ABSTRACT
Epstein-Barr virus (EBV) is a well-characterized oncovirus, associated with several malignancies. The complex and heterogeneous nature of colorectal cancer (CRC) has led to many epidemiological causal associations with CRC. However, a direct causal link between microbial infections and CRC has not been established yet. Our review indicates that the current evidence for the presence and role in EBV in CRC is insufficient and contradictory. The design of the analyzed studies, sample size as well as methodology used for EBV detection varied markedly and consequently may not lead to meaningful conclusions. The presence of EBV in other colorectal tumors (lymphomas, smooth muscle tumors) is in line with their status at other anatomic locations and may have therapeutic implications with EBV-specific vaccines. On the other hand, studies exploring EBV in colorectal adenoma-carcinoma sequence and its molecular genetic characteristics are largely missing and may significantly contribute to a better understanding of the role of EBV in CRC.
Introduction
Colorectal cancer (CRC) is one of the major cancer problems worldwide due to its high prevalence and mortality rates. In most countries, CRC represents the third most common cancer and third leading cause of cancer-related deaths in both men and women.Citation1,Citation2
Advances in the pathogenesis, diagnosis and treatment of CRC have had a major impact on the overall management of CRC. These include substantial advances in CRC screening and prevention as well as advances related to novel biomarker and genomic analyses, personalized therapies and chemotherapy, which altogether have significantly affected the outcome of the patients with CRC. Despite these advances, many CRC patients, especially those with advanced and/or metastatic CRCs will eventually die of the disease.
Colorectal cancer (CRC) is a complex and multifactorial disease resulting from multiple interactions between hereditary, lifestyle, (epi)genetic, and environmental factors.Citation3 Among others, the concept of microbial-epithelial interactions as a potential oncogenic trigger for the development of CRC has also been proposed (reviewed by Collins et al.).Citation4 In contrast to gastric carcinoma and lymphoma (MALT type) both of which are strongly associated with Helicobacter Pylori infection,Citation4 a direct causal link between various microbial infections [e.g. Helicobacter pylori, Streptococcus bovis, Escherichia Coli, Bacteroides, JC virus, cytomegalovirus, human papillomavirus (HPV), Epstein-Barr virus (EBV)] and CRC has not been established yet.Citation4,Citation5 The oncogenic effects of bacteria and viruses are different. Thus, bacteria may induce chronic inflammation or produce mutagenic toxins while most oncogenic viruses are small DNA viruses, which may interfere with the cell cycle of normal cells. Oncoviruses inhibit some key tumor suppressors such as TP53 and RB1 genes, avoiding the control of cell-cycle checkpoints and entering the S phase to replicate the viral DNA.Citation4
Epstein-Barr virus (EBV) is the first recognized human oncovirus. It belongs to a group of gamma-herpes viruses and is ubiquitously present in the adult population, primarily via salivary transmission.Citation6 The estimated prevalence of EBV infection appears to reach > 90% of adults by the age of 35 years.Citation7,Citation8
The EBV genome consists of double-stranded DNA, whose length is approximately 172 kb.Citation6 It encodes viral oncogenes such as EBV-encoded nuclear antigens [EBNAsCitation1-Citation3] and latent membrane proteins [LMPCitation1,Citation2].Citation9 Interactions of EBV’s surface protein gp350 with CD21 receptor and HLA class II on B-lymphocytes enables the entrance of the virus into B-lymphocytes.Citation6 Apart from B-cells, targets of EBV infection may include other human cell types such as epithelial cells as well as other hematopoietic cells (T cells, granulocytes, and natural killer cells).Citation8 However, the mechanisms of the infection of these cells may be different from CD21-mediated internalization, which is typically seen in B cells.Citation8
The EBV exists in two different forms: latent and lytic replication.Citation10 In its latent form, the EBV DNA (enclosed in a circular plasmid) behaves like host chromosomal DNA. In contrast, in the replication (lytic) form, the EBV genome is dramatically amplified (up to 1000-fold) by EBV replication machinery. This process occurs at the replication compartments within the nuclei and the lytic program arrests cell cycle progression and affects the cellular processes significantly.Citation10 The lytic form of EBV infection is considered a mechanism through which EBV induces neoplastic transformation in EBV-associated malignancies (carcinomas and lymphomas).Citation10 However, latent EBV forms (e.g. EBNA-1, EBERs, LMP-1) may be present in various EBV-related malignancies such as Burkitt lymphoma, Hodgkin’s disease and nasopharyngeal carcinoma.Citation8 EBV exhibits four types of latent gene expression, three of which (Latency I, II and III) have been described in EBV-related malignancies.Citation7 Latency I is usually associated with Burkitt lymphoma; Latency II has been found in Hodgkin’s disease, T-cell non-Hodgkin’s lymphomas and nasopharyngeal carcinoma whereas latency III predominantly affects immunocompromised patients (e.g. post-transplant and AIDS-related lymphoproliferative disorders).Citation8
EBV causes infectious mononucleosis (benign, self-limited disease) and several lymphoproliferative and epithelial malignancies including B-cell lymphomas (Burkitt lymphoma, Hodgkin lymphoma, post-transplant lymphoproliferative disorder), T-cell/NK lymphomas, nasopharyngeal and gastric carcinomas.Citation11-Citation13 In addition, EBV expression has also been demonstrated in several other carcinomas such as breast, prostate, oral, cervical and salivary gland carcinomas;Citation14-Citation18 Nevertheless no causal relationship has been established yet.
