ABSTRACT
Objective: Antibody persistence evaluation for all antigens of a fully liquid DTaP-IPV-HB-PRP~T vaccine at 3.5 and 4.5 y of age following different primary series and booster schedules in South Africa and Latin America.
Methods: Participants had completed one of two previous studies (Study 1-South Africa; Study 2-Latin America). In Study 1, participants who had not received HB vaccine at birth received a 6–10-14 week primary series of DTaP-IPV-HB-PRP~T or DTwP/PRP~T-Hib+HB+OPV and a third group who had received HB vaccine at birth received a 6–10-14 week primary series of DTaP-IPV-HB-PRP~T; all received a booster (15–18 months) of the primary series vaccine(s) except for HB in the DTwP/PRP~T-Hib group. In Study 2, participants received HB vaccine at birth, a 2–4-6 month primary series of DTaP-IPV-HB-PRP~T or DTaP-HB-IPV//PRP~T, and a DTaP-IPV-HB-PRP~T or DTaP-HB-IPV//PRP~T booster (12–24 months). Participants were followed up at 3.5 and 4.5 y of age for antibody persistence.
Results: Approximately 80% of eligible participants were assessed. In Study 1, a birth dose of HB increased anti-HBs persistence (≥10 mIU/mL) following DTaP-IPV-HB-PRP~T primary and booster vaccination from 76.3% to 96.1% at 3.5 y of age and from 73.3% to 96.1% at 4.5 y of age; in Study 2, anti-HBs persistence was high and similar in each group. For the other antigens, there were no differences between groups or studies at 3.5 or 4.5 y.
Conclusion: Good persistence of antibodies to each antigen in the DTaP-IPV-HB-PRP~T vaccine up to pre-school age, irrespective of the vaccination schedule during the first 2 y of life.
Introduction
Pediatric combination vaccines that include diphtheria (D), tetanus (T), pertussis (acellular [aP] or whole cell [wP]), inactivated poliovirus [IPV], hepatitis B [HB], and Haemophilus influenzae type b [Hib] antigens are crucial for the maintenance of high global coverage of protection against these infectious diseases. Commonly such vaccines are coadministered with other age-recommended pediatric vaccines against meningococcal disease, pneumococcal disease, rotavirus, measles, mumps, rubella, and varicella. Combination vaccines facilitate compliance to increasingly crowded pediatric vaccination schedules, usually using a 2- or 3-dose primary infant series followed by a toddler booster in the second year of life, by administering multiple antigens in a single vaccination.Citation1 While immunogenicity and safety data from primary vaccination series and toddler boosters of hexavalent vaccines have been widely published, few data are available to describe the long-term persistence of antibodies although this is an important aspect when considering continued protection up to pre-school booster age.
A fully liquid DTaP-IPV-HB-PRP~T hexavalent vaccine (Hexaxim™, Hexyon™, or Hexacima™, depending on the country of sale) was first licensed in 2012, is now approved in more than 110 countries worldwide with >42 million doses distributed, and has been pre-qualified by the World Health Organization.Citation2–Citation6 This vaccine builds on the success of established DTaP-IPV tetravalent and DTaP-IPV//PRP~T pentavalent vaccines (Tetraxim and Pentaxim, respectively)Citation7,Citation8 by the addition of 10 µg Hansenula polymorpha-derived HB surface antigen (HBsAg) with proven immunogenicity and safety.Citation9 The only other widely available comparable hexavalent vaccine outside the European Union is a DTaP-IPV-HB//PRP~T vaccine, which is reconstituted prior to use (Infanrix hexa™).Citation10–Citation12
The DTaP-IPV-HB-PRP~T vaccine has undergone an extensive global program of clinical studies both prior to and post-licensure, including demonstration of good and consistent immunogenicity and safety in various infant primary series schedules at 2, 3, 4 months of age,Citation13,Citation14 6, 10, 14 weeks of age,Citation15,Citation16 2, 4, 6 months of age,Citation17–Citation22 and 3, 5 months of ageCitation23 both with and without the administration of a standalone HB vaccine at birth and followed by a toddler booster as well as in a mixed hexavalent-pentavalent-hexavalent 3-dose infant schedule. Good antibody persistence for all antigens prior to a booster vaccine administered in the second year of life has also been consistently demonstrated as well as good immunogenicity and safety following booster vaccination.Citation13,Citation17,Citation20,Citation24
The long-term persistence of HB antibodies at 9–10 y of age and strong anti-HB response to subsequent HB vaccination have recently been reported following administration of the DTaP-IPV-HB-PRP~T vaccine in a 2, 4, 6 month of age primary series with a standalone HB vaccine at birth but no booster.Citation25 However, no long-term immunogenicity data for the other antigens contained in the DTaP-IPV-HB-PRP~T vaccine have previously been published. Two clinical studies from the clinical development program were chosen since they represented two different primary series and booster vaccine regimens, with different comparator vaccines, and were conducted in geographically distinct locations, thereby providing a broad perspective on pre-school antibody persistence to each antigen at 3.5 and 4.5 y of age in two distinct situations.Citation16,Citation20,Citation24
Results
Participants studied
In Study 1, 622 participants received a 6, 10, 14 week primary series of DTaP-IPV-HB-PRP~T (N = 243), DTwP/PRP~T + OPV + HB (N = 242), or DTaP-IPV-HB-PRP~T following HB at birth (N = 137), of whom 218, 219, and 130 participants received a booster vaccination of DTaP-IPV-HB-PRP~T or DTwP/PRP~T-Hib at 15–18 months of age. A total of 173, 177, and 103 participants were assessed at 3.5 y of age and 167, 167, and 102 participants were assessed at 4.5 y of age ().
