ABSTRACT
Dendritic cell (DC)-based immunotherapy is a promising strategy for the treatment of HIV-infected individuals. Different from the conventional protocol for DC differentiation based on the cytokine IL-4 (IL4-DCs), several studies have suggested obtaining DCs by culturing monocytes with type I IFN (IFN-α) to yield IFN-DCs, as performed in cancer therapy. To evaluate the phenotypic and functional characteristics, monocytes from HIV-infected subjects were differentiated into IFN-DCs or IL4-DCs, pulsed with chemically inactivated HIV and stimulated with pro-inflammatory cytokines. A comparative analysis between both types of monocyte-derived DCs (MoDCs) showed that immature IFN-DCs were phenotypically distinct from immature IL4-DCs at the baseline of differentiation, presenting a pre-activated profile. From the functional profile, we determined that IFN-DCs were capable of producing the cytokine IL-12 p70 and of inducing the production of IFN-γ by CD4 + T lymphocytes but not by TCD8+ lymphocytes. Our results suggest that IFN-DCs derived from HIV-infected individuals are able to recognize and present viral antigens to induce TCD4+ cellular immunity to HIV.
Introduction
Highly active antiretroviral therapy (HAART) has made human immunodeficiency virus (HIV) infection a chronic disease and, if treated and managed properly, could decrease morbidity and death. However, HAART is unable to eradicate HIV reservoirs and cannot completely restore the immune response.Citation1 In addition, patient adherence to HAART can be impaired by the side effects.Citation2 Accordingly, efforts have been made to reduce viral replication and improve patients’ quality of life, such as immunotherapy protocols based on autologous monocyte-derived dendritic cells (MoDCs).Citation3,Citation4
MoDCs have been used as a therapeutic vaccine because of their unique capacity to induce the primary immune response to establish immunological memory and their potent antigen-presenting ability, as well as their ability to induce, sustain and regulate the immune response.Citation5 Two major subtypes of DCs include myeloid DCs (mDCs), a professional antigen-presenting cell (APC) capable of initiating a cellular immune response, and plasmacytoid DCs (pDCs), which are related to the antiviral response by producing high levels of type I interferon (IFN), such as IFN-α.Citation6 In the context of HIV infection, there is a reduction in the absolute number of DCs, particularly in patients with active HIV-1 replication.Citation7 In addition, HIV infection promotes suboptimal maturation of DCs, resulting in poor generation of an antiviral immune response. Together, these facts suggest a key role for DC in controlling viral load. In this context, DC-based immunotherapy aims to restore DC function through the adoptive transfer of cells. In fact, in recent years, an increasing number of DC-based clinical trials have enhanced the general interest in DC biology and the interaction between this innate immune cell type and HIV-1.Citation8
MoDCs obtained through the cultivation of peripheral blood monocytes with interleukin (IL)-4 and granulocyte-macrophage colony stimulating factor (GM-CSF), referred to as IL4-DCs, have been implicated in immunotherapy for cancerCitation9,Citation10 and HIV infectionCitation3 and have been used as the gold standard in DC-based immunotherapy. Although MoDCs could provide many positive functions to immunotherapy applications, a portion of vaccinated individuals do not respond to treatment.Citation8,Citation11
Alternative protocols to obtain MoDCs include the use of IFN-α instead of IL-4 (referred to as IFN-DCs).Citation12,Citation13 Type I IFNs markedly promote the differentiation of peripheral blood monocytes into DCs. Using a murine model, studies have demonstrated that MoDCs differentiated with type I IFN are able to induce a strong cytotoxic response and cross priming of CD8 + T lymphocytes against viral or tumour antigens in both in vitro and in vivo assays.Citation14–Citation16 Additionally, IFN-DCs exhibit a combined phenotype of mDCs and pDCs associated with characteristics of natural killer (NK) cells.Citation17,Citation18
Considering the ongoing research in DC manipulation and therapeutic strategies for acquired immunodeficiency syndrome (AIDS) involving DC-based vaccines, alternative protocols to obtain DCs able to stimulate an anti-HIV immune response could improve immunotherapy.
Although many researchers have demonstrated the application of DCs obtained through IFN stimulation in cancer trials, few have used this type of DC in the HIV context to evaluate the quality of IFN-DCs derived from infected individuals. Several studies have revealed an impaired response of MoDCs from HIV-infected individuals; therefore, it is important to evaluate the feasibility of differentiating IFN-DC in this population. In addition, there is no paired comparison between IFN-DC and conventional cells in the context of HIV infection that could guide a better evaluation of the advantages and disadvantages of each type of MoDC.
In the current study, we compared several phenotypic and functional features of IFN-DCs and conventional IL4-DCs from HIV-infected individuals.
Results
IFN-DCs derived from HIV-infected individuals expressed some distinct morphological and phenotypic markers
To characterize the phenotypic profile of IFN-DCs derived from HIV-infected individuals, we evaluated the morphological aspects and expression of typical surface molecules.
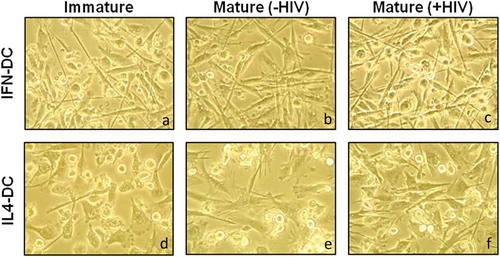
The morphological profile of immature IFN-DCs (IFN-iDCs) showed a fusiform cell body with elongated cytoplasmic processes ()) similar to that of mature IL4-DCs [IL4-mDC(-HIV)] or pulsed IL4-DCs [IL4-mDC(+HIV)] (, respectively), suggesting that IFN-iDCs are already activated even in unstimulated conditions. Similar morphological aspects were observed after activation stimulus and/or HIV pulsing (,)). However, immature IL4-DCs (IL4-iDCs) showed rounded cell bodies and short cytoplasmic processes, which are typical of immature DCs ()). After stimulation, IL4-mDC(-HIV) and IL4-mDC(+HIV) became thinner with longer processes than IL4-iDCs but not IFN-mDC(-HIV) or IFN-mDC(+HIV). A similar morphological profile was observed in MoDCs derived from healthy donors (Supplementary Figure 1).
Figure 1. Morphology of IFN-DCs and IL4-DCs derived from HIV-infected individuals. On day 7 of culture, iDC (a and d), mDC (-HIV) (b and e) and mDC (+HIV) (c and f) morphology of both IFN-DCs and IL4-DCs were analysed by inverted optical microscopy, and images were taken at x400 magnification. Images are shown from a representative HIV-infected individual from a pool of 12 individuals.