Presently, data on the role and expression of EBV in colorectal cancer are sparse and contradictory without a clear evidence of the active role of EBV in colorectal carcinogenesis.Citation4 In addition, detection assays for EBV presence vary significantly across studies, which may have a significant impact on the obtained results.Citation4
Here, we reviewed and critically appraised the studies reporting the status and the potential role of EBV in CRC carcinogenesis and other colorectal tumors with a possible link to EBV infection (lymphomas/lymphoproliferative disorders, smooth muscle tumors). We also explored the EBV status in CRC in relationship to its molecular genetic characteristics (e.g. mutational profile, microsatellite instability status). In addition, we analyzed the methodology used for EBV detection with emphasis on differences in EBV status between neoplastic and inflammatory (stromal) cells.
Methods
Search strategy and selection criteria
Data for this review were identified by searches of MEDLINE/PubMed/PubMed Central for the terms “Epstein Barr Virus”, “EBV”, “Colorectal cancer/carcinoma”, “colon cancer/carcinoma”, and “rectal cancer/carcinoma”, and “colon tumors”. Only articles/abstracts published in English were included in the review. The latest literature search was performed on July 1, 2018.
Results
The total number of extracted studies was 141. After careful evaluation and selection, only 50 articles were considered relevant for the present review (). The remaining 91 studies were excluded as most of them were not focused on EBV in colorectal cancer but on other malignancies (e.g. gastric or head and neck carcinomas or malignant lymphomas/other lymphoproliferative diseases beyond the gastrointestinal tract), in addition to experimental studies exploring EBV and/or other viruses on non-colorectal cell lines.
Figure 1. Flow diagram presenting the identified studies on PubMed/MEDLINE/PubMed Central on EBV and colorectal cancer.
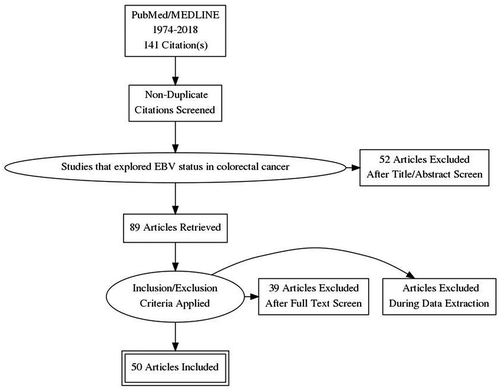
Twenty-seven studies explored EBV status in CRC (summarized in ). In addition, we found 23 studies (mainly case reports and small case series) exploring the EBV status in other colorectal malignancies, predominantly in lymphoproliferative diseases [Hodgkin lymphoma and non-Hodgkin lymphomas (B- and T-cell lineage)] and other rare large bowel tumors (e.g. smooth muscle tumors and inflammatory pseudotumor-like follicular dendritic cell sarcoma) (summarized in ).
Table 1. Studies (n = 27) that explored EBV status in colorectal cancer. The studies are listed in chronological order (from the newest to the oldest).
Table 2. Studies (n = 23) that explored EBV status in other colorectal malignancies/disorders. The studies are listed in chronological order (from the newest to the oldest).
Status of EBV in colorectal cancer
A summary of the identified studies (n = 27) with key findings is presented in . The methods used for EBV detection in human samples (blood, tissues) included immunohistochemistry (IHC), in situ hybridization (ISH), and PCR-based assays. Several studies used combined assays (IHC and ISH) (see and ).
The current literature review reveals a contradictory data on the presence of EBV in colorectal cancer samples. Thus, a positivity rate for EBV is CRC varies in a broad range from 0% (reported byCitation21,Citation27,Citation39,Citation40,Citation43,Citation44 up to 46% reported by Sole et al.Citation24 The largest single study (n = 274 samples) conducted by Cho et al.Citation40 using immunohistochemistry failed to detect EBV in cancer cells in any of the tested cases. However, they found tumor-infiltrating lymphocytes (TIL) to be positive in 12.8% of the cases. Studies of Vilor et al.,Citation42 Fiorina et al.Citation25 and Kojima et al.Citation33 also reported only TILs to be positive in CRC samples.
Most positive studies revealed EBV positivity rate to be ~ 20–40% of the cases.Citation20,Citation23,Citation26,Citation29,Citation30,Citation32,Citation34 A study of Salyakina et al.Citation26 reported a common co-infection of EBV with other viruses in 20% of the CRC samples (Cytomegalovirus and Human Herpesvirus 6B/HHV-6B/). An interesting case study by Chang et al.Citation28 on collision colorectal tumors (adenocarcinoma and non-Hodgkin lymphoma) revealed serum EBV positivity. However, the authors did not examine the EBV presence in cancer cells of either malignancy. Wong et al.Citation36 studied IBD-related colorectal carcinomas and lymphomas (n = 26) using in-situ hybridization assay (ISH). They confirmed the EBV presence in 66% of non-Hodgkin lymphomas but failed to detect it in colorectal carcinoma samples.
Mehrabani-Khasraghi et al.Citation21 explored EBV in both colon adenomas and carcinomas. The authors used PCR to examine 35 colon samples (Fifteen CRCs and twenty adenomas) and failed to detect any positive cases. Similar results were obtained in a study by Sarvari et al.Citation19 ().