Figure 1. Participant disposition. Primary series and booster data from Madhi et al (Study 1, South Africa)Citation16,Citation24 and Lopez et al (Study 2, Colombia) Citation20.
*, coadministered with MMR-V; †, coadministered with PCV7 (2, 4, 6 months) and rotavirus vaccine (2, 4 months); ‡, coadministered with PCV7 and MMR-V; #, data only available for Colombia at 3.5 years and 4.5 years of age in Study 2 (due to closure of site in Costa Rica)
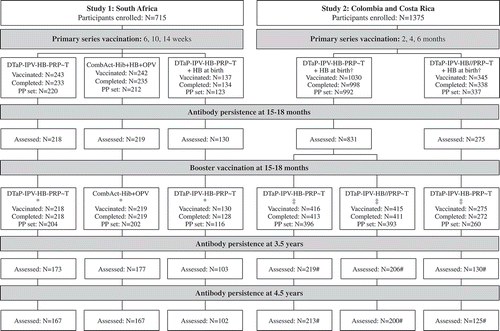
In Study 2, 1375 participants received a 2, 4, 6 month primary series of DTaP-IPV-HB-PRP~T (N = 1030) or DTaP-IPV-HB//PRP~T (N = 345). Of those who received a primary series of DTaP-IPV-HB-PRP~T, 416 participants received a DTaP-IPV-HB-PRP~T booster and 415 participants received a DTaP-IPV-HB//PRP~T booster at 12–24 months of age. Of those who received a primary series of DTaP-IPV-HB//PRP~T, 275 participants received a DTaP-IPV-HB-PRP~T booster at 12–24 months of age. A total of 219, 206, and 130 participants from the Colombian part of the study were assessed 3.5 y of age and 213, 200, and 125 participants were assessed at 4.5 y of age ().
Overall, in Study 1, of the 567 participants who were eligible a total of 453 (79.9%) and 436 (76.9%) participants were assessed at 3.5 y of age and 4.5 y of age, respectively. In Study 2, of the 699 participants who were eligible (participants from Colombia only), a total of 555 (79.4%) and 538 (77.0%) were assessed at 3.5 y of age and 4.5 y of age, respectively.
Immunogenicity
Hepatitis b
In Study 1, for participants not vaccinated with a standalone HB vaccine at birth, 76.3% and 73.3% vaccinated with DTaP-IPV-HB-PRP~T and 72.7% and 68.5% vaccinated with DTwP/PRP~T + HB + OPV demonstrated anti-HB ≥ 10 mIU/mL at 3.5 y of age and 4.5 y of age, respectively. The addition of a HB vaccination at birth increased the anti-HB seroprotection (SP) rate (≥10 mIU/mL) to 96.1% at both 3.5 and 4.5 y of age following vaccination with DTaP-IPV-HB-PRP~T. Similarly, GMCs persisted at higher levels when HB was given at birth ().
Table 1. Anti-hepatitis B antibody response post-primary vaccination, pre- and post-booster in second year of life, and persistence at 3.5 and 4.5 years of age.
In Study 2, anti-HB ≥ 10 mIU/mL and GMCs were high and similar in each study group at both 3.5 y of age (>95% of participants with anti-HB ≥ 10 mIU/mL) and 4.5 y of age (>92% of participants with anti-HB ≥ 10 mIU/mL) ().
Diphtheria
In Study 1, anti-D ≥ 0.01 IU/mL (≥97.0%) and ≥0.1 IU/mL (≥64.4%) was higher at 3.5 and 4.5 y of age following a primary series and booster of DTaP-IPV-HB-PRP~T than following a primary series and booster vaccination of DTwP/PRP~T (≥87.5% [≥0.01 IU/mL] and ≥33.1% [≥0.1 IU/mL]). Similarly, GMCs were higher for DTaP-IPV-HB-PRP~T than for DTwP/PRP~T ().
