?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Pregnant women are at increased risk of morbidity and mortality from influenza and are recognized as a priority group for influenza vaccination. Despite this, uptake is often poor and one reason cited for this is concerns about safety. The objective of this study was to perform a systematic review of the safety of inactivated influenza vaccination (IIV) in pregnancy. Studies were included if they were: (i) observational or experimental design; (ii) included a comparator group comprising of unvaccinated pregnant women; (iii) comprised of either seasonal IIV or monovalent H1N1 IIV (including adjuvanted vaccines); and (iv) addressed one of the following outcomes: preterm birth (PTB), small for gestational age (SGA), fetal death (including stillbirth or spontaneous abortion), low birth weight (LBW) or congenital abnormalities. Two reviewers screened abstracts and titles and selected full texts for retrieval. Crude odds ratios were calculated from reported event rates, using binomial standard errors. Adjusted odds ratios, hazard ratios and relative rates were extracted as reported in each paper. After removal of duplicates and full text eligibility assessment, 40 studies remained. The aOR for PTB was 0.87 (0.78–0.96), for LBW 0.82 (0.76–0.89), congenital abnormality 1.03 (0.99–1.07), SGA 0.99 (0.94–1.04) and stillbirth 0.84 (0.65–1.08). This study contributes to the increasing body of safety data for IIV in pregnancy and reports a protective effect on PTB and LBW.
Introduction
Severe pandemic H1N1 influenza infection has well-described adverse effects on pregnant women and pregnancy outcomes, which are preventable through vaccination. An association between milder seasonal influenza and severe maternal disease and poor birth outcomes is less clear. Influenza vaccination across all trimesters has been recommended since the 1960sCitation1 however uptake continued to be low until the 2009 H1N1 influenza pandemic. Despite pregnant women being nominated as a priority group for vaccination,Citation2 most studies suggest that uptake remains below 50%.Citation3
More recently, data have suggested that in addition to maternal benefits, there are benefits for the fetus and neonate.Citation4 The landmark study by Zaman et al demonstrated that infants born to vaccinated mothers were 63% less likely to have laboratory confirmed influenza in the first six months of life.Citation5 More recently, there have been three randomized controlled trials published, reporting a vaccine efficacy against laboratory confirmed influenza in infants of vaccinated mothers under 6 months of age ranging from 30% to 43.1%.Citation6–Citation8
One barrier to improving influenza vaccine uptake in pregnant women includes concerns regarding safety amongst consumers and healthcare providers. Consumer concerns have been reported consistently across multiple studies.Citation9 The language and content of the product information or product monograph makes it difficult to reconcile positive recommendation for vaccination in highly respected clinical guidelines and policy recommendations with information provided by the manufacturer. A published review by Proveaux et al reported on 96 separate influenza vaccines and found that 20 of these (21%) included language suggesting that official recommendations should be “considered”, half of the manufacturers suggest users consult a health care provider to determine whether the product should be given during pregnancy and only 10/98 product information suggested use during pregnancy.Citation10 A subsequent study of 141 maternal health-care providers from 49 countries in all six World Health Organization (WHO) regions suggested that health-care providers perceive product information as contradicting WHO and national immunisation recommendations and that this could affect their decision to recommend the vaccine to pregnant women.Citation11
In 2011, the WHO Strategic Advisory Group of Experts (SAGE) on Immunization asked the Global Advisory Committee on Vaccine Safety (GACVS) to review the evidence on safety of vaccination in pregnant and lactating women. This report included studies examining influenza vaccination in pregnancy and various outcome measures (including maternal morbidity and mortality, miscarriage/stillbirth, prematurity, small size for gestational age and congenital anomalies) and did not find any safety concerns.Citation12
Since publication of the GACVS report in 2014, there have been five systematic reviews of influenza vaccine safety in pregnancy.Citation13-Citation17 Given maternal immunisation is such a rapidly evolving field, and uptake of influenza vaccine continues to be suboptimal, it is important to continue to review the safety data to be able to support policy recommendations and reassure both healthcare providers and consumers about the safety of vaccination during pregnancy. This systematic review includes five pregnancy outcomes of interest including small for gestational age which was not included in the previous cited systematic reviews, and includes new publications from the previous three years.
Methods
Literature search methods
Systematic literature searches were conducted by a medical librarian in key bibliographic databases including OVID Medline (1946-April Week 3 2017), OVID Embase (1974- Week 18 2017), Cochrane Library databases including Database of Systematic Reviews (Issue 5 of 12, May 2017), Central Register of Controlled Trials (Issue 4 of 12, April 2017), Database of Abstracts of Reviews of Effects (Issue 2 of 4, April 2015), NHS Economic Evaluation Database (Issue 2 of 4, April 2015) and Health Technology Assessments (Issue 4 of 4, October 2016 and SCOPUS (1823-May 2017). Publications after May 2017 were not included in this systematic review.
From search results, duplicates were removed and two independent reviewers screened all abstracts and titles. Full studies selected for retrieval were assessed by two independent reviewers prior to inclusion in the review. Studies that reported on outcomes in different cohorts (eg in different seasons, or using different vaccines) were treated as separate, independent studies.
Inclusion criteria
Studies were included if they were: (i) observational or experimental design (including cohort, case-control, cross-sectional, randomized controlled clinical trial); (ii) there was a comparator group comprising of unvaccinated pregnant women; (iii) the intervention comprised of either seasonal influenza vaccine or monovalent pandemic H1N1 influenza vaccine; (iv) the vaccine was adjuvanted or non adjuvanted and (v) if the study addressed one of the following outcomes: preterm birth (PTB), small for gestational age (SGA), fetal death (including stillbirth or spontaneous abortion), low birth weight (LBW) or congenital abnormalities.
Excluded studies
Case reports and case series were not included. Articles written in a language other than English were excluded.
Definitions related to outcomes of interest
The Global Alignment of Immunization safety Assessment in pregnancy (GAIA) project, published in 2016, sought to improve the quality of outcome data from clinical vaccine trials in pregnant women, with a specific focus on safety monitoring in low and middle-income countries.Citation18 Twenty-one standardized case definitions for obstetric outcomes and neonatal outcomes were developed and standardized case definitions for preterm birth, stillbirth, congenital abnormalities, spontaneous abortion, small for gestational age and low birth weight have been published.Citation19-Citation24
Preterm birth (PTB)
For this review PTB has been defined as any live birth prior to 37 completed gestational weeks.