The cell viability of both types of MoDCs was evaluated by the Trypan blue exclusion test. IFN-DCs showed a survival average of 73.8% and 80%, while the average survival of IL4-DCs was approximately 81.5% and 88% in HIV-infected individuals and healthy donors, respectively. Subsequently, IFN-DCs and IL4-DCs were acquired and analysed by flow cytometry, presenting a well-defined population and elevated CD11c expression within which all other markers were evaluated ()). We found that IFN-DCs, as well as IL4-DCs, presented reduced CD14 expression (a typical monocyte marker) ()), indicating a successful differentiation process. Likewise, expression of HLA-DR, CD1a, CD86, CD40, CCR7, BDCA-2 and BDCA-4 did not present statistically significant differences between IFN-DCs and IL4-DCs ()).
Figure 2. Immunophenotypic profile of IFN-DCs in comparison to IL4-DCs derived from HIV-infected individuals. On day 7 of culture, iDCs, mDCs (-HIV) and mDCs (+HIV) of both IFN-DCs and IL4-DCs (n = 12) were harvested and labelled with monoclonal antibodies specific to the molecules CD11c, CD14, HLA-DR, CD1a, CD80, CD86, CD83, CD40, CCR7, BDCA-2, BDCA-4 and CD56. Flow cytometry analysis gating strategies performed in FlowJo software are shown in (a): a time gate was initially applied to exclude any electronic noise followed by a gate applied to select the DC population, followed by selection of CD11c-expressing cells. Finally, all the markers above were evaluated in a double-positive population (CD11c+ and FITC+ or PE+ or APC+). The gate strategy was defined based on the unlabelled cells (grey population shown in SSCxCD11c, FITCxPE-Cy5/ PE/ APC dot plots). In B, representative histograms from a pool of 12 individuals are shown. Histograms of non-stained DCs (negative control), iDCs, mDCs (-HIV) and mDCs (+HIV) are represented as dashed, opened, tinted and filled histograms. The one-way ANOVA test was used to compare the different conditions. Statistical significance was observed in the percentage (b) and median fluorescence intensity (C) of CD80, CD83 and CD56 expression. The results are expressed as the mean ± SEM (*p < 0.05, **p < 0.01, or ***p < 0.0001).
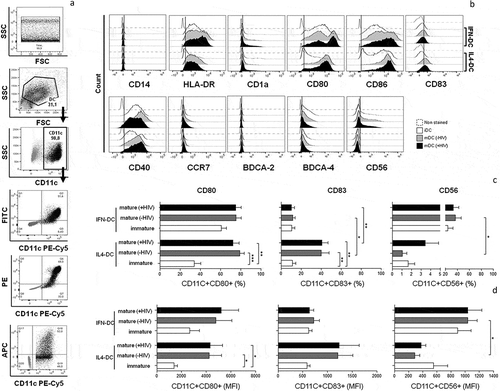
On the other hand, several differences between IFN-DCs and IL4-DCs were observed in the expression of CD80, CD83 and CD56 molecules (,) and Supplementary Figure 2C and 2D). Regarding CD80 (,), left graphs), comparable levels of expression were observed in IFN-mDC(-HIV) and IFN-mDC(+HIV), as well as in IFN-iDCs. These results were different from obtained with IL4-DCs, which showed typical low expression in the immature state and increasing levels after maturation (,), left graphs).
The expression of the DC activation marker CD83 (,), middle graphs) was low (approximately 10% in IFN-DCs), independent of maturation stage, compared to the expression in IL4-iDCs (), middle graphs). In turn, IL4-DCs presented a significant increase in CD83 expression after maturation stimulation (), middle graph).
CD56 (,), right graphs) was almost exclusively expressed by IFN-DCs. Approximately 40% of IFN-DCs presented this marker, and the levels were notably similar in all maturation stages (,), right graphs). Conversely, IL4-DCs had a very low frequency of CD56 expression (approximately 4%) and low mean fluorescence intensity (MFI) values (,), right graphs).
It is worth mentioning that the expression profile of CD80 and CD83 by IFN-DCs and IL4-DCs derived from healthy donors (Supplementary Figure 2C and 2D) was similar to that observed for HIV patients (Supplementary Figure 2C and 2D). The expression of CD56 could not be determined for healthy donor samples.
IFN-DCs induce a pro-Th1 response-promoting cytokine environment
To evaluate the IFN-DC cytokine secretion profile, we measured IL-12 p70 (Th1 promoting cytokine) and IL-10 (regulatory cytokine) levels in supernatants collected from both IFN-DCs and IL4-DCs exposed or not exposed to the pro-inflammatory stimuli ().
In general, the results presented high interindividual variability and low production of IL-12 p70 (). We observed that the IFN-iDCs derived from infected individuals were capable of produce some amount of this cytokine, whereas IFN-mDC(-HIV) showed a tendency to yield an increased production of this cytokine. In contrast, it appears that the viral pulse promoted a moderate reduction in IFN-mDC(+HIV) compared with IFN-mDC(-HIV). Similar results were observed with the healthy donor samples, but we did not observe the production of IL12p70 by IFN-iDCs (Supplementary Figure 3).
The IL-12 production levels obtained with IL4-DCs were close to or less than the limit of detection of the test, and this finding was obtained for all conditions tested. Similar results were also obtained for IL4-DCs derived from uninfected individuals.
No detectable levels of IL-10 were observed (data not shown).
IFN-DCs were capable of inducing an allogeneic T response
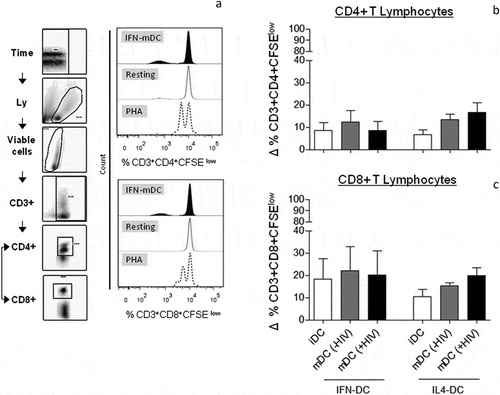
The functional ability of MoDCs to stimulate T lymphocyte proliferation was evaluated by mixed leukocyte reaction (MLR). IFN-DCs and IL4-DCs obtained from HIV-infected individuals were co-cultured for 6 days with allogeneic lymphocytes obtained from healthy donors.