The study of Karpinski et al.Citation29 was based on the fact that integration of viral genome into the host may cause alterations of the methylation pattern, which is a common molecular event in CRC pathogenesis.Citation68 Although they found EBV in 19% of the tested samples (n = 186), EBV presence along with the John Cunningham virus (JCV) were unrelated to the methylation status of the six CpG island methylator phenotype (CIMP)-specific genes (MLH1, CACNA1G, NEUROG1, IGF2, SOCS1, RUNX3) and seven cancer-related genes (p16, MINT1, MINT2, MINT31, EN1, SCTR and INHBB).Citation29 On the other hand, a study by Park et al.Citation30 assessed the EBV status in colorectal cancers in renal transplant patients. They found EBV in 30% of the samples using immunohistochemistry and in-situ hybridization assays. Similarly, Albright et al.Citation57 reported EBV-positive post-transplant lymphoproliferative tumor of the cecum (). Elawabdeh et al.Citation54 reported two EBV-positive small muscle tumors of the colon in the post-transplant pediatric patients. Lee et al.Citation66 reported similar findings in three post-transplant patients with small muscle tumors of the colon.
Another type of primary colorectal cancer is lymphoepithelioma-like carcinoma (LEC) or poorly differentiated adenocarcinoma with lymphoid stroma, which is an extremely rare subtype.Citation41 LEC of the colon is morphologically similar to poorly differentiated nasopharyngeal carcinomas that are strongly associated with EBV infection.Citation69 Nevertheless, a study of Delaney et al.Citation27 involving a case of LEC of the colon revealed no EBV presence. The authors also reviewed previously published five cases of colon LEC of which only one case had equivocal EBV positivity.Citation27 A study of Samaha et al. involving one LEC revealed no EBV in cancer cells by IHC but only by PCR.Citation41 This discrepancy may reveal the potential bias caused by DNA contamination by EBV-positive inflammatory cells in PCR assays, while IHC, enables a precise identification of the location of EBV presence (cancer vs. inflammatory and/or stromal cells). Thus far, Kon et al.Citation38 conducted the only study that confirmed EBV presence in cancer cells of the colon LEC. Taken together, the results on EBV in LEC of the colon are strikingly different from those on EBV of the LECs affecting other anatomic locations, e.g. lacrimal and salivary glands, esophagus, pancreas and middle ear.Citation70-Citation75 Notably, our recent review on LEC of uterine cervix revealed no EBV infection in cancer cells in this rare primary cervical malignancyCitation76 .
Status of EBV in hematologic malignancies and other tumors of the colon
A summary of the identified studies (n = 23) with key findings is presented in . Given that the primary non-epithelial malignancies of the colon are very rare, it is not surprising that most of the published reports are either case reports or small case series. A vast majority of EBV-positive colorectal non-epithelial malignancies are hematological malignancies, predominantly lymphomas, both Hodgkin and non-Hodgkin of B-, T- and NK-origins (). Hodgkin lymphoma (HL) is a well-known hematologic malignancy, associated with EBV infection in 50% of the cases (particularly lymphocyte-depleted and mixed-cellularity variants). HLs are characterized by the presence of Reed-Sternberg (RS) cells, typical B-transformed malignant cells, which are infected by EBV. In addition, EBV has been demonstrated in other hematologic malignancies such as Burkitt lymphoma, post-transplant lymphoproliferative disorders, and various T-cell/NK lymphoproliferative disorders. Therefore, it is expected that EBV-associated hematologic malignancies located in the colon are frequently EBV positive as their counterparts elsewhere in the body ().
Specifically for colon, several studies explored the EBV status in lymphoproliferative neoplasms in association with inflammatory bowel disease (IBD) (Crohn’s disease and ulcerative colitis). Most of these studies confirm EBV presence in lymphoproliferative diseases associated with IBD.Citation36,Citation50,Citation51,Citation53,Citation58,Citation60,Citation62,Citation65 Van Hauwaert et al.Citation56 reported the only case of EBV-negative Hodgkin lymphoma in a patient with ulcerative colitis and primary sclerosing cholangitis.
EBV status in smooth muscle tumors of the colon was explored in three separate small studies (total number of patients was six, predominantly pediatric, post-transplant patients).Citation54,Citation61,Citation66 All three studies confirmed the presence of EBV (100%) in this rare primary colon tumor.
Discussion
Although some comprehensive association studies revealed a positive association between EBV infection and CRC,Citation22 based on data analysis from gene expression profiling, protein-protein interactions, transcriptional and post-transcriptional regulation data; Nevertheless, currently, scientific evidence for the presence and role in EBV in colorectal cancer is insufficient and contradictory. The design of the analyzed studies (retrospective nature, selection bias), sample type (paraffin-embedded tissue block vs. fresh tissue; endoscopic vs. surgical biopsy), sample size (small cohorts), disease stage as well as the methodology used for EBV detection varied markedly and may not consequently lead to any meaningful conclusions. Several studies reporting a positive association were based on PCR assay, which may be contaminated by EBV-positive inflammatory cells, but not cancer cells. In addition, the role of EBV in tumor infiltrating cells (lymphocytes) is unclear; some well documented studies (e.g. Fiorina et al.)Citation25 revealed the presence of EBV in its latent, not lytic form in lymphoid aggregates within the tumor mass, which led the authors to argue against the active oncogenic role of EBV in colorectal carcinogenesis.
A substantial proportion of the analyzed studies (8/27) used immunohistochemistry (IHC) alone or combined with other techniques for EBV detection. Since EBV assessment by IHC is not routinely employed in CRC and other colorectal malignancies, the thresholds for positivity varied across the studies (1–10%) while several studies did not report the threshold for positivity.Citation20,Citation25,Citation27,Citation30
We point out here the importance of the method of EBV detection as it may play a key role in elucidating not only the presence but also the potential contribution of EBV to colorectal carcinogenesis. Most studies used PCR and/or IHC for EBV detection. Given that both assays may be biased by only selecting/targeting a certain EBV gene (due to different primers) or protein (different antibodies), therefore, more state-of-the art methods with higher sensitivity would avoid such biases should they be applied to detect EBV in colorectal and other cancers.Citation77 A less biased approach should include a full sequencing of colorectal cancer cells and search for any EBV nucleic acid fragments present. One such methodology that allows for an unbiased approach is next-generation sequencing (NGS) assay, a recent high-throughput DNA assay.Citation77 Likewise, other PCR-based techniques and NGS assays are not necessarily devoid of the contamination by infiltrating immune cells (lymphocytes), which implies that additional approaches (e.g. microdissection of cancer cells or single cell analysis) may help in circumventing the detection bias. A number of different NGS platforms are currently available for diagnostic and theranostic purposes and their detailed discussion is beyond the scope of this article.