Stillbirth and spontaneous abortion
Stillbirth refers to death of the fetus. However, miscarriage (spontaneous abortion) also refers to death of the fetus. There is no universally accepted definition of when a fetal death is called a stillbirth versus spontaneous abortion. In addition, limitations in capacity to use tools, such as ultrasound, to accurately determine gestational age can impact on the quality of data for this outcome measure. Existing definitions for stillbirth include > 20 weeks (USA CDC), > 22 weeks (WHO/ICD for general statistics and registration), > 22 weeks (European Medicines Agency), > 24 weeks (UK) and > 28 weeks (WHO/ICD for international comparison and reporting). The case definition determined by the Brighton Collaboration Stillbirth Working Group does not use a specific gestational age cut off to distinguish between miscarriage (spontaneous abortion) and stillbirth, but rather considers variability based on viability cut-offs in different settings.Citation20 For this review stillbirth was defined as after 22 weeks gestation and spontaneous abortion defined as prior to 22 weeks gestation.
Congenital abnormalities
For this review, we defined congenital abnormalities (also referred to as birth defects, congenital malformations or congenital anomalies) as a condition that developed in utero, was present at birth and can impact on the infant’s health.Citation21
Low birth weight
For this review we defined low birth weight as less than 2500 gm.
Small for gestational age
For this review we defined small for gestational age as weight below the 10th percentile for gestational age as assessed against a validated global, regional or local standard defined in the study.
Background rate of outcomes of interest
The source of the background rate used to compare the outcomes of interest for the calculations related to number needed to vaccinate was derived from the Australian National Perinatal Data collection published in 2018. This was chosen as it is a national dataset, therefore collecting data on all public and private births, including all indigenous and ethnic groups, all socioeconomic groups in a setting where 99.9% of women have at least one antenatal visit and only 0.3% of births occur outside of facilities. Background rates from a resource rich setting were selected to be comparable with the settings for the majority of included studies.
Data extraction and assessment of methodological quality
We developed a standard data collection form to extract study information including: study design, setting, time period, participants, vaccine type, timing of exposure per trimester if reported, comparator, outcome, definition of outcome used in the study, events in the vaccinated group, events in the comparator group, adjusted effect including upper and lower confidence interval limits.
Each reviewer independently assessed quality using the Newcastle-Ottawa Scale. This is a scale developed by the Cochrane review group on Effective Practice and Organisation of care to assess the quality of non-randomized studies to determine the potential for selection bias, information bias and residual confounding.Citation25 It is a nine point scale that evaluates studies on representativeness of the study population, selection of controls, ascertainment of exposure, baseline assessment, comparability of cohorts and assessment of outcome, duration of follow up and adequacy of follow up. Studies are assessed on the potential for selection bias (up to 4 stars), comparability (up to 2 stars) and exposure ascertainment (up to 3 stars). We deemed the most important potential confounder as maternal age. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to synthesise the overall quality of evidence and implications for practice.Citation26 This process provides a framework for summarizing evidence, assessing the quality of evidence and formulating recommendations. In assessing the quality of evidence, we downgraded quality based on all factors (risk of bias, inconsistency, indirectness, imprecision and publication bias).
Statistical analysis
Crude odds ratios for influenza vaccinated versus non influenza vaccinated cohorts for each outcome measure were calculated from reported event rates in each study, using binomial standard errors. Adjusted odds ratios, hazard ratios and relative rates were extracted as reported in each study. Outcomes were reported according to the most detailed information provided on trimester of exposure: first trimester, second trimester, and third trimester; second and third trimester together; or at any time during pregnancy. Pooled event rates were estimated using random effects models. Forest plots were generated and pooled estimates were calculated using the metan module in Stata 14.2 (College Station, Texas, United States). The number needed to vaccinate (NNV), where is the background rate and
is the odds ratio, was calculated using the formula:
Results
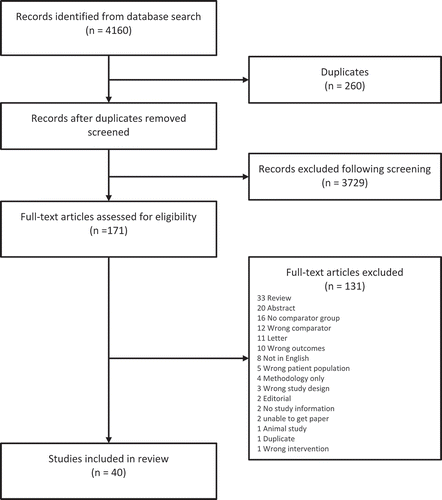
A total of 4160 publications were identified. After duplicates were removed 3900 records remained. Of these, 3729 records were excluded after abstract screening, leaving 171 records for full article eligibility assessment. In total, 131 full-text articles were excluded. The most common reasons for exclusion were: (i) the article was a review rather than a primary study (n = 33); (ii) the publication was an abstract with insufficient results to calculate an odds ratio (n = 20); (iii) there was no comparator (n = 16); (iv) the comparator was inappropriate for this review (n = 12); and (v) the publication was a letter that did not provide adequate information (n = 11). The remaining reasons for exclusion are listed in the PRISMA diagram ().
Of the 40 included studies, 25 were retrospective cohort studies, 9 were prospective cohort studies, three were case control studies, two were cross sectional and one was a randomized controlled trialCitation27-Citation66 (Supplementary Table 1). Only 15 of 40 studies included vaccine brand-specific information, of which three used both adjuvanted and non-adjuvanted vaccines. No studies used quadrivalent influenza vaccine. All studies were conducted between 1976 and 2015. The majority of studies were conducted in high-income countries with the exception of South Africa, Laos, Taiwan, China and Argentina. The results for assessment of quality according to the Newcastle-Ottawa scale are in presented in Supplementary Table 2.
Preterm birth
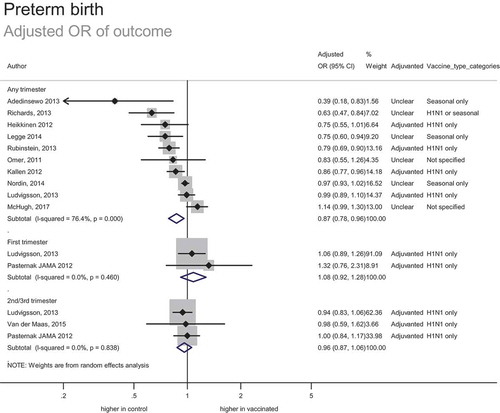
In total, 26 studies included data on 184,305 women vaccinated during pregnancy (including 9,280 known to be vaccinated in the first trimester, 1,177 vaccinated in the second trimester, 11,314 vaccinated in either the second or third trimester, and 711 vaccinated in the third trimester). This included 173,131 women vaccinated during pregnancy in risk adjusted analyses (12 studies reporting adjusted odds ratios and 12 studies reporting adjusted relative risks or hazard ratios).