Proliferating CD4+ and CD8 + T lymphocytes were considered CFSElow staining cells () and Supplementary Figure 4A). The data are presented as the delta (Δ) percentage based on the proliferation of resting lymphocytes. Phytohaemagglutinin (PHA)-stimulated lymphocytes were considered positive controls. We observed that both IFN-DCs and IL4-DCs were able to induce some CD4 + T lymphocyte proliferation, but no difference was achieved between them, independent of maturation stage or HIV pulse ()). Similar results were observed for CD8 + T lymphocyte proliferation ()), although with further high interindividual variability for IFN-DC-stimulated cultures ()). MoDCs derived from healthy donors induced T lymphocyte proliferation, similar to MoDCs derived from patients, although with little interindividual variability, and presented higher proliferation levels than MoDCs from HIV-infected patients, with no statistical significance (Supplementary Figure 3A and 3B). As HLA-DR molecules are major MLR target antigens, the potential correlation between the expression levels of HLA-DR molecules by all MoDCs studied and the proliferation levels of allogeneic lymphocytes was evaluated; however, we did not observe statistical significance (data not shown). Taken together, our results suggested that IFN-DCs are capable of inducing an allogeneic response at similar levels as IL4-DCs and that the viral pulse does not seem to interfere with this response.
Figure 3. Ability of IL-12 p70 production by IFN-DCs in comparison to IL4-DCs derived from HIV-infected individuals. On day 7 of culture, culture supernatants of iDCs, mDCs (-HIV) and mDCs (+HIV) of both IFN-DCs and IL4-DCs (n = 9) were obtained to measure IL-12 p70 levels by ELISA. The dashed line indicates the sensitivity of the test (4 pg/mL). The results are expressed as the average concentration (pg/mL) ± SEM. One-way ANOVA was used to compare the different conditions.
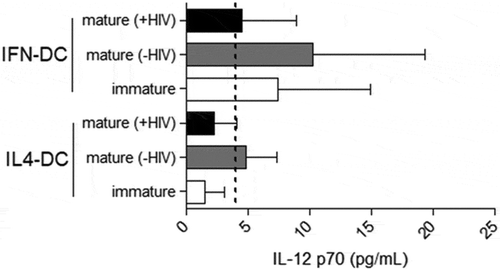
Figure 4. Ability of IFN-DCs to induce allogeneic T lymphocyte proliferation in comparison to IL4-DCs derived from HIV-infected individuals. Allogeneic T lymphocytes previously stained with 2 μM CFSE were co-cultured with iDCs, mDCs (-HIV) and mDCs (+HIV) of both IFN-DCs and IL4-DCs (n = 5). The negative and positive controls were composed of resting lymphocytes and PHA-stimulated lymphocytes, respectively. On day 6 (144 hours) of co-culture, the cells were harvested, stained with viability marker and labelled with monoclonal antibodies specific to CD3, CD4 and CD8. The frequency of proliferative cells was assessed by flow cytometry and analysed in FlowJo software (FlowJo, LLC, USA) according to the gate strategy shown in the density graphs (A, left graphs): a time gate was initially applied to exclude any electronic noise followed by a gate applied to select lymphocyte populations, followed by a viability gate to exclude any dead cells. Then, a gate was applied to select CD3-expressing cells, followed by gates selecting double-positive populations of CD3+ CD4+ cells and/or CD3+ CD8+ cells. Proliferating cells were considered CFSElow staining cells as shown in the histograms (A, right graphs). Histograms of IFN-DC-stimulated lymphocytes, resting lymphocytes and PHA-stimulated lymphocytes (positive control) are represented as filled, opened and dashed histograms, respectively (A, right graphs). The same analysis was used for IL4-DCs. The panel in (a) shows a representative HIV-infected individual from a pool of 5 individuals. The proliferation levels were assessed based on the proliferation of resting lymphocytes. Therefore, the frequency of resting lymphocyte proliferation was considered as the threshold of MoDC-stimulated lymphocyte proliferation, which is represented by delta (Δ) (b and c). The frequency of CD4+ and CD8 + T lymphocyte proliferation is shown in (b) and (c). One-way ANOVA was used to compare the different conditions. No statistical significance was observed.
IFN-DCs were able to stimulate IFN-γ production by CD4 + T lymphocytes
The ability of IFN-DCs to stimulate specific cellular immune responses was assessed by co-cultivation of these cells with autologous T lymphocytes following the evaluation of intracellular IFN-γ production by CD4+ and CD8 + T lymphocytes. To assess CD4 + T lymphocytes, we used the CD3+ CD8- T cell population. This strategy was used because phorbol myristate acetate (PMA)/ionomycin stimulation, when used as a positive control, downregulates the expression of CD4 molecules on human T cells. Background IFN-γ levels from cultures, determined in non-DC-stimulated lymphocytes, were very low and are represented as a cut-off line.
shows the results. Although the data revealed interindividual variation, we observed that compared to non-pulsed IFN-mDCs(-HIV), IFN-mDCs(+HIV) were capable of stimulating CD4 + T lymphocytes and significantly increasing IFN-γ production ()). The magnitude of the response was quite similar to that observed for IL4-DCs; however, no significant difference was observed for these cells ()).
Figure 5. Ability of IFN-DCs to induce IFN-γ production by autologous T lymphocytes in comparison to IL4-DCs obtained from HIV-infected individuals. Autologous lymphocytes were co-cultured with iDCs, mDCs (-HIV) and mDCs (+HIV) of both IFN-DCs and IL4-DCs (n = 9). The positive control was composed of PMA/ionomycin-stimulated lymphocytes. After 5 days (120 hours) of co-culture, the cells were harvested and labelled with monoclonal antibodies specific to the surface molecules CD3 and CD8 and to the cytokine IFN-γ. The frequency of IFN-γ-producing lymphocytes was assessed by flow cytometry and analysed according to the gate strategy shown in the dot plot graphs (a). The panel in (a) shows a representative HIV-infected individual from a pool of 9 individuals. The CD4 + T lymphocytes were determined as CD8- T lymphocytes. The frequency of IFN-γ-producing CD4+ and CD8 + T lymphocytes is shown in (b) and (c). The dashed line represents the average proliferation obtained in resting lymphocytes (b and c). Significant differences are shown (**p < 0.01) in (b). A t-test was used to compare the stimulating potential between mDC(HIV-) and mDC(HIV+).