Table 2. Anti-diphtheria antibody response post-primary vaccination, pre- and post-booster in second year of life, and persistence at 3.5 and 4.5 years of age.
In Study 2, anti-D ≥ 0.01 IU/mL (≥99.5% at 3.5 y of age and ≥ 98.0% at 4.5 y of age), ≥0.1 IU/mL (≥67.0% at 3.5 y of age and ≥48.2% at 4.5 y of age), and GMCs were similar in each group ().
Tetanus
In both studies, anti-T ≥ 0.01 IU/mL (≥99.0%) and ≥0.1 IU/mL (≥76.8%) was high and similar in each group at 3.5 and 4.5 y of age, and GMCs were in the same range in each group ().
Table 3. Anti-tetanus antibody response post-primary vaccination, pre- and post-booster in second year of life, and persistence at 3.5 and 4.5 years of age.
Poliovirus
Anti-polio 1, 2, and 3 were not assessed in Study 1 due to the occurrence of OPV administrations during National Immunization Days. In Study 2, anti-polio 1, 2, and 3 ≥ 8 (1/dil) was high and similar in each group and at 3.5 y of age (≥98.5%) and 4.5 y of age (≥99.0%). The GMCs in each group were similar for each poliovirus type and slightly higher at 3.5 y of age than 4.5 y of age ().
Table 4. Anti-poliovirus 1, 2, and 3 antibody response post-primary vaccination, pre- and post-booster in second year of life, and persistence at 3.5 and 4.5 years of age.
Pertussis
In Studies 1 and 2 for anti-PT GMCs and in Study 1 for anti-FHA, GMCs were slightly higher at 3.5 y of age than 4.5 y of age; in Study 2 anti-FHA GMCs were slightly higher at 4.5 y of age than at 3.5 y of age. Both anti-PT and anti-FHA GMCs were generally similar between groups on each occasion ().
Haemophilus influenzae type b
In both studies, the majority of children had anti-PRP ≥ 0.15 µg/mL and ≥1.0 µg/mL at 3.5 y of age and 4.5 y of age, with no differences between groups (Study 1: ≥98.3% and ≥98.8% [≥0.15 µg/mL] and ≥87.0% and 78.4% [≥0.1 µg/mL]; Study 2: ≥99.2% and 100.0% [≥0.15 µg/mL] and ≥86.8% and ≥84.4% [≥0.1 µg/mL]). The GMCs were similar in each group at 3.5 y of age and 4.5 y of age with no difference between groups in each study ().
Safety
No SAEs occurred in any group since the booster part in either study.
Discussion
A high rate of follow-up of approximately 80% of participants was achieved at 3.5 and 4.5 y of age, which was similar in each study. Good antibody persistence was demonstrated for all antigens in each group in both studies. Due to the differences in study design and vaccines administered (due to the different immunization regimens in South Africa [Study 1] versus Colombia and Costa Rica [Study 2]) a numerical comparison between studies is not valid, and evaluation of anti-PT and anti-FHA was limited to GMCs due to the lack of a correlate of protection for these pertussis antigens.
The results confirm good antibody persistence up to pre-school age following a primary series of the DTaP-IPV-HB-PRP~T vaccine with a booster in the second year of life, even following the less immunogenic 6, 10, 14 week infant primary series schedule. Although it is not possible to fully assess any potential impact of the coadministered vaccines in the two studies, the antibody responses post-primary series, pre-booster, and post-boosterCitation16,Citation20,Citation24 are aligned with results from a wide range of studies evaluating the immunogenicity of the DTaP-IPV-HB-PRP~T vaccine in a range of schedules, countries, and with and without coadministered vaccines.Citation13–Citation15,Citation17–Citation19,Citation21–Citation23 It is therefore unlikely that there would be a clinically important effect of the coadministered vaccines on antibody persistence at 3.5 and 4.5 y of age.
Anti-HBs antibodies are of particular interest since the HB antigen is the new inclusion in the DTaP-IPV-HB-PRP~T vaccine. At 3.5 and 4.5 y of age the percentage of vaccinees with anti-HBs ≥10 mIU/mL was similar for both DTaP-IPV-HB-PRP~T and DTwP/PRP~T + HB + OPV when given in a 6, 10, 14 week infant primary series schedule (with a HB booster at 15–18 months of age in the DTaP-IPV-HB-PRP~T group) if no HB vaccine was administered at birth (Study 1) (≥68.5%). In the DTaP-IPV-HB-PRP~T group the proportion of participants with anti-HBs ≥10 mIU/mL was higher when standalone HB vaccine was given at birth (≥96.1%).