The estimated adjusted odds of preterm birth for women who received any influenza vaccine during pregnancy was 0.87 (0.78–0.96) (). This reduced risk of preterm birth associated with vaccination equates to a number needed to benefit of 98 (one preterm birth would be prevented for every 98 women vaccinated), based on a background rate of preterm birth of 8.6%. The possible increased risk of preterm birth with first trimester vaccination is not statistically significant in the adjusted odds ratio and adjusted relative risk analyses, however the estimates are imprecise. The strength of evidence was assessed as moderate; despite the inherent potential for bias associated with observational data, studies were generally consistent and provided a precise estimate of effect.
Low birth weight
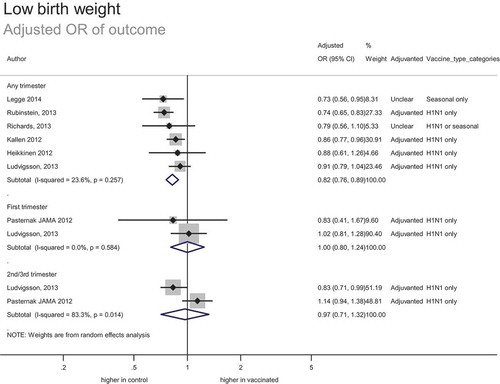
In total, 12 studies included 84 314 women vaccinated during pregnancy (including 8169 known to be vaccinated in the first trimester). This included 81 609 women vaccinated during pregnancy in adjusted analyses (7 studies reporting adjusted odds ratios and 3 studies reporting adjusted relative risks or hazard ratios).
The estimated adjusted odds ratio of outcome was 0.82 (0.76–0.89) (). This suggests a reduced risk of low birth weight associated with vaccination, with number needed to benefit of 96, based on a background rate of 6.2%. The strength of evidence was assessed as high; despite the inherent risk of bias associated with observational data, studies were consistent and provided a precise estimate effect.
Congenital abnormalities
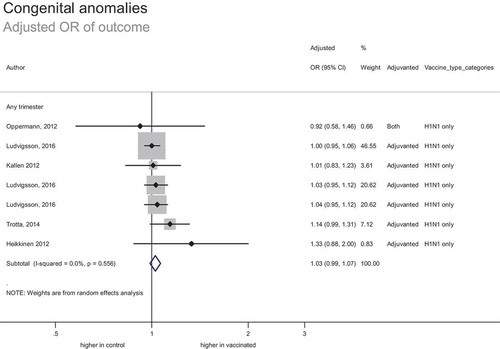
In total, 12 studies included 169 828 women vaccinated during pregnancy (including 765 vaccinated in the first trimester, and 2548 vaccinated in the second or third trimesters). This included 157,601 women vaccinated during pregnancy in risk adjusted analyses (7 studies reporting adjusted odds ratios and 2 studies reporting adjusted relative risks or hazard ratios).
For the 16 studies that looked at congenital abnormality as an outcome, only 5 performed an analysis including first trimester exposure as a stand-alone group not combined with any other trimesters of exposure. This is important given the highest risk period for congenital abnormality arising from teratogen exposure is thought to be during embryogenesis and early fetal development.
The estimated adjusted odds ratio of outcome was 1.03 (95% CI: 0.99–1.07) (). This suggests no significant increase or decrease in the risk of congenital abnormalities associated with vaccination. Hypothetically, based on the upper limit of confidence interval and baseline incidence of 308 congenital abnormalities per 100,000 births, the number needed to harm is unlikely to be less than 5428. The strength of evidence was moderate; despite the inherent risk of bias associated with observational data, studies were generally consistent and provided a precise estimate of effect.
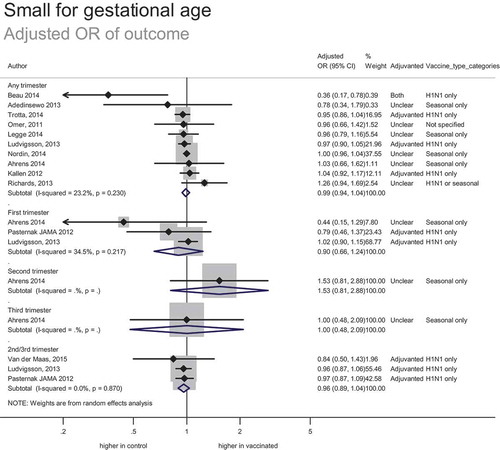
Small for gestational age
In total, 17 studies included 176 486 women vaccinated during pregnancy (including 8912 known to be vaccinated in the first trimester, 754 vaccinated in the second trimester, 7765 vaccinated in either the second or third trimester, and 431 vaccinated in the third trimester). This included 164,966 women vaccinated during pregnancy in risk-adjusted analyses (12 studies reporting adjusted odds ratios and 7 studies reporting adjusted relative risks or hazard ratios).
The estimated adjusted odds ratio of outcome was 0.99 (95% CI: 0.94–1.04) (). This suggests no significant increase or decrease in the risk of small for gestation age births associated with vaccination. Hypothetically, based on the upper limit of the confidence interval and baseline risk of 9%, the number needed to harm is unlikely to be less than 444. The strength of evidence was low; in addition to the inherent risk of bias associated with observational data, studies were not completely consistent although the data provided a precise estimate of effect.
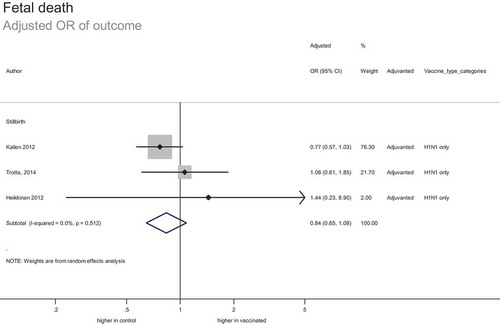
Fetal death
In total, 20 studies included 152,713 women vaccinated during pregnancy (including 8654 known to be vaccinated in the first trimester and 3385 vaccinated in either the second or third trimester) comparing the risk of stillbirth to the risk in unvaccinated pregnant women. This included 145,185 women vaccinated during pregnancy in risk-adjusted analyses. Additionally, eight studies included 6,471 women vaccinated during pregnancy comparing risk of spontaneous abortion to the risk in unvaccinated pregnant women.