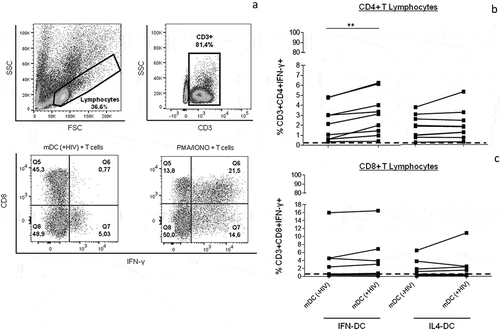
Similarly, mDC(-HIV)- and mDC(+HIV)-stimulated CD8 + T lymphocytes showed no differences in IFN-DCs and IL4-DCs ()).
Taken together, our results suggested that IFN-DCs were able to stimulate a specific CD4 + T cell response but not a CD8 + T cell response.
Discussion
MoDC-based vaccines for treating HIV-infected individuals have been investigated over the past decade, with limited efficacy of clinical results. Accordingly, attempts to improve current MoDC-based protocols are desirable. In general, protocols have used MoDCs differentiated with IL-4 and GM-CSF and activated with inflammatory cytokines (gold standard MoDCs). Alternatively, type I IFN has been shown to be a potent stimulus to differentiate or activate MoDCs, as demonstrated in both in vitro and clinical protocols for cancer treatment.Citation19–Citation25
In the HIV context, few studies have evaluated the potential of MoDCs differentiated with IFNs inducing an anti-HIV immune response.Citation26–Citation28 Thus, in this study, we focused our attention on evaluating the phenotypic and functional profiles of monocyte-derived IFN-DCs from HIV-infected individuals compared to IL-4-differentiated DCs. We found that both shared typical DC characteristics, such as the lack of CD14 expression and high levels of HLA-DR, CD86 and CD40 molecules, which play an important role in antigen presentation. The expression of CD1a and CCR7 was low in both. However, a distinct profile was observed for CD80, CD83 and CD56 expression. In particular, IFN-DCs presented low expression of the activation marker CD83, even after exposure to maturation stimuli, suggesting that the presence of IFN-α could have interfered with the expression of this marker. However, high levels of CD80 were observed at all differentiation/maturation stages, suggesting that these cells presented a pre-activated profile. A morphological analysis corroborated these data: the immature-stage IFN-iDCs were quite similar to the mature-stage IL4-mDCs. Additionally, CD56 was found to be an almost exclusive marker of IFN-DCs.
It is worth mentioning that comparisons of DCs derived from infected patients and healthy donors did not show any significant difference when different molecules were analysed (Supplementary data).
Based on the phenotypic profile, our data suggest that it is feasible to differentiate monocytes in IFN-DCs from HIV-infected individuals, as already demonstrated for IL4-DCs.
In the functional analysis, we evaluated the ability of IFN-DCs to produce IL-12 p70 and IL-10 and found that IFN-DCs tended to produce some amount of IL-12, whereas IL4-DCs yielded values close to the minimum limit of detection. Additionally, the production of IL-12 p70 by IFN-iDCs, even at lower levels, somehow corroborated the pre-activated profile suggested by the phenotypic analysis. No IL-10 levels were detected in our assays.
It is worth mentioning that our data presented high variability; thus, the results should be interpreted with caution. This variability could be due to the monocyte isolation method used (plastic adherence), the number of monocytic precursors, and the final number of MoDCs that produce cytokines, which might vary among individuals. To minimize this bias, all cell plating and washing procedures were carefully standardized, but the individual monocyte adhesion capacity could affect the results.
The IL-12 p70 subunit is secreted by APCs during antigen presentation and induces IFN-γ production by T and NK cells. In HIV infection, HIV-specific Th1 cells can produce high levels of IFN-γ and can induce an HIV-specific CTL response. In contrast, IL-10 is recognized for its ability to inhibit the activation and effector functions of T cells, monocytes, macrophages, and DCs. Although IL-12 p70 was secreted, even at lower levels, by IFN-DCs, IL-10 was not detectable in the culture supernatants from either IFN-DC or IL4-DC cultures (the levels were below the detection limit of the kit, and the data are thus not shown), contributing to a pro-inflammatory Th1-driven microenvironment. It has been suggested that higher ratios of IL-12:IL-10 levels reflect the functional potency of DCs that could be related to reactivation of latent HIV.Citation28 This subject is a timely and important issue, since activation of latent proviruses could be considered the first step to eliminating HIV reservoirs, which in turn can promote a cure for HIV infection.
The functional profile of IFN-DCs was also evaluated to check its ability to stimulate a cellular immune response based on allogeneic T lymphocyte proliferation and IFN-γ production by autologous T lymphocytes.
In the MLR assessment, contrary to our expectations, we observed a low allogeneic T cell stimulation capacity, even with mature MoDCs, which presented stimulation levels comparable to those of immature MoDCs. We can speculate that the lack of prostaglandin E2 in the activation cocktail used for both MoDCs cultures could have affected this response.Citation29,Citation30 Additionally, because HLA-DR molecules are major MLR target antigens, we expected the allogeneic response levels induced by mature MoDCs to be higher because these cells express higher levels of HLA-DR molecules; however, we did not find a statistically significant difference between immature and mature MoDCs. Additionally, no significant correlation was observed between HLA-DR expression in IFN-DCs or IL4-DCs and lymphoproliferation levels. In general, we observed that IFN-DCs presented similar allogeneic ability to IL4-DCs. Additionally, the HIV pulse did not interfere with this response. We are aware that this result should be interpreted with caution because only a few samples (n = 5) were analysed.
Concerning the HIV-specific response to DC stimuli, we found that IFN-DCs were able to induce specific IFN-γ production at variable intensities and numbers of patients, suggesting that these cells were capable of effectively processing and presenting HIV antigens to autologous lymphocytes, at least in some patients. The ability of IFN-DCs to produce IL-12 over IL-10 corroborates these data.
Curiously, some patients presented higher levels of IFN-γ-producing lymphocytes stimulated by non-pulsed DCs. In this sense, it is worth mentioning that DCs and their monocytic precursors are reservoirs for HIV and can process and present their antigens. As these cells were derived from HIV-infected subjects, endogenously produced HIV by DC/monocytes could potentially induce IFN-γ production by lymphocytes, even if these DCs were not pulsed with additional HIV [non-pulsed DCs – mDC(-HIV)]. Considering this context, potential stimuli promoted by DCs could have been masked by this high “basal” condition. Unfortunately, we were not able to measure the cell virus titre to assess the basal contribution of endogenous virus to the lymphocyte response, which would be an interesting approach.