Therefore, we believe that studies exploring EBV presence and role in colorectal adenoma-carcinoma sequence are largely missing and may significantly contribute to a better knowledge on the role of EBV in colorectal carcinogenesis. A recent study of Sarvari et al.Citation19 revealed very low EBV positivity in invasive colorectal carcinomas (1.4%) while all corresponding colorectal adenomas (n = 70) as well as adjacent normal colonic mucosa (n = 70) were negative for EBV. Similarly, Mehrabani-Khasraghi et alCitation21 reported no EBV expression in any carcinomas or adenomas they investigated. In addition, studies exploring the relationship between EBV presence and molecular genetic characteristics of CRC (e.g. mutational profile, microsatellite instability status, immune status, e.g. checkpoint regulators PD-1 and PD-L1) are completely missing. Primary non-epithelial cancers affecting colon are very rare. Most non-epithelial colon malignancies are hematologic cancers (lymphomas) some of which may be associated with IBD. In this regard, it is well known that immunosuppressive drugs used for the treatment of IBD and organ transplant recipients may reactivate viral diseases such as EBV and trigger their oncogenic role in the development of various malignancies.Citation78 Our review confirms that the EBV-status in lymphoproliferative disorders affecting colon is similar to those occurring elsewhere in the body (e.g. classical Hodgkin lymphoma and other B-cell lymphomas/lymphoproliferative disorders may be EBV positive in a significant proportion of the cases).Citation79 Interestingly, EBV presence was also confirmed in six cases of smooth muscle tumors of the colon including those affecting post-transplant pediatric patients.Citation54,Citation61,Citation66 This is in line with EBV positivity in smooth muscle tumors at other anatomic locations, particularly among the immunocompromised and/or post-transplant patients (e.g. head and neck, vulva).Citation80-Citation83 The presence of EBV in non-epithelial colonic tumors may also have therapeutic implications given the recent success with an EBV specific vaccine that was successfully used in the treatment of EBV-positive nasopharyngeal carcinoma.Citation84,Citation85 Another alternative for the treatment may be the prophylactic EBV vaccine, which is based on virus-like particles that mimic the structure of EBV but lacks EBV genome. This approach has been shown to be effective in preclinical models.Citation86
Conclusions
The current scientific evidence for the presence and role of EBV in colorectal cancer pathogenesis is insufficient and contradictory. The design of the analyzed studies, their sample size as well as the methodology used for EBV detection vary markedly and may not consequently lead to meaningful conclusions. Several studies reporting positive associations were based on PCR assay, which may be contaminated by EBV-positive inflammatory, but not cancer, cells. In this regard, the presence of EBV in inflammatory cells (lymphocytes) may not be an indication of an oncogenic effect given that the virus may be in its latent form. Apparently, high sensitivity assays (e.g. NGS platforms) that allow for a full sequencing of colorectal cancers for any EBV load are critically important to elucidate the potential contribution of EBV in colorectal oncogenesis. The presence of EBV in other colorectal tumors (lymphomas, smooth muscle tumors) is in line with the EBV status in these tumors at other anatomic locations. Nevertheless, studies exploring EBV presence and role in colorectal adenoma-carcinoma sequence are largely missing and may significantly contribute to a better knowledge on the possible role of EBV in colorectal carcinogenesis. In addition, studies exploring the relationship between EBV and molecular genetic characteristics of CRC (e.g. mutational profile, microsatellite instability status, immune checkpoint regulators/PD-1 and PD-L1) are also warranted. Most non-epithelial colon malignancies are lymphoproliferative diseases (lymphomas) some of which may be associated with inflammatory bowel disease. Their EBV-status in colon is similar to those occurring elsewhere in the body and unrelated to inflammatory bowel disease. In any case, future studies are necessary to confirm the importance of EBV vaccines in the treatment of such malignancies.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author.
Acknowledgments
We are thankful to Mrs. Amal Kassab for her comments and proofreading of the manuscript.
References
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–197. DOI:10.1055/s-0029-1242458
- Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7(3):105–114.
- Aran V, Victorino AP, Thuler LC, Ferreira CG. Colorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer. 2016;15(3):195–203. DOI:10.1016/j.clcc.2016.02.008
- Collins D, Hogan AM, Winter DC. Microbial and viral pathogens in colorectal cancer. Lancet Oncol. 2011;12(5):504–512. DOI:10.1016/S1470-2045(10)70186-8
- Burnett-Hartman AN, Newcomb PA, Potter JD. Infectious agents and colorectal cancer: a review of helicobacter pylori, streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2970–2979. DOI:10.1158/1055-9965.EPI-08-0571
- Mui UN, Haley CT, Tyring SK. Viral oncology: molecular biology and pathogenesis. J Clin Med. 2017;6(12). DOI:10.3390/jcm6120111
- Balfour HH Jr., Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W. Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6-19 years in the United States and factors affecting its acquisition. J Infect Dis. 2013;208(8):1286–1293. DOI:10.1093/infdis/jit321
- Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004;10(3):803–821.