Despite the smaller number of studies including spontaneous abortion as an outcome, the available data suggested an overall protective effect (crude OR 0.27, 95% CI: 0.14, 0.52). Three of the eight studies individually demonstrated a statistically significant reduction in spontaneous abortion.
The estimated adjusted odds ratio of stillbirth was 0.84 (95% CI: 0.65–1.08) (). This suggests no significant increase or decrease in the risk of fetal death associated with vaccination. Based on the confidence intervals and baseline risk of 7 per 1000 births, the number needed to vaccinate to prevent one stillbirth is estimated at 900, and number needed to harm unlikely to be less than 3597. The strength of evidence was assessed as high; despite the inherent risk of bias associated with observational data, studies were consistent and provided a precise estimate of effect.
Adjuvanted versus non-adjuvanted vaccines
Results in studies that examined adjuvanted vaccines were similar for SGA (pooled adjusted OR 0.98, 95% CI: 0.93, 1.03); PTB (0.86, 95% CI: 0.77, 0.99), LBW (0.83, 95% CI: 0.75, 0.92), fetal death (0.84, 95% CI: 0.65, 1.08) and congenital abnormalities (1.02, 95% CI: 0.98, 1.06).
Discussion
The findings from this systematic review not only confirm the safety of influenza vaccination in pregnancy but provide evidence to support a protective effect against preterm birth and low birth weight. Specifically, vaccination was not associated with an increase in preterm birth, low birth weight, small for gestational age newborns, congenital abnormalities, spontaneous abortion or stillbirth. This is based on multiple studies involving more than 100,000 pregnant women, with the exception of low birth weight (n =84314 women) and spontaneous abortion (n = 6471 women).
In comparison to no influenza vaccination in pregnancy, we found high quality evidence that the use of inactivated influenza vaccine is associated with a reduction in incidence of low birth weight newborns, and no significant difference in the incidence of stillbirth. We found moderate quality evidence that the use of influenza vaccine is associated with reduction in preterm births and no difference in congenital abnormalities. We also found low quality evidence of the effect of influenza vaccination on spontaneous abortion, which suggested a protective effect, and on small for gestational age births which suggested no difference. We did not find any differences in the risk of adverse pregnancy outcomes when the analysis was restricted to adjuvanted influenza vaccines versus no vaccine.
Maternal immunisation is a rapidly evolving area. In comparison to previous systematic reviews published in 2015–2016,Citation13-Citation17 our manuscript includes small for gestational age as an outcome of interest, 40 publications along with a meta- analysis, and ten papers published between 2015–2017 (at closure of our search date). We also performed a sub-analysis examining adjuvanted vaccines versus no vaccine which was only included in one of the previous systematic reviews. Since undertaking our literature search (which ended May 2017), there have been a number of important publications which were not included but are worthy of mention. There have been two randomized controlled trials in pregnant women published which compared IIV to placebo.Citation6,Citation7 Specifically the study by Nunes and colleagues,Citation6 reported a vaccine efficacy of 43% against all-cause acute lower respiratory tract infection and hospitalization in the first 6 months of life and no difference in rates of preterm birth and low birth weight between the vaccinated and unvaccinated groups. This study was conducted in South Africa and included 1026 vaccinated women. In the study by Steinhoff,Citation7 influenza vaccination reduced maternal febrile influenza-like illness with an overall efficacy of 19% and for laboratory confirmed influenza infections in infants less than 6 months of age, immunisation had an overall efficacy of 30%. In this randomized controlled trial conducted in Nepal, which included 1847 pregnant women, maternal immunisation reduced the rate of low birth weight by 15% but did not modify the rate of small for gestational age. The number of adverse obstetric events such as miscarriage, stillbirth and congenital abnormalities was not different between the placebo and vaccinated groups. Although these studies were not included in our systematic review due to date of publication they further strengthen the safety data we report and add additional high quality evidence for benefits to the newborn against respiratory illness in the first 6 months of life.
Of all the outcomes of interest included in this systematic review, stillbirth and spontaneous abortion were the most challenging to assess, due to a lack of consistent definition and limited data. Many studies differed on the cut off gestational age that defines a stillbirth, ranging from fetal death after 20 weeks to after 28 weeks. Spontaneous abortion was not commonly included in observational studies, as reflected by the smallest number of vaccinated women included in the analysis (6471 compared to > 100,000 for all other outcomes) and often studies did not include a definition pertaining to this term. Other factors that make this outcome challenging to assess include the timing of pregnancy and diagnostic confirmation, and non-healthcare seeking behavior.
In 2017, Donahue and colleagues reported an association of spontaneous abortion with receipt of inactivated influenza vaccine containing A/H1N1pdm2009 antigen.Citation67 This case-control study, conducted over two influenza seasons, reported an adjusted odds ratio of 2.0 (95% CI 1.1–3.6) for vaccine receipt within 28 days of spontaneous abortion in women who had received an influenza vaccine in the previous season in a post hoc analysis. There was no association for any other exposure window found. This is in contrast to our findings, and to other studies which have reported no increased risk.Citation13,Citation15 Similarly, a study published in 2017 of 102 spontaneous, pregnancy specific reports of adverse events following influenza vaccination submitted to the United States Vaccine Adverse Events Reporting System found no increase in spontaneous abortion.Citation68 Although Donahue and colleagues state that their results do not confirm causality, the findings do highlight the importance of ongoing vigilance in reporting safety of influenza vaccines in pregnancy.
As maternal immunization gains momentum as a promising intervention to reduce neonatal mortality and morbidity from a number of diseases the increased interest requires a consistent approach to monitoring safety. The Global Alignment of immunization safety assessment in pregnancy (GAIA) project was formed to improve the outcome data quality from clinical vaccine trials in pregnant women with a specific focus on safety monitoring in low and middle-income countries.Citation18 The three main objectives of the GAIA project are to: (i) improve comparability of safety data across products, programs and populations; (ii) optimize the value of local investigations by global harmonization of methods; and (iii) increase analytic power. As part of this, development of standardised case definitions has been a priority. GAIA will be very important for future studies to adopt to facilitate easier comparisons and meta-analyses, but has limited capacity to apply to past studies.