In particular, we found that IFN-mDCs(+HIV) could stimulate specific IFN-γ production by CD4 + T lymphocytes, as observed by the significant difference between basal and stimulated conditions. In fact, an HIV-1-specific CD4+ helper T cell response is known to be crucial to achieving a sustained and effective HIV-1-specific CD8+ CTL response able to control HIV-1 replication.Citation31–Citation33 In HIV infection, DCs can produce IL-12, which induces CD4 + T lymphocyte polarization to Th1, which in turn is able to induce IFN-γ production as well as activate CD8 + T lymphocytes, the CTLs.
However, contrary to our expectations, we did not observe an increasing frequency of IFN-γ+ CD8 + T lymphocytes in cultures stimulated by IFN-mDCs(+HIV). Previous studies showed that IFN-DCs are able to augment antigen-specific CD8 + T lymphocytes. Lapenta et al.Citation15 observed that immunization of hu-PBL-SCID mice with IFN-DCs pulsed with AT-2-HIV could promote an increased frequency of IFN-γ and granzyme production in HIV-1-specific CD8 + T lymphocytes. We speculate that the time of co-culture could be insufficient to improve IFN-γ production by CD8 + T cells, which is dependent on CD4 + T cell activation and cytokine production. Another possibility is that IFN-DCs cannot improve the production of IFN-γ by CD8 + T lymphocytes. In contrast, we should also consider the phenotypic pre-activated profile of IFN-DC promoted by type I IFN, which could interfere with the functionality of these cells because they were derived from a previously activated environment (from HIV-infected individuals).
In this context, considering the chronic activation state of the immune system observed in HIV patients, the time spent to differentiate IFN-DC in this activated environment may have been sufficiently long that it induced an exhausting effect after 7 days of culture.Citation14,Citation34–Citation37 In fact, we observed some differences in the viability of the cells.
Cancer trials have demonstrated the potential of type I IFN to induce IFN-DCs capable of stimulating a strong CD8 + T response, which is desirable in the context of HIV, and thus several alterations could be made. Protocols that require 3 days to obtain DCs could be one alternative to overcome this limitation in time to differentiation.Citation15,Citation16,Citation38–Citation40 Another possibility could be to use type I IFN to activate rather than to differentiate DCs. This could prevent a pre-activated profile during differentiation and improve the activation profile in the last hours of obtaining DCs.
Our study has some limitations regarding the small sample size and high data variability, which may have made it difficult to interpret some of the results. Additionally, the potential contribution of endogenous virus to our co-culture assays, which could potentially interfere with our specific immune response data, remains to be determined.
Finally, in this study, we demonstrated that IFN-DCs derived from HIV-infected individuals display a mature phenotypic profile, one able to perform antigenic presentation and predominant Th1 cytokine production. These cells were able to stimulate HIV-specific autologous CD4+ but not CD8 + T lymphocytes.
Conclusions
Compared to IL4-DCs, IFN-DCs derived from HIV-infected individuals present several distinct phenotypic features and similar allostimulatory profiles. Additionally, IFN-DCs were able to prime specific autologous CD4 + T lymphocytes to produce IFN-γ in response to HIV stimulation.
Materials and methods
Human subjects
HIV-1-infected, HAART naive subjects (n = 12) were recruited at the Hospital das Clínicas (University of São Paulo, Brazil). The characteristics of the patients are described in . All patients had been infected with HIV for more than 5 years and did not manifest AIDS-specific clinical symptoms. Written informed consent was obtained according to the protocols of the Clinics Hospital Ethical Committee (CAPPesq) (Sao Paulo, Brazil) under approval protocol number 0756/11 and the Commission on Ethics with Human Research of Institute of Biomedical Sciences (ICB-USP; Sao Paulo, Brazil) under reference number 412.005. All participants who agreed to participate in the study gave informed consent at the time of recruitment into the study.
Table 1. Baseline characteristics of patients.
Autologous virus production and chemical inactivation
Virus isolation and expansion were performed according to a previously described protocol.Citation41 Briefly, peripheral blood mononuclear cells (PBMCs) from infected individuals were cultured with 20 IU/mL IL-2 (Roche, Baden-Württemberg, GE) in RPMI-1640® medium (Gibco, NY, USA) supplemented with 10% fetal bovine serum (Gibco, NY, USA). Cells were fed every 7 days with 2 × 107 PBMCs obtained from healthy donors previously activated with 1% PHA (Gibco, NY, USA) and 20 IU/mL of IL-2 for 48 hours. A portion of the supernatant samples was separated to monitor virus production using HIV-1 p24 antigen capture immunoassay (ZeptoMetrix Corporation, NY, USA), and the rest of the supernatant was frozen. Positive cultures were expanded through weekly feeding with allogeneic PBMCs.
Supernatants from the virus culture were thawed and inactivated using 1 mM aldrithiol-2 (AT-2; Sigma-Aldrich, MO, USA), as described by Rossio et al.,Citation42 and concentrated through Centricon® Millipore filters (100 kDa; Millipore, Co. Cork, IR). AT-2-inactivated virus (AT-HIV) was finally ultracentrifuged on a 20% sucrose-Tris NaCl EDTA/TNE solution for virus purification. The virus titre was measured by Versant® HIV-1 RNA 3.0 Assay using a System 340 bDNA Analyser (Siemens, BY, GE) and expressed as particles/mL (prt/mL).
Generation and activation of monocyte-derived dendritic cells (MoDCs)
PBMCs isolated by centrifugation over a Ficoll-Paque® (GE Healthcare, Uppsala, SE) gradient were cultured at a concentration of 5 × 106 cells/mL in RPMI-1640® medium (Gibco) and incubated on a plastic surface for approximately 2 hours to obtain adherent cells (monocytes). Lymphocytes (non-adherent cells) were removed and cryopreserved using FBS containing 10% dimethylsulfoxide (DMSO; Labsynth, SP, BR). Monocytes were cultured in AIM-V® medium (Gibco, NY, USA) containing 50 ng/mL GM-CSF (PeproTech, NJ, USA) and 1,000 UI/mL IFN-α (Schering-Plough, NJ, EUA) or 50 ng/mL IL-4 (PeproTech) (for IFN-DC and IL4-DC, respectively) and incubated for 5 days at 37 °C and 5% CO2 atmosphere to obtain IFN-iDCs and IL4-iDCs, respectively. All of these cytokines were replenished after 5 days at the same concentration.