In Study 2 (2, 4, 6 month primary series with HB vaccination at birth and a booster in the second year of life), anti-HBs SP rates were high (>92%) in each group, with no difference between participants who received DTaP-IPV-HB-PRP~T/DTaP-IPV-HB-PRP~T, DTaP-IPV-HB-PRP~T/DTaP-IPV-HB//PRP~T, or DTaP-IPV-HB//PRP~T/DTaP-IPV-HB-PRP~T infant/toddler series. The high persisting anti-HB SP rate in Study 2 is consistent with that from Study 1 when a primary series and booster of DTaP-IPV-HB-PRP~T was given following HB vaccination at birth, and higher than the DTaP-IPV-HB-PRP~T group in Study 1 that did not receive HB vaccine at birth.
Anti-HBs antibody persistence, in terms of antibody titers ≥10 mIU/mL, after a 2, 4, 6 month primary series of DTaP-IPV-HB-PRP~T with HB vaccine administered at birth and with no booster vaccination has recently been shown to be maintained at 9–10 y of age in approximately 50% of participants.Citation25 Furthermore, a strong anamnestic response was reported following HB vaccine challenge re-vaccination at 9–10 y of age despite the reduced SP rate. These data are consistent with previous studies that have shown the persistence of anti-HB T-cell and B-cell immune memory for decades following vaccination with HB-containing combination vaccines, even without regular booster vaccination.Citation26–Citation30 As such, a strong anti-HBs response would be expected following subsequent HB exposure even in individuals with an anti-HBs antibody titer <10 mIU/mL, and the WHO have stated that ‘the substantial body of evidence does not provide a compelling basis for recommending a booster dose of hepatitis B vaccine after completion of the primary vaccination series for persons with normal immune status’.Citation31
Overall, very good antibody persistence was demonstrated in two different vaccination schedules and in two different populations (South Africa and Latin America) with a variety of primary series/booster sequences, indicating that mixed primary series/booster schedules are equally immunogenic as the administration of the same vaccine in a primary series and as a booster. These data add to those recently reported that show good primary series and booster immunogenicity following a mixed infant primary series and booster schedule (hexavalent-pentavalent-hexavalent primary series and pentavalent booster). There was no clinically important difference in antibody persistence between the two populations, irrespective of the different environmental factors in the different regions.
Limitations of this analysis include the evaluation of only two primary series schedules and no available data for HB immunogenicity after a 2, 4, 6 month primary series and booster without a HB vaccination at birth. However, good primary series and booster immunogenicity has been shown consistently for the DTaP-IPV-HB-PRP~T vaccine, as well as good long-term HB immunogenicity with an anamnestic response on challenge re-vaccination,Citation25 and so it would be expected that the data described can be extrapolated to other primary series and booster schedules. Second, in Study 2, the window for booster administration was 12–24 months of age, which meant that subjects assessed at 3.5 and 4.5 y of age could have received the booster from 1.5–2.5 y prior to the assessment at 3.5 y of age and from 2.5–3.5 y prior to the assessment at 4.5 y of age. However, for a descriptive analysis such as that reported this was considered acceptable. Third, in Study 2, due to the closure of the study site in Costa Rica, it was only possible to collect follow-up data at 3.5 and 4.5 y of age from participants in the Colombian part of the study. It is, however, appropriate to consider these data in the context of primary and booster series data from both Colombia and Costa Rica due to the similar ethnicity and due to the descriptive nature of the analysis at 3.5 and 4.5 y of age.
These results are the first to describe antibody persistence for the fully liquid DTaP-IPV-HB-PRP~T vaccine at 3.5 and 4.5 y of age following primary series and booster vaccination. These encouraging results add to a growing body of literature describing the good immunogenicity of this vaccine in a variety of primary series and booster schedules.