There are several limitations related to the findings of this systematic review. The first is that only one randomized controlled study was identified. Secondly, the majority of studies were conducted in high income settings. Although maternal influenza vaccination is not currently recommended in many low and middle income countries, it is being considered along with a number of other maternal vaccines, so the publication of safety data from these settings will be essential for the future confidence by policy makers. The underrepresentation from low and middle income countries has been identified by the WHO Working Group convened between 2014–2017 to evaluate influenza disease burden and vaccine efficacy to inform estimates of maternal influenza immunisation programs.Citation69 Of the 40 included studies in this review, only 15 contained brand specific information. In addition, only 13 specified use of adjuvanted vaccines and 6 non-adjuvanted vaccines. This means that the power to draw conclusions for individual vaccines or for adjuvanted versus non-adjuvanted is reduced. In addition, the background rate of adverse pregnancy outcomes used to calculate the number needed to vaccinate to harm is based on published data from a resource rich setting.Citation70 The authors acknowledge that the rate of preterm birth, congenital abnormalities, stillbirth, small for gestational age and low birth weight vary across settings and this figure may under or over represent the potential benefit/harm depending on the chosen comparator.
In addition, given influenza vaccine is recommended in all trimesters of pregnancy, there is considerable interest in outcomes according to timing of maternal vaccination. In particular, there is debate as to whether vaccination in the second and third trimesters is relevant to the outcome of congenital abnormality compared with vaccination in the first trimester. In this systematic review 24 of the 40 studies did not assess the outcome of interest according to timing of maternal vaccination. Ten studies performed the analysis by trimester, but there were still inconsistencies with three of these ten combining second and third trimesters together. As many studies do not examine the outcome according to gestation of vaccination, when this is applied, the numbers of exposed, particularly in the first trimester are significantly reduced, thereby reducing the power to detect differences between the exposed and control group.
In addition, for outcomes such as preterm birth, most studies accounted for potential confounding by factors such as maternal age, socioeconomic status, past history of preterm birth or smoking but few studies stratified their analysis by gestational age at the time of vaccination or period of influenza activity. Given that preterm birth has been linked to a pro-inflammatory milieu which may be induced by influenza infection,Citation71 and numerous observational studies have reported higher rates of preterm birth associated with hospitalization for respiratory illness during pregnancy,Citation72 it is biologically plausible that there is a protective effect of maternal influenza vaccination on preterm birth particularly during periods of influenza circulation. Inconsistencies in different study results may be attributable to differences in baseline immunity to influenza in the study population, degree of match between vaccine and circulating strains for the season under investigation, seasonal variation in pathogenicity and study design, which could not be assessed from the available published data. In addition, some authors have highlighted the importance of considering immortal time bias when considering preterm birth.Citation73 Immortal time in cohort studies is the time period of follow up during which the study outcome of interest cannot occur by study design. So, in studies looking for an association between influenza vaccination exposure and an outcome such as preterm birth, the immortal time is that between conception and vaccination (during which the participant is considered in the unexposed group). To account for this vaccination status can be considered a time varying variable. When this is done the results may shift from a decreased risk of preterm birth to no association.Citation73
Overall, our findings are consistent with previous reports; that there is no increased risk of adverse outcome associated with vaccination during pregnancy, and in fact may protect against some of these outcomes.
Conclusion
A large number of studies over decades investigating the safety of influenza vaccination in pregnancy, and the findings of our review, affirm that there is no evidence of an increased risk of adverse pregnancy outcomes following influenza vaccination in pregnancy. Despite this reassuring finding, there remain some important areas where more data from ongoing surveillance and formal research projects for pregnancy-related safety of vaccination would be beneficial. This includes further research on the safety of adjuvanted vaccines in pregnancy, particularly their use in first trimester, to establish a more comprehensive safety profile. In addition, future studies should aim to analyse outcomes according to consistent, reproducible definitions and according to trimester of exposure, thus allowing application of meta-analyses to harmonized aggregate data on outcome measure across multiple settings. Furthermore, interpreting the results in relation to the circulating influenza activity that season and the relative match of the vaccine with circulating virus strain would be valuable to provide greater understanding to the potential benefit (as compared to lack of increased risk) for pregnancy outcomes such as low birth weight and preterm birth.
Inclusion of national or WHO recommendations for vaccine use during pregnancy in the product information, when aligned with the product’s safety profile and supported by quality evidence review, may help improve vaccine uptake in pregnancy. The results from this systematic review can be included as further evidence that the large body of evidence confirms no increased risk of adverse pregnancy outcomes, particularly for non-adjuvanted inactivated influenza vaccines. Vaccine manufacturers are encouraged to consider this body of evidence and include it when writing their product information for pregnant women.
The policy implications of this systematic review relate to translation of safety data to healthcare providers and consumers to improve uptake. This is challenging however, and needs to be considered in addition to other factors that may impact on uptake during pregnancy such as accessibility, affordability and healthcare provider recommendation.
Contribution to Authorship
MG and SK were involved in planning and carrying out the screening of abstract and titles. They were also involved in assessing full texts for retrieval, assessing their quality, collecting the data, interpreting the analysis and writing up of the manuscript.
KM was involved in planning of the study, interpreting the analysis and writing up of the manuscript.
AC was involved in carrying out the analysis, interpreting the data and writing up of the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Ethics Approval
Ethics approval was not required for the purpose of this systematic review
Supplemental Material
Download Zip (253.4 KB)Acknowledgments
The authors would like to acknowledge Catherine King, librarian, with the National Centre for Immunisation Research and Surveillance for undertaking the literature search. The authors would also like to acknowledge Dr Clayton Chiu for his review and input into the study design and manuscript.
Supplementary material
Supplemental data can be accessed at tandfonline.com/khvi
Additional information
Funding
References
- Burney LE. Influenza immunization: statement. Public Health Rep. 1960;75:944. doi:10.2307/4590965.
- World Health Organization. The weekly epidemiological record. Geneva. 2012;87:201–216.
- Centers for Disease Control and Prevention. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunisation Practices (ACIP) 2009. MMWR Recomm Rep. 2009;58:1–86.
- Fell DB, Bhutta ZA, Hutcheon JA, Karron RA, Knight M, Kramer MS, Monto AS, Swamy GK, Ortiz JR, Savitz DA. Report of the WHO technical consultation on the effect of maternal influenza and influenza vaccination on the developing fetus: montreal, Canada, September 30-October 1, 2015. Vaccine. 2017 April 28;35(18):2279–2287. doi:10.1016/j.vaccine.2017.03.056.
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunisation in mothers and infants. N Engl J Med. 2008;359:1555–1564. doi:10.1056/NEJMoa0708630.
- Nunes MC, Cutland CL, Jones S, Downs S, Weinberg A, Ortiz JR, Neuzil KM, Simoes E, Klugman KP, Madhi SA. Efficacy of maternal influenza vaccination against all-cause lower respiratory tract infection hospitalizations in young infants: results from a randomized controlled trial. Clin Inf Dis. 2017;65(7):1066–1071. doi:10.1093/cid/cix497.