After differentiation, these cells were pulsed by further exposure to 1 × 108 AT-2-inactivated HIV-1 particles in each well for 2 hours followed by stimulus with a cocktail of pro-inflammatory cytokines (50 ng/mL TNF-α, 10 ng/mL IL-1β and 100 ng/mL IL-6; PeproTech) for an additional 48 hours to obtain pulsed DCs, called IFN-mDC(+HIV) or IL4-mDC(+HIV). As a control, a portion of iDCs were treated only with a pro-inflammatory cocktail (without HIV pulse) and were defined as IFN-mDC(-HIV) or IL4-mDC(-HIV).
Cytokine measurement by ELISA
Supernatants were harvested from immature, mature (-HIV) and mature (+HIV) IFN-DCs and IL4-DCs and were analysed by ELISA for IL-12 p70 (Human IL-12p70 ELISA Ready-SET Go! Kit®, eBioscience, San Diego, CA, USA) and IL-10 (Human IL-10 Quantikine ELISA Kit®, R&D systems, MN, USA) according to the manufacturer’s instructions. The detection limits of the IL-12 p70 and IL-10 ELISA kits were 4– 500 pg/mL and 31– 2,000 pg/mL, respectively.
Mixed leukocyte reaction
To evaluate the ability of MoDCs to stimulate T lymphocyte proliferation, IFN-DCs and IL4-DCs obtained from HIV-infected individuals were co-cultured with allogeneic lymphocytes, which were obtained from PBMCs by plastic adherence using non-adherent cells. Lymphocytes were labelled with 2 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen, CA, USA) according to the manufacturer’s instructions and plated at 2 × 105 per well in AIM-V supplemented with 10% AB-Serum (Sigma-Aldrich) in a 96-well flat-bottom plate. Immature, mature (-HIV) or mature (+HIV) IFN-DCs or IL4-DCs were added to the wells at a MoDC/lymphocyte ratio of 1:5. The proliferation of CD4+ and CD8 + T lymphocytes was analysed using flow cytometry after 6 days (144 hours). PHA (1%)-stimulated lymphocytes (for 72 hours) were used as a positive control. Resting lymphocytes were used as a negative control, and their proliferation rate was subtracted from that of the other conditions to generate delta (Δ) data.
On day 6 (144 hours) of co-culture, lymphocytes were harvested and stained for 30 minutes using the LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Life Technologies, Eugene, OR, USA) to determine the cell viability. After washing, lymphocytes were stained for 20 minutes with anti-CD3 PE-Cy5 (UCHT1), anti-CD4 PE (RPA-T4) and anti-CD8 APC-H7 (SK1) monoclonal antibodies (BD Bioscience, CA, USA). At least 60,000 lymphocytes were acquired using an LSRFortessa (BD Bioscience) flow cytometer. Analysis was performed using FlowJo 7.6.5 software.
Co-culture for HIV-specific T lymphocyte induction
To evaluate the ability of MoDCs to stimulate HIV-specific Th1 and CTL responses, IFN-DCs and IL4-DCs obtained from HIV-infected individuals were co-cultured with autologous lymphocytes to assess IFN-γ intracellular production. Lymphocytes were thawed at 37 °C and rested in RPMI-1640® medium (Gibco) supplemented with 10% FBS (Gibco) a day before the beginning of co-cultivation. For the co-culture, lymphocytes were plated at 2 × 105 per well in AIM-V supplemented with 10% AB-Serum (Sigma-Aldrich) in a 96-well U-bottom plate to optimize the interaction between DCs and specific lymphocytes, which is relatively rare among total lymphocytes. Immature, mature (-HIV) and mature (+HIV) IFN-DCs or IL4-DCs were added to the wells at a MoDC/lymphocyte ratio of 1:5. The HIV-specific Th1 and CTL responses were analysed using flow cytometry after 5 days (120 hours). Non-stimulated lymphocytes were used as a negative control. The positive control was composed of PMA (2 µg/Ml; Sigma-Aldrich) and ionomycin (10 µg/mL; Sigma-Aldrich)-stimulated lymphocytes for 25 hours. The stop Golgi complex brefeldin A (10 µg/mL; Sigma-Aldrich) was used for the last 24 hours. On day 5 (120 hours) of co-culture, lymphocytes were harvested and stained with anti-CD3 PE-Cy5 (UCHT1) and anti-CD8 APC-H7 (SK1) monoclonal antibodies followed by anti-IFN-γ PE (MHCIFG043) (BD Bioscience, CA, USA). BD Cytofix/Cytoperm® Fixation and Permeabilization Solution (BD Cytofix/Cytoperm Plus Kit, BD Bioscience) reagents were used for intracellular staining. At least 60,000 lymphocytes were acquired using an LSRFortessa (BD Bioscience) flow cytometer. Analysis was performed with FlowJo 7.6.5 software.
Flow cytometry
Anti-human CD11c PE-Cy5 (B-ly6), HLA-DR FITC (G46-6), CD1a PE (HI149), CD86 FITC (2331/FUN-1), CD80 PE (L307.4), CD83 FITC (HB15e), CD40 FITC (5C3), CCR7 PE (3D12), CD14 PE (M5E2) (BD Bioscience), BDCA-2 FITC (AC144), BDCA-4 PE (AD5-17F6) and CD56 APC (AF12-7H3) (Miltenyi Biotec) and anti-human CD3 PE-Cy5 (UCHT1) and CD8 APC-H7 (SK1) (BD Bioscience) mAb were used. Anti-IFN-γ PE (MHCIFG043) (BD Bioscience), BD Cytofix/Cytoperm® Fixation and Permeabilization Solution (BD Cytofix/Cytoperm Plus Kit, BD Bioscience), and brefeldin A (Sigma-Aldrich) reagents were used for intracellular staining. Viability was determined using the LIVE/DEAD® Fixable Aqua Dead Cell Stain Kit (Life Technologies) following the manufacturer’s instructions. Cells were acquired using an LSRFortessa flow cytometer (BD Bioscience) and analysed using FlowJo 7.6.5 (FlowJo, LLC; Ashland, OR, USA).
Statistics
Statistical significance (p-value) was calculated using one-way ANOVA with a non-parametric Kruskal-Wallis test to compare different groups. The differences were considered statistically significant when the p obtained was < 0.05. All analyses and graphical representations were performed using the GraphPad Prism v.5 programme (GraphPad® Software Inc., USA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
BTS and TMO conceived and designed the experiments and drafted the manuscript; BTS, DSR, LTS and NTR performed the experiments, data analysis and interpretation, and TMO and ADJS provided intellectual guidance during the project.