- Guidry JT, Scott RS. 2017. The interaction between human papillomavirus and other viruses. Virus Res. 231:139–147. DOI:10.1016/j.virusres.2016.11.002
- Tsurumi T, Fujita M, Kudoh A. Latent and lytic Epstein-Barr virus replication strategies. Rev Med Virol. 2005;15(1):3–15. DOI:10.1002/rmv.441
- de Lima MAP, Neto PJN, Lima LPM, Goncalves Junior J, Teixeira Junior AG, Teodoro IPP, Facundo HT, da Silva CG, Lima MV. Association between Epstein-Barr virus (EBV) and cervical carcinoma: a meta-analysis. Gynecol Oncol. 2018;148(2):317–328. DOI:10.1016/j.ygyno.2017.10.005
- Vedham V, Verma M, Mahabir S. Early-life exposures to infectious agents and later cancer development. Cancer Med. 2015;4(12):1908–1922. DOI:10.1002/cam4.538
- Vockerodt M, Yap LF, Shannon-Lowe C, Curley H, Wei W, Vrzalikova K, Murray PG. The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol. 2015;235(2):312–322. DOI:10.1002/path.4459
- She Y, Nong X, Zhang M, Wang M, Gershburg E. Epstein-Barr virus infection and oral squamous cell carcinoma risk: a meta-analysis. PLoS One. 2017;12(10):e0186860. DOI:10.1371/journal.pone.0186860
- Whitaker NJ, Glenn WK, Sahrudin A, Orde MM, Delprado W, Lawson JS. Human papillomavirus and Epstein Barr virus in prostate cancer: koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate. 2013;73(3):236–241. DOI:10.1002/pros.22562
- Al Moustafa AE, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother. 2016;12(7):1936–1939. DOI:10.1080/21645515.2016.1139255
- Mozaffari HR, Ramezani M, Janbakhsh A, Sadeghi M. Malignant salivary gland tumors and Epstein-Barr Virus (EBV) Infection: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2017;18(5):1201–1206. DOI:10.22034/APJCP.2017.18.5.1201
- Al-Thawadi H, Ghabreau L, Aboulkassim T, Yasmeen A, Vranic S, Batist G, Moustafa A. Co-Incidence of Epstein-barr virus and high-risk human papillomaviruses in cervical cancer of syrian women. Front Oncol. 2018;8:250. DOI:10.3389/fonc.2018.00250
- Sarvari J, Mahmoudvand S, Pirbonyeh N, Safaei A, Hosseini SY. The very low frequency of Epstein-Barr JC and BK Viruses DNA in colorectal cancer tissues in Shiraz, Southwest Iran. Pol J Microbiol. 2018;67(1):73–79. DOI:10.5604/01.3001.0011.6146
- Al-Antary N, Farghaly H, Aboulkassim T, Yasmeen A, Akil N, Al Moustafa AE. Epstein-Barr virus and its association with Fascin expression in colorectal cancers in the Syrian population: a tissue microarray study. Hum Vaccin Immunother. 2017;13(7):1573–1578. DOI:10.1080/21645515.2017.1302046
- Mehrabani-Khasraghi S, Ameli M, Khalily F. Demonstration of herpes simplex virus, cytomegalovirus, and Epstein-Barr Virus in colorectal cancer. Iran Biomed J. 2016;20(5):302–306.
- Guan X, Yi Y, Huang Y, Hu Y, Li X, Wang X, Fan H, Wang G, Wang D Revealing potential molecular targets bridging colitis and colorectal cancer based on multidimensional integration strategy. Oncotarget. 2015;6(35):37600–37612. DOI:10.18632/oncotarget.6067
- Tafvizi F, Fard ZT, Assareh R. Epstein-Barr virus DNA in colorectal carcinoma in Iranian patients. Pol J Pathol. 2015;66(2):154–160.
- Sole CV, Calvo FA, Ferrer C, Alvarez E, Carreras JL, Ochoa E. Human cytomegalovirus and Epstein-Barr virus infection impact on (18)F-FDG PET/CT SUVmax, CT volumetric and KRAS-based parameters of patients with locally advanced rectal cancer treated with neoadjuvant therapy. Eur J Nucl Med Mol Imaging. 2015;42(2):186–196. DOI:10.1007/s00259-014-2910-8
- Fiorina L, Ricotti M, Vanoli A, Luinetti O, Dallera E, Riboni R, Paolucci S, Brugnatelli S, Paulli M, Pedrazzoli P, Baldanti F. Systematic analysis of human oncogenic viruses in colon cancer revealed EBV latency in lymphoid infiltrates. Infect Agent Cancer. 2014;9:18. DOI:10.1186/1750-9378-9-18
- Salyakina D, Tsinoremas NF. 2013. Viral expression associated with gastrointestinal adenocarcinomas in TCGA high-throughput sequencing data. Hum Genomics. 7:23. DOI:10.1186/1479-7364-7-4
- Delaney D, Chetty R. Lymphoepithelioma-like carcinoma of the colon. Int J Clin Exp Pathol. 2012;5(1):105–109.