Materials and methods
Study design and participants
Phase III, randomized, observer-blind, controlled, primary vaccination and booster clinical studies that are reported elsewhere were conducted separately in South Africa (Study 1)Citation16,Citation24 and Colombia and Costa Rica (Study 2).Citation20
In Study 1, participants in South Africa who had not received standalone HB vaccine at birth were randomized to receive a 6, 10, 14 week primary series of either DTaP-IPV-HB-PRP~T or DTwP/PRP~T + HB + OPV, and a third group of participants who had received a standalone HB vaccine at birth received a 6, 10, 14 week primary series of DTaP-IPV-HB-PRP~T;Citation16 all participants were to receive a booster vaccination of the same vaccine(s) administered in the primary series, co-administered with measles, mumps, rubella, and varicella vaccines (MMR-V).Citation24
In Study 2, all participants in Colombia and Costa Rica received a standalone HB vaccination at birth and were randomized to receive a primary series of either DTaP-IPV-HB-PRP~T or DTaP-HB-IPV//PRP-T at 2, 4, 6 months of age co-administered with pneumococcal (PCV) 7 vaccine (2, 4, 6 months) and rotavirus vaccine (2 and 4 months); in the second year of life, participants who had received DTaP-IPV-HB-PRP~T in the primary series were randomized to receive a booster of DTaP-IPV-HB-PRP~T or DTaP-HB-IPV//PRP-T, and those who had received a primary series of DTaP-HB-IPV//PRP-T were to receive a booster of DTaP-IPV-HB-PRP~T (booster vaccines were co-administered with PCV7; additionally, MMR-V and PCV13 vaccines were available at 15 months of age in Costa Rica, but not in Colombia, in accordance with the national recommendations).Citation20
Participants in each study were followed up at 3.5 y of age and 4.5 y of age to assess antibody persistence (UTNs: U1111-1111–5789 and NCT01105559 [South Africa] and U1111-1122–2457 and NCT01983540 [Colombia and Costa Rica]). These antibody persistence data are reported in this article. For the study in Colombia and Costa Rica (Study 2) only participants in Colombia were followed up at 3.5 y of age and 4.5 y of age due to the closure of the study site in Costa Rica (due to a lack of proposed clinical studies), so only data from Colombian participants are presented at these time points for Study 2.
All study protocols and amendments were approved by independent ethics committees and studies were performed according to local regulations, Good Clinical Practice, and the ethical principles of Declaration of Helsinki (Edinburgh revision, October 2000). Prior to enrolment an informed consent form was signed by at least one parent or legally acceptable representative and an independent witness if applicable according to the appropriate local regulations. Participants attended the study sites to provide blood samples for 3.5 year and 4.5 year antibody persistence between April 2010 and September 2011 (Study 1) and between October 2013 and April 2015 (Study 2).
Participants were included in the primary series and booster parts according to standard inclusion and exclusion criteria described elsewhere,Citation16,Citation20,Citation24 and all were healthy infants born at full term (≥37 weeks) with birth weight ≥2.5 kg. For the 3.5 and 4.5 y of age follow-up assessments, participants were 3.5 y of age at enrolment and had received a complete 3-dose primary series and booster vaccination according to the study protocols. Exclusion criteria at 3.5 y of age included participation in another clinical trial in the previous 4 weeks; history of diphtheria, tetanus, pertussis, poliomyelitis, Haemophilus influenzae, or hepatitis B, or vaccination against any of these since the booster vaccination; any vaccination within the previous 30 d (except measles, monovalent polio, of pandemic influenza vaccines that were prohibited within 2 weeks of inclusion); receipt of blood products or immunosuppressant drugs in the previous 3 months; acquired immunodeficiency or hepatitis C infection since the booster vaccination; chronic illness following the booster vaccination (e.g. leukemia, lymphoma, Crohn’s disease); and any acute illness or febrile illness.
Vaccines and vaccine administration
The composition and batch numbers of the vaccines administered in the primary series and booster parts are described elsewhere for Study 1 (DTaP-IPV-HB-PRP~T [Hexaxim; Sanofi Pasteur], DTwP/PRP~T [CombAct-Hib; Sanofi Pasteur], HB [Engerix B; GlaxoSmithKline], OPV [Sanofi Pasteur], MMR [ROR, Sanofi Pasteur], and V [Varilrix, GlaxoSmithKline])Citation16,Citation24 and Study 2 (DTaP-IPV-HB-PRP~T [Hexaxim; Sanofi Pasteur], DTaP-IPV-HB//PRP~T [Infanrix hexa; GlaxoSmithKline], PCV7 [Prevenar; Pfizer], and rotavirus [Rotarix; GlaxoSmithKline]).Citation20
The DTaP-IPV-HB-PRP~T, DTaP-IPV-HB//PRP~T, PCV7, and HB vaccines were administered intramuscularly, MMR was administered either intramuscularly or subcutaneously, V was administered subcutaneously, and rotavirus vaccine was administered orally (see for subjects disposition).
Serology
Blood samples post-primary series, pre-booster and post-booster were collected and analyzed as reported elsewhere.Citation16,Citation20,Citation24
Blood samples were collected at 3.5 y of age (5 mL) and 4.5 y of age (5 mL) for determination of anti-HB, anti-D, anti-T, anti-polio 1, 2 and 3, anti-PT, anti-FHA, and anti-PRP antibodies. Anti-polio 1, 2, and 3 were not evaluated in Study 1 due to the possibility that participants could have received an extra dose of OPV since the booster vaccination as part of a national vaccination campaign against polio.