Supplemental Material
Download MS Word (1.5 MB)Acknowledgments
The authors thank all study participants, Dr Sadia Samer for critical comments, and Dr Alexandre de Almeida, Dr Jorge Casseb and Mariana Monteiro for patient recruitment.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Kumari G, Singh RK. Anti-HIV drug development: structural features and limitations of present day drugs and future challenges in the successful HIV/AIDS treatment. Curr Pharm Des. 2013;19(10):1767–1783. PubMed PMID: WOS:000316455200002.
- Murphy DA, Roberts KJ, Martin DJ, Marelich W, Hoffman D. Barriers to antiretroviral adherence among HIV-infected adults. AIDS Patient Care STDS. 2000;14(1):47–58. doi:10.1089/108729100318127. PubMed PMID: WOS:000085014300007.
- Garcia F, Plana M, Climent N, Leon A, Gatell JM, Gallart T. Dendritic cell based vaccines for HIV infection The way ahead. Hum Vaccin Immunother. 2013;9(11):2445–2452. doi:10.4161/hv.25876. PubMed PMID: WOS:000330382300029.
- Garcia F, Routy JP. Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection Workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine. 2011;29(38):6454–6463. doi:10.1016/j.vaccine.2011.07.043. PubMed PMID: WOS:000295148800009.
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YT, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi:10.1146/annurev.immunol.18.1.767. PubMed PMID: WOS:000087236500026.
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–1837. doi:10.1126/science.284.5421.1835. PubMed PMID: WOS:000080809000050.
- Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187(1):26–37. doi:10.1086/345957. PubMed PMID: WOS:000179809900005.
- Coelho AVC, de Moura RR, Kamada AJ, da Silva RC, Guimaraes RL, Brandao LAC, de Alencar LCA, Crovella S. Dendritic cell-based immunotherapies to fight HIV: how far from a success story? A systematic review and meta-analysis. Int J Mol Sci. 2016;17(12):1985. doi:10.3390/ijms17121985. PubMed PMID: WOS:000392280500029.
- Shang N, Figini M, Shangguan J, Wang B, Sun C, Pan L, Ma QH, Zhang ZL. Dendritic cells based immunotherapy. Am J Cancer Res. 2017;7(10):2091–2102. PubMed PMID: WOS:000414291400010.
- Constantino J, Gomes C, Falcao A, Neves BM, Cruz MT. Dendritic cell-based immunotherapy: a basic review and recent advances. Immunol Res. 2017;65(4):798–810. doi:10.1007/s12026-017-8931-1. PubMed PMID: WOS:000407325000004.
- Leal L, Lucero C, Gatell JM, Gallart T, Plana M, Garcia F. New challenges in therapeutic vaccines against HIV infection. Expert Rev Vaccines. 2017;16(6):587–600. doi:10.1080/14760584.2017.1322513. PubMed PMID: WOS:000401437800007.
- Della Bella S, Nicola S, Riva A, Biasin M, Clerici M, Villa ML. Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon-alpha. J Leukoc Biol. 2004;75(1):106–116. doi:10.1189/jlb.0403154. PubMed PMID: WOS:000188741200014.
- Paquette RL, Hsu NC, Kiertscher SM, Park AN, Tran L, Roth MD, Glaspy JA. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 1998;64(3):358–367. PubMed PMID: WOS:000075745100011.
- Lapenta C, Santini SM, Logozzi M, Spada M, Andreotti M, Di Pucchio T, Parlato S, Belardelli F. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J Exp Med. 2003;198(2):361–367. doi:10.1081/jem.20021924. PubMed PMID: WOS:000184368200018.
- Lapenta C, Santini SM, Spada M, Donati S, Urbani F, Accapezzato D, Franceschini D, Andreotti M, Barnaba V, Belardelli F. IFN-alpha-conditioned dendritic cells are highly efficient in inducing cross-priming CD8(+) T cells against exogenous viral antigens. Eur J Immunol. 2006;36(8):2046–2060. doi:10.1002/eji.200535579. PubMed PMID: WOS:000239855900006.
- Parlato S, Romagnoli G, Spadaro F, Canini I, Sirabella P, Borghi P, Ramoni C, Filesi I, Biocca S, Gabriele L, et al. LOX-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood. 2010;115(8):1554–1563. doi:10.1182/blood-2009-07-234468. PubMed PMID: WOS:000274974200014.
- Korthals M, Safaian N, Kronenwett R, Maihofer D, Schott M, Papewalis C, Blanco ED, Winter M, Czibere A, Haas R, et al. Monocyte derived dendritic cells generated by IFN-alpha acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J Transl Med. 2007;5:46. doi:10.1186/1479-5876-5-46. PubMed PMID: WOS:000250873000001.
- Farkas A, Kemeny L. Interferon-alpha in the generation of monocyte-derived dendritic cells: recent advances and implications for dermatology. Br J Dermatol. 2011;165(2):247–254. doi:10.1111/j.1365-2133.2011.10301.x. PubMed PMID: WOS:000292926900006.
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: A novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64(17):5934–5937. doi:10.1158/0008-5472.can-04-1261. PubMed PMID: WOS:000223603200005.
- Hansen M, Hjorto GM, Donia M, Met O, Larsen NB, Andersen MH, Straten PT, Svane IM. Comparison of clinical grade type I polarized and standard matured dendritic cells for cancer immunotherapy. Vaccine. 2013;31(4):639–646. doi:10.1016/j.vaccine.2012.11.053. PubMed PMID: WOS:000314625900010.
- Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, et al. Induction of CD8(+) T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with alpha-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant Glioma. J Clin Oncol. 2011;29(3):330–336. doi:10.1200/jco.2010.30.7744. PubMed PMID: WOS:000286319000024.
- Rozera C, Cappellini GA, D’Agostino G, Santodonato L, Castiello L, Urbani F, Macchia I, Arico E, Casorelli I, Sestili P, et al. Intratumoral injection of IFN-alpha dendritic cells after dacarbazine activates anti-tumor immunity: results from a phase I trial in advanced melanoma. J Transl Med. 2015;13. doi:10.1186/s12967-015-0473-5. PubMed PMID: WOS:000369795900001.
- Rizza P, Moretti F, Capone I, Belardelli F. Role of type I interferon in inducing a protective immune response: perspectives for clinical applications. Cytokine Growth Factor Rev. 2015;26(2):195–201. doi:10.1016/j.cytogfr.2014.10.002. PubMed PMID: WOS:000353730700012.