- Chang H, Chuang WY, Shih LY, Tang TC. Collision in the colon: concurrent adenocarcinoma and diffuse large B-cell lymphoma in the same tumour. Acta Clin Belg. 2011;66(4):302–304. DOI:10.2143/ACB.66.4.2062573
- Karpinski P, Myszka A, Ramsey D, Kielan W, Sasiadek MM. Detection of viral DNA sequences in sporadic colorectal cancers in relation to CpG island methylation and methylator phenotype. Tumour Biol. 2011;32(4):653–659. DOI:10.1007/s13277-011-0165-6
- Park JM, Choi MG, Kim SW, Chung IS, Yang CW, Kim YS, Jung CK, Lee KY, Kang JH. Increased incidence of colorectal malignancies in renal transplant recipients: a case control study. Am J Transplant. 2010;10(9):2043–2050. DOI:10.1111/j.1600-6143.2010.03231.x
- Nishigami T, Kataoka TR, Torii I, Sato A, Tamura K, Hirano H, Hida N, Ikeuchi H, Tsujimura T. Concomitant adenocarcinoma and colonic non-Hodgkin’s lymphoma in a patient with ulcerative colitis: a case report and molecular analysis. Pathol Res Pract. 2010;206(12):846–850. DOI:10.1016/j.prp.2010.07.007
- Militello V, Trevisan M, Squarzon L, Biasolo MA, Rugge M, Militello C, Palù G, Barzon L. Investigation on the presence of polyomavirus, herpesvirus, and papillomavirus sequences in colorectal neoplasms and their association with cancer. Int J Cancer. 2009;124(10):2501–2503. DOI:10.1002/ijc.24224
- Kojima Y, Mogaki M, Takagawa R, Ota I, Sugita M, Natori S, Hamaguchi Y, Kurosawa H, Fukushima T, Masui H, Fukazawa S. A case of lymphoepithelioma-like carcinoma of the colon with ulcerative colitis. J Gastroenterol. 2007;42(2):181–185. DOI:10.1007/s00535-006-1981-0
- Song LB, Zhang X, Zhang CQ, Zhang Y, Pan ZZ, Liao WT, Li MZ, Zeng MS. 2006. Infection of Epstein-Barr virus in colorectal cancer in Chinese. Ai Zheng. 25(11):1356–1360.
- Liu HX, Ding YQ, Li X, Yao KT. Investigation of Epstein-Barr virus in Chinese colorectal tumors. World J Gastroenterol. 2003;9(11):2464–2468.
- Wong NA, Herbst H, Herrmann K, Kirchner T, Krajewski AS, Moorghen M, Niedobitek F, Rooney N, Shepherd NA, Niedobitek G. Epstein-Barr virus infection in colorectal neoplasms associated with inflammatory bowel disease: detection of the virus in lymphomas but not in adenocarcinomas. J Pathol. 2003;201(2):312–318. DOI:10.1002/path.1442
- Grinstein S, Preciado MV, Gattuso P, Chabay PA, Warren WH, De Matteo E, Gould VE. 2002. Demonstration of Epstein-Barr virus in carcinomas of various sites. Cancer Res. 62(17):4876–4878.
- Kon S, Kasai K, Tsuzuki N, Nishibe M, Kitagawa T, Nishibe T, Sato N. Lymphoepithelioma-like carcinoma of rectum: possible relation with EBV. Pathol Res Pract. 2001;197(8):577–582. DOI:10.1078/0344-0338-00130
- Kijima Y, Hokita S, Takao S, Baba M, Natsugoe S, Yoshinaka H, Aridome K, Otsuji T, Itoh T, Tokunaga M, Eizuru Y. 2001. Epstein-Barr virus involvement is mainly restricted to lymphoepithelial type of gastric carcinoma among various epithelial neoplasms. J Med Virol. 64(4):513–518.
- Cho YJ, Chang MS, Park SH, Kim HS, Kim WH. In situ hybridization of Epstein-Barr virus in tumor cells and tumor-infiltrating lymphocytes of the gastrointestinal tract. Hum Pathol. 2001;32(3):297–301. DOI:10.1053/hupa.2001.22766
- Samaha S, Tawfik O, Horvat R, Bhatia P. Lymphoepithelioma-like carcinoma of the colon: report of a case with histologic, immunohistochemical, and molecular studies for Epstein-Barr virus. Dis Colon Rectum. 1998;41(7):925–928.
- Vilor M, Tsutsumi Y. Localization of Epstein-Barr virus genome in lymphoid cells in poorly differentiated adenocarcinoma with lymphoid stroma of the colon. Pathol Int. 1995;45(9):695–697.
- Yuen ST, Chung LP, Leung SY, Luk IS, Chan SY, Ho J. In situ detection of Epstein-Barr virus in gastric and colorectal adenocarcinomas. Am J Surg Pathol. 1994;18(11):1158–1163.
- Boguszakova L, Hirsch I, Brichacek B, Faltyn J, Fric P, Dvorakova H, Vonka V. 1988. Absence of cytomegalovirus, Epstein-Barr virus, and papillomavirus DNA from adenoma and adenocarcinoma of the colon. Acta Virol. 32(4):303–308.
- Nonoyama M, Kawai Y, Huang CH, Pagano JS, Hirshaut Y, Levine PH. Epstein-Barr virus DNA in Hodgkin’s disease, American Burkitt’s lymphoma, and other human tumors. Cancer Res. 1974;34(5):1228–1231.
- Kazemimood R, Saei Hamedani F, Sharif A, et al. A rare case of Epstein-Barr Virus negative inflammatory pseudotumor-like follicular dendritic cell sarcoma presenting as a solitary colonic mass in a 53-year-old woman; case report and review of literature. Appl Immunohistochem Mol Morphol. 2017;25(5):e30–e3. DOI:10.1097/PAI.0000000000000405
- Shimoyama S, Kuroda H, Yoshida M, Usami M, Sakamoto H, Yamada M, Fujii S, Maeda M, Fujita M, Nakano T, Kanari Y. [RS3PE syndrome associated with senile Epstein-Barr virus-positive diffuse large B cell lymphoma of a patient with colon cancer]. Rinsho Ketsueki. 2015;56(11):2329–2335. DOI:10.11406/rinketsu.56.2329
- Zheng X, Xie J, Zhou X. Epstein-Barr virus associated T-cell lymphoproliferative disease misdiagnosed as ulcerative colitis: a case report. Int J Clin Exp Pathol. 2015;8(7):8598–8602.