All assays were performed at either the Sponsor’s Global Clinical Immunology (GCI) laboratory (Swiftwater, PA, USA) or at qualified contract laboratories approved by GCI. Anti-D antibody concentrations (IU/mL) and anti-polio 1, 2, 3 antibody titers (1/dil) were measured by a microneutralization assay (with an assay against Mahoney, MEF-1 and Saukett poliovirus strains), anti-T (IU/mL), anti-PT (EU/mL), and anti-FHA (EU/mL) concentrations by an enzyme linked immunosorbent assay (ELISA), anti-PRP-T (µg/mL) concentrations by a radioimmunoassay, and anti-HB concentrations (mIU/mL) by a commercially available chemiluminescence assay (VITROS ECi/ECiQ).
Safety
Since the 3.5 year of age and 4.5 year of age assessments were non-interventional no safety data were recorded (other than ongoing SAE long-term monitoring after the primary series and booster vaccinations).
Statistical analyses
The objective of these two studies was to describe the long-term antibody persistence and 3.5 y of age and 4.5 y of age following primary series and booster vaccinations that are described elsewhere.Citation16,Citation20,Citation24 There were no formal statistical tests performed for this assessment of long-term antibody persistence and all analyses were descriptive.
Antibody thresholds and criteria used to define the SP rates are presented for anti-HB, anti-D, anti-T, anti-polio 1, 2 and 3, and anti-PRP antibodies in , , , , and 6, respectively. Additionally, geometric mean titers (GMTs) are presented for IPV and geometric mean concentrations (GMCs) are presented for HB, PRP, D, T, PT, and FHA ( to ). Previously published data are presented for the primary series and booster vaccinations in order to provide the full profile for each antigen.Citation16,Citation20,Citation24 All data are presented with their 95% confidence intervals (CIs) calculated using the exact binomial distribution (Clopper-Pearson)Citation32 for proportions and the normal approximation method for GMCs and GMTs.
Table 5. Anti-pertussis (anti-PT and anti-FHA) antibody geometric mean concentrations post-primary vaccination, pre- and post-booster in second year of life, and persistence at 3.5 and 4.5 years of age.
Table 6. Anti-PRP antibody response post-primary vaccination, pre- and post-booster in second year of life, and persistence at 3.5 and 4.5 years of age.
No calculation for sample size was performed since these were descriptive studies. The sample size in each study was based on the available participants who had completed the prior primary series and booster vaccinations, i.e. a maximum of 567 participants for Study 1 and a maximum of 699 participants in Study 2 (see ).
All statistical analyses were performed under the responsibility of the Sponsor’s Biostatistics
Platform using SAS® software, Version 9.1 or 9.2 (SAS Institute, Cary, North Carolina, USA).
Authors Contributions
SM, SB’C, EJ, and EF contributed to the conception, design, and clinical conduct of Study 1.
PL, BZ, SB’C, EJ, and FN contributed to the conception, design, and clinical conduct of Study 2.
All authors contributed to the analysis and interpretation of study data presented in this article and all approved the final version of this article.
Disclosure of potential conflicts of interest
No clinical investigator (SM and PL) involved in these studies received any direct payment from Sanofi Pasteur with regard to their contribution to this manuscript, but received funding for their institutions from Sanofi Pasteur to support their work in their respective studies and could receive expenses for conference attendance for the presentation of data from these studies.
BZ, EJ, SB’C, FN, and EF are employees of Sanofi Pasteur.
Acknowledgments
The authors acknowledge all enrolled infants and parents/legal guardian(s) for their participation.
The authors would also like to thank the co-investigators for the primary series and booster studies in South Africa (Ismail Mitha MD, Anthonet Koen MD, Clare Cutland MD, and Michelle Groome MD) and Costa Rica (Adriano Arguedas Mohs MD, Arturo Abdelnour Vásquez MD) and all study personnel for the conduct of the studies.
At Sanofi Pasteur, the authors thank Fabrice Bailleux, Xavier DaCosta, Séverine Paulhac, Eduardo Santos-Lima, Maria Consuelo Miranda, and the Hexaxim clinical team for their valuable input into the conception, execution, and analysis of the clinical trials described in this manuscript.
This manuscript was prepared with the assistance of a professional medical writer, Dr Andrew Lane (Lane Medical Writing) funded by Sanofi Pasteur, in accordance with the European Medical Writers Association guidelines and Good Publication Practice.
Additional information
Funding
References
- Decker MD, Edwards KM, Howe BJ. Combination vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Vaccines. 7th ed. PA (USA): Elsevier; 2018. p. 198–227.
- Lyseng-Williamson KM, McCormack PL. DTaP-IPV-Hep B-Hib vaccine (Hexyon®/Haxacima®): a guide to its use in the primary and booster vaccination of infants and toddlers in Europe. Drugs Ther Perspect. 2013;29(11):329–335. doi:10.1007/s40267-013-0078-0.