- Steinhoff MC, Katz J, Englund JA, Khatry SK, Shrestha L, Kuypers J, Stewart L, Mullany LC, Chu HY, LeClerq SC, et al. Year-round influenza immunisation during pregnancy in Nepal: a phase 4, randomised, placebo-controlled trial. Lancet Infect Dis. 2017;17:981–989. doi:10.1016/S1473-3099(17)30252-9.
- Tapia MD, Sow SO, Tamboura B, Teguete I, Pasetti MF, Kodio M, Onwuchekwa LI, Tennant SM, Blackwelder WC, Coulibaly F, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis. 2016;16:1026–1035. doi:10.1016/S1473-3099(16)30054-8.
- Yuen CY, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women - a systematic review. Vaccine. 2014 Aug 6;32(36):4602–4613. doi:10.1016/j.vaccine.2014.06.067.
- Proveaux T, Lambach P, Ortiz JR, Hombach J, Halsey NA. Review of prescribing information for influenza vaccines for pregnant and lactating women. Vaccine. 2016 Oct 26;34(45):5406–5409. doi:10.1016/j.vaccine.2016.08.042.
- Top KA, Arkell C, Scott H, Mannerfeldt J, Ortiz JR, Lambach P, MacDonald N. Effect of package insert language on health-care providers’ perceptions of influenza vaccination safety during pregnancy. Lancet. 2016;4(10):e690–e691.
- Global Advisory Committee on Vaccine Safety. Safety of Immunization during Pregnancy. A review of the evidence. World Health Organization, Geneva; 2014.
- McMillan M, Porritt K, Kralik D, Costi L, Marshall H. Influenza vaccination during pregnancy: a systematic review of fetal death, spontaneous abortion and congenital malformation safety outcomes. Vaccine. 2015;33:2108–2117. doi:10.1016/j.vaccine.2015.02.068.
- Nunes MC, Aqil AR, Omer SB, Madhi SA. The effects of influenza vaccination during pregnancy on birth outcomes: a systematic review and meta-analysis. Am J Perinatol. 2016;33:1104–1114. doi:10.1055/s-0036-1586101.
- Bratton KN, Wardle MT, Orenstein WA, Omer SB. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: a systematic review and meta-analysis. Clin Infect Dis. 2015;60. doi:10.1093/cid/ciu915.
- Fell DB, Platt RW, Lanes A, Wilson K, Kaufman JS, Basso O, Buckeridge D. Fetal death and preterm birth associated with maternal influenza vaccination: systematic review. Bjog. 2015;122(1):17–26. doi:10.1111/1471-0528.12977.
- Polyzos KA, Konstantelias AA, Pitsa CE, Falagas ME. Maternal influenza vaccination and risk for congenital malformations a systematic review and meta-analysis. Obstet Gynecol. 2015;126:1075–1084. doi:10.1097/AOG.0000000000001068.
- Bonhoeffer J, Kochhar S, Hirschfeld S, Heath PT, Jones CE, Bauwens J, Honrado A, Heininger U, Munoz FM, Eckert L, et al. Global alignment of immunization safety assessment in pregnancy- the GAIA project. Vaccine. 2016;34:5993–5997. doi:10.1016/j.vaccine.2016.07.006.
- Quinn JA, Munoz F, Gonik B, Frau L, Cutland C, Mallet-Moore T, Kissou A, Wittke F, Das M, Nunes T, et al. Preterm birth: case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2016;24:6047–6056. doi:10.1016/j.vaccine.2016.03.045.
- De Silva FT, Gonik B, McMillan M, Keech C, Dellicour S, Bhange S, Tila M, Harper DM, Woods C, Kawai AT, et al. Stillbirth: case definition and guidelines for data collection, analysis and presentation of maternal immunization safety data. Vaccine. 2016;34:6057–6068. doi:10.1016/j.vaccine.2016.03.044.
- De Silva M, Munoz F, McMillan M, Kawai AT, Marshall H, Maccartney KK, Joshi J, Oneko M, Rose AE, Dolk H, et al. Congenital anomalies: case definition and guidelines for data collection, analysis and presentation of immunization safety data. Vaccine. 2016;34:6015–6026. doi:10.1016/j.vaccine.2016.03.047.
- Rouse CE, Eckert LO, Babarinsa I, Fay E, Gupta M, Harrison MS, Kawai AT, Kharbanda EO, Kucuko M, Meller L, et al. Spontaneous abortion and ectopic pregnancy: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35:6563–6574. doi:10.1016/j.vaccine.2017.01.047.
- Cutland CL, Lackritz EM, Mallett-Moore T, Bardaji A, Chandrasekaran R, Lahariya C, Nisar MI, Tapia MD, Pathirana J, Kochlar S, et al. Low birth weight: case definition & guidelines for data collection, analysis and presentation of maternal immunization safety data. Vaccine. 2017;35:6492–6500. doi:10.1016/j.vaccine.2017.01.049.
- Schlaudecker EP, Munoz FM, Bardaji A, Boghossian NS, Khalil A, Mousa H, Nesin M, Nisar MI, Pool V, Spiegel HML, et al. Small for gestational age: case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine. 2017;35:6518–6528. doi:10.1016/j.vaccine.2017.01.040.
- Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman OG. International stroke trial collaborative group; European carotid surgery trial collaborative group. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:27.
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated. The GRADE Working Group, 2013; Oct 2013.
- Cantu J, Biggio J, Jauk V, Wetta L, Andrews W, Tita A. Selective uptake of influenza vaccine and pregnancy outcomes. J Maternal-Fetal Med. 2013;26(12):1207–1211. doi:10.3109/14767058.2013.775419.
- Adedinsewo DA, Noory L, Bednarczyk RA, Steinhoff MC, Davis R, Ogbuanu C, Omer SB. Impact of maternal characteristics on the effect of maternal influenza vaccination on fetal outcomes. Vaccine. 2013;31(49):5827–5833. doi:10.1016/j.vaccine.2013.09.071.
- Ahrens KA, Louik C, Kerr S, Mitchell AA, Werler MM. Seasonal influenza vaccination during pregnancy and the risks of preterm delivery and small for gestational age birth. Paediatr Perinat Epidemiol. 2014;28(6):498–509. doi:10.1111/ppe.2014.28.issue-6.
