB, Roshanaei G, Mazdeh M. Multiple sclerosis and immunological-related risk factors: results from a case-control study. Human Antibodies. 2015;23(1,2):31–36.
- Hernán MA, S S J, Olek MJ, Jick H. Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study. Neurology. 2004;63(5):838–842.
- Langer-Gould A, Qian L, Tartof SY, Brara SM, Jacobsen SJ, Beaber BE, Sy LS, Chao C, Hechter R, Tseng HF. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA neurol. 2014;71(12):1506–1513. doi:10.1001/jamaneurol.2014.2633.
- Mikaeloff Y, Caridade G, Rossier M, Suissa S, Tardieu M. Hepatitis B vaccination and the risk of childhood-onset multiple sclerosis. Arch Pediatr Adolesc Med. 161(12):1176–1182. doi:10.1001/archpedi.161.12.1176.
- Ramagopalan SV, Valdar W, Dyment DA, DeLuca GC, Yee IM, Giovannoni G. Ebers GC Group Canadian Collaborative Study. Association of infectious mononucleosis with multiple sclerosis. A population-based study. Neuroepidemiology. 2009;32(4):257–262. doi:10.1159/000201564.
- Touzé E, Fourrier A, Rue-Fenouche C, Rondé-Oustau V, Jeantaud I, Bégaud B, Alpérovitch A. Vaccination and first central nervous system demyelinating event: acase-control study. Neuroepidemiology. 2002;21(4):180–186.
- Rutschmann OT, McCrory DC, Matchar DB; Guidelines IPotMSCfCP. Immunization and MS A summary of published evidence and recommendations. Neurology. 2002;59(12):1837–1843. doi:10.1212/WNL.59.12.1837.
- Belbasis L, Bellou V, Evangelou E, Ioannidis JPA. Tzoulaki, I Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263–273. doi:10.1016/S1474-4422(14)70267-4.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Andersson T, Ahlbom A. Meta-analysis. In: Rothman K, editor. EPISHEET: Spreadsheets for the analysis of epidemiological data. Boston; 2003.