- Haramura T, Haraguchi M, Irie J, Ito S, Tokai H, Noda K, Kitajima M, Minami S, Inoue K, Sasaki Y, Oshima K. Case of plasmablastic lymphoma of the sigmoid colon and literature review. World J Gastroenterol. 2015;21(24):7598–7603. DOI:10.3748/wjg.v21.i24.7598
- Moran NR, Webster B, Lee KM, Trotman J, Kwan YL, Napoli J, Leong RW. Epstein Barr virus-positive mucocutaneous ulcer of the colon associated Hodgkin lymphoma in Crohn’s disease. World J Gastroenterol. 2015;21(19):6072–6076. DOI:10.3748/wjg.v21.i19.6072
- Rasmussen SL, Thomsen C. 2015. Rectal Hodgkin lymphoma in a patient with ulcerative colitis: a case study. Diagn Pathol. 10:25. DOI:10.1186/s13000-015-0271-7
- Ambrosio MR, Rocca BJ, Barone A, Mastrogiulio MG, Costa A, Bellan C, Primary anorectal Hodgkin lymphoma: report of a case and review of the literature. Hum Pathol. 2014;45(3):648–652. DOI:10.1016/j.humpath.2013.09.015
- Allen PB, Laing G, Connolly A, O’Neill C. EBV-associated colonic B-cell lymphoma following treatment with infliximab for IBD: a new problem? BMJ Case Rep. 2013;2013. DOI:10.1136/bcr-2013-200423
- Elawabdeh N, Cone BM, Abramowsky CR, Wrubel DM, Grossniklaus H, Walrath J, Bashir MZ, Shehata BM. Epstein-Barr virus associated smooth muscle tumors in post transplant pediatric patients two cases of rare locations, and review of the literature. Fetal Pediatr Pathol. 2013;32(3):184–191. DOI:10.3109/15513815.2012.701265
- Da Costa AA, Flora AC, Stroher M, Soares FA, de Lima VC. Primary Hodgkin’s lymphoma of the rectum: an unusual presentation. J Clin Oncol. 2011;29(10):e268–70. DOI:10.1200/JCO.2010.32.2073
- Van Hauwaert V, Meers S, Verhoef G, Tousseyn T, Sagaert X, Vermeire S, Rutgeerts P, Van Assche G. Rectal non-Hodgkin’s lymphoma in an infliximab treated patient with ulcerative colitis and primary sclerosing cholangitis. J Crohns Colitis. 2010;4(6):683–686. DOI:10.1016/j.crohns.2010.06.006
- Albright JB, Bonatti H, Stauffer J, Dickson RC, Nguyen J, Harnois D, Jeanpierre C, Hinder R, Steers J, Chua H, Aranda‐Michel J. Colorectal and anal neoplasms following liver transplantation. Colorectal Dis. 2010;12(7):657–666. DOI:10.1111/j.1463-1318.2009.01840.x
- Kakimoto K, Inoue T, Nishikawa T, Ishida K, Kawakami K, Kuramoto T, Abe Y, Morita E, Murano N, Toshina K, Murano M. Primary CD56+ NK/T-cell lymphoma of the rectum accompanied with refractory ulcerative colitis. J Gastroenterol. 2008;43(7):576–580. DOI:10.1007/s00535-008-2192-7
- Ogasawara T, Kimura A, Yasuyama M, Ohtuka K, Aiba M, Kawauchi K. [2-year complete remission after withdrawal of methotrexate in a rectal methotrexate-associated diffuse large B-cell lymphoma]. Rinsho Ketsueki. 2007;48(6):485–490.
- Bai M, Katsanos KH, Economou M, Kamina S, Balli C, Briasoulis E, Kappas AM, Agnantis N, Tsianos EV. Rectal Epstein-Barr virus-positive Hodgkin’s lymphoma in a patient with Crohn’s disease: case report and review of the literature. Scand J Gastroenterol. 2006;41(7):866–869. DOI:10.1080/00365520500529629
- Medlicott SA, Devlin S, Helmersen DS, Yilmaz A, Mansoor A. Early post-transplant smooth muscle neoplasia of the colon presenting as diminutive polyps: a case complicating post-transplant lymphoproliferative disorder. Int J Surg Pathol. 2006;14(2):155–161. DOI:10.1177/106689690601400212
- Daniel F, Damotte D, Moindrot H, Molina T, Berger A, Cellier C. A steroid-refractory ulcerative colitis revealing Epstein-Barr virus/cytomegalovirus-positive colonic lymphoma. Int J Colorectal Dis. 2006;21(3):288–290. DOI:10.1007/s00384-004-0719-9
- Valbuena JR, Gualco G, Espejo-Plascencia I, Medeiros LJ. Classical Hodgkin lymphoma arising in the rectum. Ann Diagn Pathol. 2005;9(1):38–42.
- Hsiao CH, Kao HL, Lin MC, Su IJ. Ulcerative colon T-cell lymphoma: an unusual entity mimicking Crohn’s disease and may be associated with fulminant hemophagocytosis. Hepatogastroenterology. 2002;49(46):950–954.
- Kumar S, Fend F, Quintanilla-Martinez L, Kingma DW, Sorbara L, Raffeld M, Banks PM, Jaffe ES. 2000. Epstein-Barr virus-positive primary gastrointestinal Hodgkin’s disease: association with inflammatory bowel disease and immunosuppression. Am J Surg Pathol. 24(1):66–73.