- Baum U, Leino T, Gissler M, Kilpi T, Jokinen J. Perinatal survival and health after maternal influenza A(H1N1)pdm09 vaccination: A cohort study of pregnancies stratified by trimester of vaccination. Vaccine. 2015;33(38):4850–4857. doi:10.1016/j.vaccine.2015.07.061.
- Beau AB, Hurault-Delarue C, Vidal S, Guitard C, Vayssiere C, Petiot D, Montastruc JL, Damase-Michel C, Lacroix I. Pandemic A/H1N1 influenza vaccination during pregnancy: A comparative study using the EFEMERIS database. Vaccine. 2014;32(11):1254–1258. doi:10.1016/j.vaccine.2014.01.021.
- Chambers CD, Johnson DL, Xu R, Luo YJ, Louik C, Mitchell AA, Schatz M, Jones KL. Safety of the 2010-11, 2011-12, 2012-13, and 2013-14 seasonal influenza vaccines in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants, a study from the cohort arm of VAMPSS. Vaccine. 2016;34(37):4443–4449. doi:10.1016/j.vaccine.2016.06.054.
- Chambers CD, Johnson D, Xu R, Luo Y, Louik C, Mitchell AA, Schatz M, Jones KL. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants. Vaccine. 2013;31(44):5026–5032. doi:10.1016/j.vaccine.2013.08.097.
- Cleary BJ, Rice U, Eogan M, Metwally N, McAuliffe F. A/H1N1 influenza vaccination in pregnancy: uptake and pregnancy outcomes - A historical cohort study. Eur J Obstetrics Gynecol Reprod Biol. 2009;2014(178):163–168.
- Deinard AS, Ogburn JP. A/NJ/8/76 Influenza vaccination program: effects on maternal health and pregnancy outcome. Am J Obstet Gynecol. 1981;140(3):240–245. doi:10.1016/0002-9378(81)90267-2.
- Dodds L, MacDonald N, Scott J, Spencer A, Allen VM, McNeil S. The association between influenza vaccine in pregnancy and adverse neonatal outcomes. J Obstetrics Gynaecol Can. 2012;34(8):714–720. doi:10.1016/S1701-2163(16)35336-1.
- Fabiani M, Bella A, Rota MC, Clagnan E, Gallo T, D’Amato M, Pezzotti P, Ferrara L, Demicheli V, Martinelli D, et al. A/H1N1 pandemic influenza vaccination: A retrospective evaluation of adverse maternal, fetal and neonatal outcomes in a cohort of pregnant women in Italy. Vaccine. 2015;33(19):2240–2247. doi:10.1016/j.vaccine.2015.03.041.
- Fell DB, Sprague AE, Liu N, Yasseen Iii AS, Wen SW, Smith G, Walker MC. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102(6):E33–E40. doi:10.2105/AJPH.2011.300606.
- Håberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, Skrondal A, Cappelen I, Engeland A, Aavitsland P, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. New England J Med. 2013;368(4):333–340. doi:10.1056/NEJMoa1207210.
- Heikkinen T, Young J, Van Beek E, Franke H, Verstraeten T, Weil JG, Della Cioppa G. Safety of MF59-adjuvanted A/H1N1 influenza vaccine in pregnancy: A comparative cohort study. Am J Obstet Gynecol. 2012;207(3):177.e1-.e8. doi:10.1016/j.ajog.2012.07.007.
- Kallen B, Olausson PO. Vaccination against H1N1 influenza with Pandemrix during pregnancy and delivery outcome: A Swedish register study. BJOG: Int J Obstetrics Gynaecol. 2012;119(13):1583–1590. doi:10.1111/j.1471-0528.2012.03470.x.
- Launay O, Krivine A, Charlier C, Truster V, Tsatsaris V, Lepercq J, Ville Y, Avenell C, Andrieu T, Rozenberg F, et al. Low rate of pandemic A/H1N1 2009 influenza infection and lack of severe complication of vaccination in pregnant women: a prospective cohort study. PloS one [Internet]. 2012;7(12):e52303. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/273/CN-00864273/frame.html.
- Legge A, Dodds L, MacDonald NE, Scott J, McNeil S. Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. Cmaj. 2014;186(4):E157–E64. doi:10.1503/cmaj.130499.
- Lin TH, Lin SY, Lin CH, Lin RI, Lin HC, Chiu TH, Cheng P-J, Lee C-N. AdimFlu-S() influenza A (H1N1) vaccine during pregnancy: the Taiwanese Pharmacovigilance Survey. Vaccine. 2012;30(16):2671–2675. doi:10.1016/j.vaccine.2012.02.008.
- Louik C, Ahrens K, Kerr S, Pyo J, Chambers C, Jones KL, Schatz M, Mitchell AA. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: exposure prevalence, preterm delivery, and specific birth defects. Vaccine. 2013;31(44):5033–5040. doi:10.1016/j.vaccine.2013.08.096.
- Louik C, Kerr S, Van Bennekom CM, Chambers C, Jones KL, Schatz M, Mitchell AA. Safety of the 2011-12, 2012-13, and 2013-14 seasonal influenza vaccines in pregnancy: preterm delivery and specific malformations, a study from the case-control arm of VAMPSS. Vaccine. 2016;34(37):4450–4459. doi:10.1016/j.vaccine.2016.06.078.
- Ludvigsson JF, Strom P, Lundholm C, Cnattingius S, Ekbom A, Ortqvist A, Feltelius N, Granath F, Stephansson O. Risk for congenital malformation with H1N1 influenza vaccine: A cohort study with sibling analysis. Ann Intern Med. 2016;165(12):848–855. doi:10.7326/M16-0139.
- Ludvigsson JF, Strom P, Lundholm C, Cnattingius S, Ekbom A, Ortqvist A, Feltelius N, Granath F, Stephansson O. Maternal vaccination against H1N1 influenza and offspring mortality: population based cohort study and sibling design. BMJ. 2015;351:h5585. doi:10.1136/bmj.h5585.
- Ludvigsson JF, Zugna D, Cnattingius S, Richiardi L, Ekbom A, Ortqvist A, Persson I, Stephansson O. Influenza H1N1 vaccination and adverse pregnancy outcome. Eur J Epidemiol. 2013;28(7):579–588. doi:10.1007/s10654-013-9813-z.
- Madhi SA, Cutland CL, Kuwanda L, Weinberg A, Hugo A, Jones S, Adrian PV, van Niekerk N, Treurnicht F, Ortiz JR, et al. Influenza vaccination of pregnant women and protection of their infants. N Engl J Med [Internet]. 2014;371(10):918–931. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/134/CN-01000134/frame.html.