- McCormack PL. DTaP-IPV-Hep B-Hib vaccine (Hexaxim®): a review of its use in primary and booster vaccination. Paediatr Drugs. 2013;15(1):59–70. doi:10.1007/s40272-013-0007-7.
- Nunes MC, Madhi SA. Review of a new fully liquid, hexavalent vaccine: hexaxim. Expert Opin Biol Ther. 2013;13(4):575–593. doi:10.1517/14712598.2013.774368.
- Santos-Lima E, B’Chir S, Lane A. Combined immunogenicity data for a new DTaP-IPV-Hep B-PRP-T vaccine (Hexaxim) following primary series administration at 2, 4, 6 months of age in Latin America. Vaccine. 2013;31(9):1255–1258. doi:10.1016/j.vaccine.2012.11.087.
- WHO. WHO prequalified vaccines. 2018 [ accessed 2018 Oct 11]. https://extranet.who.int/gavi/PQ_Web/
- Plotkin SA, Liese J, Madhi SA, Ortiz E. A DTaP-IPV//PRP~T vaccine (Pentaxim): a review of 16 years’ clinical experience. Expert Rev Vaccines. 2011;10(7):981–1005. doi:10.1586/erv.11.72.
- Vidor E, Plotkin SA. Immunogenicity of a two-component (PT & FHA) acellular pertussis vaccine in various combinations. Hum Vaccin. 2008;4(5):328–340.
- Tregnaghi MW, Voelker R, Santos-Lima E, Zambrano B. Immunogenicity and safety of a novel yeast Hansenula polymorpha-derived recombinant Hepatitis B candidate vaccine in healthy adolescents and adults aged 10-45 years. Vaccine. 2010;28(20):3595–3601. doi:10.1016/j.vaccine.2010.02.049.
- Baldo V, Bonanni P, Castro M, Gabutti G, Franco E, Marchetti F, Prato R, Vitale F. Combined hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b vaccine; Infanrix™ hexa. Hum Vaccin Immunother. 2014;10(1):129–137. doi:10.4161/hv.26269.
- Dhillon S. DTPa-HBV-IPV/Hib vaccine (Infanrix hexa): A review of its use as primary and booster vaccination. Drugs. 2010;70(8):1021–1058. doi:10.2165/11204830-000000000-00000.
- Zepp F, Schmitt HJ, Cleerbout J, Verstraeten T, Schuerman L, Jacquet JM. Review of 8 years of experience with Infanrix hexa (DTPa-HBV-IPV/Hib hexavalent vaccine). Expert Rev Vaccines. 2009;8(6):663–678. doi:10.1586/erv.09.32.
- Ceyhan M, Yildirim I, Tezer H, Devrim I, Feroldi E. 2017. A fully liquid DTaP-IPV-HB-PRP-T hexavalent vaccine for primary and booster vaccination of healthy Turkish infants and toddlers. Turkish J Med Sci. 47:1247–1256. doi:10.3906/sag-1609-62.
- Vesikari T, Borrow R, Da Costa X, Richard P, Eymin C, Boisnard F, Lockhart S. Concomitant administration of a fully liquid, ready-to-use DTaP-IPV-HB-PRP-T hexavalent vaccine with a meningococcal serogroup C conjugate vaccine in infants. Vaccine. 2017;35(3):452–458. doi:10.1016/j.vaccine.2016.11.053.
- Chhatwal J, Lalwani S, Vidor E. Immunogenicity and safety of a liquid hexavalent vaccine in Indian infants. Indian Pediatr. 2017;54:15–20.
- Madhi SA, Mitha I, Cutland C, Groome M, Santos-Lima E. Immunogenicity and safety of an investigational fully liquid hexavalent combination vaccine versus licensed combination vaccines at 6, 10, and 14 weeks of age in healthy South African infants. Pediatr Infect Dis J. 2011;30(4):e68–74. doi:10.1097/INF.0b013e31820b93d2.
- Aquino AG, Brito MG, Doniz CE, Herrera JF, Macias M, Zambrano B, Plennevaux E, Santos-Lima E. A fully liquid DTaP-IPV-Hep B-PRP-T hexavalent vaccine for primary and booster vaccination of healthy Mexican children. Vaccine. 2012;30(45):6492–6500. doi:10.1016/j.vaccine.2012.07.040.
- Kosalaraksa P, Thisyakorn U, Benjaponpitak S, Chokephaibulkit K, Santos-Lima E. Immunogenicity and safety study of a new DTaP-IPV-Hep B-PRP-T combined vaccine compared to a licensed DTaP-IPV-Hep B//PRP-T comparator, both concomitantly administered with a 7-valent pneumococcal conjugate vaccine at 2, 4, and 6 months of age in Thai infants. Int J Infect Dis. 2011;15(4):e249–56. doi:10.1016/j.ijid.2010.12.004.