- Lee ES, Locker J, Nalesnik M, Reyes J, Jaffe R, Alashari M, Nour B, Tzakis A, Dickman PS. 1995. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med. 332(1):19–25.
- Verastegui E, Ortega V, Soler C. Lazo de la Vega S, Ocadiz R, Meneses A, et al. Primary B Cell Lymphoma Rectum Patient Coinfected with HIV-1 HTLV-I Rev Invest Clin. 1992;44(3):387–392.
- Tse JWT, Jenkins LJ, Chionh F, Mariadason JM. Aberrant DNA methylation in colorectal cancer: what should we target?. Trends Cancer. 2017;3(10):698–712. DOI:10.1016/j.trecan.2017.08.003
- Young LS, Dawson CW. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):581–590. DOI:10.5732/cjc.014.10197
- Maeda H, Yamashiro T, Yamashita Y, Hirakawa H, Agena S, Uehara T, Matayoshi S, Suzuki M. Lymphoepithelial carcinoma in parotid gland related to EBV infection: A case report. Auris Nasus Larynx. 2018;45(1):170–174. DOI:10.1016/j.anl.2016.12.010
- Kermani W, Belcadhi M, Sriha B, Abdelkefi M. Epstein-Barr virus-associated lymphoepithelial carcinoma of the larynx. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132(4):231–233. DOI:10.1016/j.anorl.2015.05.004
- Samdani RT, Hechtman JF, O’Reilly E, DeMatteo R, Sigel CS. EBV-associated lymphoepithelioma-like carcinoma of the pancreas: case report with targeted sequencing analysis. Pancreatology. 2015;15(3):302–304. DOI:10.1016/j.pan.2015.03.016
- Keelawat S, Tirakunwichcha S, Saonanon P, Maneesri-leGrand S, Ruangvejvorachai P, Lerdlum S, Thorner PS. Cytokeratin-negative undifferentiated (Lymphoepithelial) carcinoma of the lacrimal sac. Ophthalmic Plast Reconstr Surg. 2017;33(1):e16–e8. DOI:10.1097/IOP.0000000000000422
- Terada T. Epstein-Barr virus associated lymphoepithelial carcinoma of the esophagus. Int J Clin Exp Med. 2013;6(3):219–226.
- Huon LK, Wang PC, Huang SH. Epstein Barr virus-associated lymphoepithelial carcinoma in the middle ear. Otolaryngol Head Neck Surg. 2011;144(2):296–297. DOI:10.1177/0194599810390479
- Vranic S, Cyprian FS, Akhtar S, Al Moustafa AE. 2018. The role of Epstein-Barr Virus in cervical cancer: a brief update. Front Oncol. 8:113. DOI:10.3389/fonc.2018.00113
- Akhtar S, Vranic S, Cyprian FS, Al Moustafa AE. 2018. Epstein-Barr Virus in Gliomas: cause, association, or artifact? Front Oncol. 8:123. DOI:10.3389/fonc.2018.00123
- Schulz TF. Cancer and viral infections in immunocompromised individuals. Int J Cancer. 2009;125(8):1755–1763. DOI:10.1002/ijc.24741
- Mathas S, Hartmann S, Kuppers R. Hodgkin lymphoma: pathology and biology. Semin Hematol. 2016;53(3):139–147. DOI:10.1053/j.seminhematol.2016.05.007
- Suwansirikul S, Sukpan K, Sittitrai P, Suwiwat S, Khunamornpong S. Epstein-Barr virus-associated smooth muscle tumor of the tonsil. Auris Nasus Larynx. 2012;39(3):329–332. DOI:10.1016/j.anl.2011.07.013
- Khunamornpong S, Sukpan K, Suprasert P, Shuangshoti S, Pintong J, Siriaunkgul S. Epstein-Barr virus-associated smooth muscle tumor presenting as a vulvar mass in an acquired immunodeficiency syndrome patient: a case report. Int J Gynecol Cancer. 2007;17(6):1333–1337. DOI:10.1111/j.1525-1438.2007.00989.x
- Cheuk W, Li PC, Chan JK. Epstein-Barr virus-associated smooth muscle tumour: a distinctive mesenchymal tumour of immunocompromised individuals. Pathology. 2002;34(3):245–249. DOI:10.1080/00313020220131309
- Yu L, Aldave AJ, Glasgow BJ. Epstein-Barr virus-associated smooth muscle tumor of the iris in a patient with transplant: a case report and review of the literature. Arch Pathol Lab Med. 2009;133(8):1238–1241. DOI:10.1043/1543-2165-133.8.1238
- Hui EP, Taylor GS, Jia H, Ma BB, Chan SL, Ho R, Wong WL, Wilson S, Johnson BF, Edwards C, Stocken DD. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 2013;73(6):1676–1688. DOI:10.1158/0008-5472.CAN-12-2448
- Taylor GS, Jia H, Harrington K, Lee LW, Turner J, Ladell K, Price DA, Tanday M, Matthews J, Roberts C, Edwards C. A recombinant modified vaccinia ankara vaccine encoding Epstein-Barr Virus (EBV) target antigens: a phase I trial in UK patients with EBV-positive cancer. Clin Cancer Res. 2014;20(19):5009–5022. DOI:10.1158/1078-0432.CCR-14-1122-T
- Ruiss R, Jochum S, Wanner G, Reisbach G, Hammerschmidt W, Zeidler R. A virus-like particle-based Epstein-Barr virus vaccine. J Virol. 2011;85(24):13105–13113. DOI:10.1128/JVI.05598-11