- Ma F, Zhang L, Jiang R, Zhang J, Wang H, Gao X, Li X, Liu Y, Hodinka RL. Prospective cohort study of the safety of an influenza A(H1N1) vaccine in pregnant Chinese women. Clin Vaccine Immunol [Internet]. 2014;21(9):1282–1287. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/821/CN-01001821/frame.html.
- McHugh L, Andrews RM, Lambert SB, Viney KA, Wood N, Perrett KP, Marshall HS, Richmond P, O’Grady K-AF. Birth outcomes for Australian mother-infant pairs who received an influenza vaccine during pregnancy, 2012-2014: the FluMum study. Vaccine. 2017;35(10):1403–1409. doi:10.1016/j.vaccine.2017.01.075.
- Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, Glezen WP. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;192(4):1098–1106. doi:10.1016/j.ajog.2004.12.019.
- Nordin JD, Kharbanda EO, Vazquez Benitez G, Lipkind H, Vellozzi C, Destefano F, Vaccine Safety Datalink. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatrics. 2014;164(5):1051–7.e2. doi:10.1016/j.jpeds.2014.01.037.
- Olsen SJ, Mirza SA, Vonglokham P, Khanthamaly V, Chitry B, Pholsena V, Chitranonh V, Omer SB, Moen A, Bresee JS, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014-2015. Clin Infect Dis. 2016;63(4):487–494. doi:10.1093/cid/ciw290.
- Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, Ramakrishnan U, Cooper BS. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: A retrospective cohort study. PLoS Med. 2011;8(5):(no pagination)(e1000441). doi:10.1371/journal.pmed.1000441.
- Oppermann M, Fritzsche J, Weber-Schoendorfer C, Keller-Stanislawski B, Allignol A, Meister R, Schaefer C. A(H1N1)v2009: A controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine. 2012;30(30):4445–4452. doi:10.1016/j.vaccine.2012.04.081.
- Pasternak B, Svanström H, Mølgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, Hviid A. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ (Online). 2012;344:7857.
- Pasternak B, Svanstrom̈ H, Mølgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, Hviid A. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA J Am Med Assoc. 2012;308(2):165–174. doi:10.1001/jama.2012.6131.
- Regan AK, Moore HC, De Klerk N, Omer SB, Shellam G, Mak DB, Effler PV. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis. 2016;62(10):1221–1227. doi:10.1093/cid/ciw082.
- Richards JL, Hansen C, Bredfeldt C, Bednarczyk RA, Steinhoff MC, Adjaye-Gbewonyo D, Ault K, Gallagher M, Orenstein W, Davis RL, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis. 2013;56(9):1216–1222. doi:10.1093/cid/cit045.
- Rubinstein F, Micone P, Bonotti A, Wainer V, Schwarcz A, Augustovski F, Pichon Riviere A, Karolinski A. Influenza A/H1N1 MF59 adjuvanted vaccine in pregnant women and adverse perinatal outcomes: multicentre study. BMJ (Online) [Internet]. 2013;346(7896). http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/224/CN-00917224/frame.html.
- Sammon CJ, Snowball J, McGrogan A, de Vries CS. Evaluating the hazard of foetal death following H1N1 influenza vaccination; A population based cohort study in the UK GPRD. PLoS ONE. 2012;7(12):(no pagination)(e51734). doi:10.1371/journal.pone.0051734.
- Sheffield JS, Greer LG, Rogers VL, Roberts SW, Lytle H, McIntire DD, Wendel GD. Effect of influenza vaccination in the first trimester of pregnancy. Obstetrics Gynecol. 2012;120(3):532–537. doi:10.1097/AOG.0b013e318263a278.
- Trotta F, Da Cas R, Spila Alegiani S, Gramegna M, Venegoni M, Zocchetti C, Traversa G. Evaluation of safety of A/H1N1 pandemic vaccination during pregnancy: cohort study. BMJ. 2014;348:g3361. doi:10.1136/bmj.g3361.
- Van Der Maas N, Dijs-Elsinga J, Kemmeren J, Van Lier A, Knol M, De Melker H. Safety of vaccination against influenza A (H1N1) during pregnancy in the Netherlands: results on pregnancy outcomes and infant’s health: cross-sectional linkage study. BJOG: Int J Obstetrics Gynaecol. 2016;123(5):709–717. doi:10.1111/1471-0528.13329.
- Donahue JG, Kieke BA, King JP, DeStefano F, Mascola MA, Irving SA, Cheetham TC, Glanz JM, Jackson LA, Klein MP, et al. Association of spontaneous abortion with receipt of inactivated influenza vaccine containing H1N1pdm09 in 2010-11 and 2011-12. Vaccine. 2017;35:5314–5322. doi:10.1016/j.vaccine.2017.06.069.
- Moro P, Baumblatt J, Lewis P, Cragan J, Tepper N, Cano M. Surveillance of adverse events after seasonal influenza vaccination in pregnant women and their infants in the vaccine adverse event reporting system, July 2010-May 2016. Drug Saf. 2017;40:145–152. doi:10.1007/s40264-016-0482-1.
- Fell DB, Azziz-Baumgartner E, Baker MG, Batra M, Beaute J, Beutels P, Bhat N, Bhutta ZA, Cohen C, De Mucio B, et al. Influenza epidemiology and immunisation during pregnancy: final report of a World Health Organization working group. Vaccine. 2017;35:5738–5750. doi:10.1016/j.vaccine.2017.08.037.
- Australian Institute of Health and Welfare. Australia’s mothers and babies 2016-in brief. Perinatal statistics series no. 34. Cat. No. PER 97. Canberra, Australia: AIHW; 2018.
- Uchide N, Ohyama K, Bessho T, Takeichi M, Toyoda H. Possible roles of proinflammatory and chemoattractive cytokines produced by human fetal membrane cells in the pathology of adverse pregnancy outcomes associated with influenza virus infection. Mediators Inflamm. 2012;270670.
- Martin A, Cox S, Jamieson DJ, Whiteman MK, Kulkarni A, Tepper NK. Respiratory illness hospitalisations among pregnant women during influenza season, 1998-2008. Matern Child Health J. 2013 Sep;17(7):1325–1331. doi:10.1007/s10995-012-1135-3.
- Zerbo O, Modaressi S, Chan B, Goddard K, Lewis N, Bok K, Fireman B, Klein NP, Baxter R. No Association between influenza vaccination during pregnancy and adverse pregnancy outcomes. Vaccine. 2017 May 31;35(24):3186–3190. doi:10.1016/j.vaccine.2017.04.074.