- Lanata C, Zambrano B, Ecker L, Amemiya I, Gil A, Santos-Lima E. 2012. Immunogenicity and safety of a fully liquid DTaP-IPV-Hep B-PRP-T vaccine at 2-4-6 months of age in Peru. J Vaccines Vaccin. 3:128. doi:10.4172/2157-7560.1000128.
- López P, Mohs A, Vásquez A, Consuelo-Miranda M, Feroldi E, Noriega F, Jordanov E, B Chir S, Zambrano B. A randomized, controlled study of a fully liquid DTaP-IPV-HB-PRP-T hexavalent vaccine for primary and booster vaccinations of healthy infants and toddlers in Latin America. Pediatr Infect Dis J. 2017;36(11):e272–e82. doi:10.1097/INF.0000000000001682.
- Macias M, Lanata CF, Zambrano B, Gil AI, Amemiya I, Mispireta M, Ecker L, Santos-Lima E. Safety and immunogenicity of an investigational fully liquid hexavalent DTaP-IPV-Hep B-PRP-T vaccine at two, four and six months of age compared with licensed vaccines in Latin America. Pediatr Infect Dis J. 2012;31(8):e126–32. doi:10.1097/INF.0b013e318258400d.
- Tregnaghi MW, Zambrano B, Santos-Lima E. Immunogenicity and safety of an investigational hexavalent diphtheria-tetanus-acellular pertussis-inactivated poliovirus-hepatitis B-Haemophilus influenzae B conjugate combined vaccine in healthy 2-, 4-, and 6-month-old Argentinean infants. Pediatr Infect Dis J. 2011;30(6):e88–96. doi:10.1097/INF.0b013e318212eb80.
- Vesikari TS, Silfverdal SA, Jordanov E, Feroldi E. A randomized, controlled study of DTaP-IPV-HB-PRP-T, a fully liquid hexavalent vaccine, administered in a 3, 5 and 11-12 month schedule. Pediatr Infect Dis J. 2017;36(1):87–93. doi:10.1097/INF.0000000000001358.
- Martinón-Torres F, Diez-Domingo J, Feroldi E, Jordanov E, B’Chir S, Da Costa X. Evaluation of a hexavalent-pentavelent-hexavalent infant primary vaccination series followed by a pentavalent booster vaccine in infants and toddlers. Pediatr Inf Dis J. 2018 (in press). doi:10.1097/INF.0000000000002231
- Madhi SA, Koen A, Cutland C, Groome M, Santos-Lima E. Antibody persistence and booster vaccination of a fully liquid hexavalent vaccine coadministered with measles/mumps/rubella and varicella vaccines at 15-18 months of age in healthy South African infants. Pediatr Infect Dis J. 2013;32(8):889–897. doi:10.1097/INF.0b013e318292f7b1.
- Kosalaraksa P, Chokephaibulkit K, Benjaponpitak S, Pancharoen C, Chuenkitmongkol S, B’Chir S, Da Costa X, Vidor E. Persistence of hepatitis B immune memory until 9-10 years of age following hepatitis B vaccination at birth and DTaP-IPV-HB-PRP~T vaccination at 2, 4 and 6 months. Hum Vaccin Immunother. 2018;14(5):1257–1265. doi:10.1080/21645515.2018.1426418.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet JM. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine. 2010;28(3):730–736. doi:10.1016/j.vaccine.2009.10.074.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Hum Vaccin Immunother. 2013;9(8):1679–1684. doi:10.4161/hv.24844.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Leroux-Roels G, Crasta PD, Hardt K. Persistence and immune memory to hepatitis B vaccine 20 years after primary vaccination of Thai infants, born to HBsAg and HBeAg positive mothers. Hum Vaccin Immunother. 2012;8(7):896–904. doi:10.4161/hv.19989.
- Van Der Meeren O, Behre U, Crasta P. Immunity to hepatitis B persists in adolescents 15-16 years of age vaccinated in infancy with three doses of hepatitis B vaccine. Vaccine. 2016;34(24):2745–2749. doi:10.1016/j.vaccine.2016.04.013.
- Van Der Meeren O, Bleckmann G, Crasta PD. Immune memory to hepatitis B persists in children aged 7-8 years, who were vaccinated in infancy with 4 doses of hexavalent DTPa-HBV-IPV/Hib (Infanrix hexa) vaccine. Hum Vaccin Immunother. 2014;10(6):1682–1687. doi:10.4161/hv.28480.
- WHO. Hepatitis B vaccines: WHO position paper - July 2017. Wkly Epidemiol Rec. 2017;92(27):369–392.
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–872.