- Hwang EC, Il Jung S, Lee HJ, Lee JJ, Kwon DD. Generation of potent cytotoxic T lymphocytes against in male patients with non-muscle invasive bladder cancer by dendritic cells loaded with dying T24 bladder cancer cells. Int Braz J Urol. 2017;43(4):615–627. doi:10.1590/s1677-5538.ibju.2016.0274. PubMed PMID: WOS:000407352000007.
- Kim YH, Tran TAT, Lee HJ, Jung SI, Lee JJ, Jang WY, Moon KS, Kim IY, Jung S, Jung TY. Branched multipeptide immunotherapy for glioblastoma using human leukocyte antigen-A*0201-restricted cytotoxic T-lymphocyte epitopes from ERBB2, BIRC5 and CD99. Oncotarget. 2016;7(31):50535–50547. doi:10.18632/oncotarget.10495. PubMed PMID: WOS:000385422000130.
- Levy Y, Thiebaut R, Montes M, Lacabaratz C, Sloan L, King B, Perusat S, Harrod C, Cobb A, Roberts LK, et al. Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load. Eur J Immunol. 2014;44(9):2802–2810. doi:10.1002/eji.201344433. PubMed PMID: WOS:000342818900026.
- Smith KN, Mailliard RB, Piazza PA, Fischer W, Korber BT, Fecek RJ, Ratner D, Gupta P, Mullins JI, Rinaldo CR. Effective cytotoxic T lymphocyte targeting of persistent HIV-1 during antiretroviral therapy requires priming of naive CD8(+) T cells (vol 7, e00473, 2016). MBio. 2016;7(4). doi:10.1128/mBio.01012-16. PubMed PMID: WOS:000383440500049.
- Macatangay BJC, Riddler SA, Wheeler ND, Spindler J, Lawani M, Hong F, Buffo MJ, Whiteside TL, Kearney MF, Mellors JW, et al. Therapeutic vaccination with dendritic cells loaded with autologous HIV type 1-Infected apoptotic cells. J Infect Dis. 2016;213(9):1400–1409. doi:10.1093/infdis/jiv582. PubMed PMID: WOS:000376295800007.
- Krause P, Bruckner M, Uermosi C, Singer E, Groettrup M, Legler DF. Prostaglandin E-2 enhances T-cell proliferation by inducing the costimulatory molecules OX40L, CD70, and 4-1BBL on dendritic cells. Blood. 2009;113(11):2451–2460. doi:10.1182/blood-2008-05-157123. PubMed PMID: WOS:000264110600013.
- Rubio MT, Means TK, Chakraverty R, Shaffer J, Fudaba Y, Chittenden M, Luster AD, Sykes M. Maturation of human monocyte-derived dendritic cells (MoDCs) in the presence of prostaglandin E-2 optimizes CD4 and CD8 T cell-mediated responses to protein antigens: role of PGE(2) in chemokine and cytokine expression by MoDCs. Int Immunol. 2005;17(12):1561–1572. doi:10.1093/intimm/dxh335. PubMed PMID: WOS:000233669100005.
- Letvin NL, Walker BD. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat Med. 2003;9(7):861–866. doi:10.1038/nm0703-861. PubMed PMID: WOS:000183979300025.
- Walker B, McMichael A. The T-Cell response to HIV. Cold Spring Harb Perspect Med. 2012;2(11):a007054–a007054. doi:10.1101/cshperspect.a007054. PubMed PMID: WOS:000314282300014.
- Porichis F, Kaufmann DE. HIV-specific CD4 T cells and immune control of viral replication. Curr Opin HIV AIDS. 2011;6(3):174–180. doi:10.1097/COH.0b013e3283454058. PubMed PMID: WOS:000295513000006.
- Kodama A, Tanaka R, Saito M, Ansari AA, Tanaka Y. A novel and simple method for generation of human dendritic cells from unfractionated peripheral blood mononuclear cells within 2 days: its application for induction of HIV-1-reactive CD4(+) T cells in the hu-PBL SCID mice. Front Microbiol. 2013;4. doi:10.3389/fmicb.2013.00292. PubMed PMID: WOS:000331444100001.
- Xu SW, Koski GK, Faries M, Bedrosian I, Mick R, Maeurer M, Cheever MA, Cohen PA, Czerniecki BJ. Rapid high efficiency sensitization of CD8(+) T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171(5):2251–2261. doi:10.4049/jimmunol.171.5.2251. PubMed PMID: WOS:000184970900010.
- Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A. Mature dendritic cells derived from human monocytes within 48 hours: A novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170(8):4069–4076. doi:10.4049/jimmunol.170.8.4069. PubMed PMID: WOS:000182171100017.
- Czerniecki BJ, Koski GK, Koldovsky U, Xu SW, Cohen PA, Mick R, Nisenbaum H, Pasha T, Xu M, Fox KR, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67(4):1842–1852. doi:10.1158/0008-5472.can-06-4038. PubMed PMID: WOS:000244289200053.
- Tyrinova TV, Leplina OY, Tikhonova MA, Mishinov SV, Stupak VV, Ostanin AA, Chernykh ER. CCL19/CCL21-Dependent chemotaxis of dendritic cells in healthy individuals and patients with brain tumors. Bull Exp Biol Med. 2015;158(6):785–788. doi:10.1007/s10517-015-2862-4. PubMed PMID: WOS:000354066800021.
- Spadaro F, Lapenta C, Donati S, Abalsamo L, Barnaba V, Belardelli F, Santini SM, Ferrantini M. IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119(6):1407–1417. doi:10.1182/blood-2011-06-363564. PubMed PMID: WOS:000300420900017.
- Leplina OY, Tyrinova TV, Tikhonova MA, Ostanin AA, Chernykh ER. Interferon alpha induces generation of semi-mature dendritic cells with high pro-inflammatory and cytotoxic potential. Cytokine. 2015;71(1):1–7. doi:10.1016/j.cyto.2014.07.258. PubMed PMID: WOS:000347584600001.
- da Silva LT, Pontillo A, da Silva WC, de Almeida A, Duarte AJD, Oshiro TM. Autologous and allogenic systems of HIV expansion: what is the better choice for clinical application in therapeutic vaccine? Immunotherapy. 2013;5(12):1305–1311. doi:10.2217/imt.13.136. PubMed PMID: WOS:000327426800012.
- Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72(10):7992–8001. PubMed PMID: WOS:000075864100033.
