ABSTRACT
We previously found that Pleurotus ferulae polysaccharides (PFPS) improved the maturation and function of dendritic cells (DCs). In this study, we investigated the effects of PFPS on the antitumor efficacy of therapeutic human papillomavirus (HPV) DC-based vaccine. PFPS stimulated DCs pulsed with HPV E6/E7 peptides were used to treat tumor mice on day 5 & 12 (HPV + PFPS-DCs early) and day 12 & 19 (HPV + PFPS-DCs late) after TC-1 cell injection. Compared to control group, both HPV + PFPS-DCs early and HPV + PFPS-DCs late strategies significantly inhibited tumor growth, which was significantly correlated with the increased activation status of both CD4+ and CD8+ T cells, the decreased frequencies of myeloid-derived suppressor cells, and the induction of HPV-specific CD8+ T cell responses. The survival of tumor mice was also greatly improved by HPV + PFPS-DCs early. Moreover, HPV + PFPS-DCs early completely inhibited the growth of second challenged TC-1 cells in tumor free mice. The results showed that PFPS improved the antitumor efficacy of therapeutic HPV DC-based vaccine, suggesting that PFPS might be a potential adjuvant for DC-based vaccines. This study provides a potential strategy for developing the therapeutic DC-based vaccine against cervical cancer.
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cells, which capture, process and present antigens to naïve T cells to generate antigen-specific immune responses. During antigen presentation, various signals including MHC-peptide complex, the interaction of co-stimulatory molecules between DCs and T cells and cytokines secreted by DCs were involved in the activation of antigen-specific T cells.Citation1 IL-12 facilitates the generation of CD4+ Th1 cells and cytotoxic T lymphocytes,Citation2–Citation4 which play pivotal roles in the treatment of tumors. DC-based vaccines are hopeful platform for the treatment of cancers due to the capacity of DCs. Antigen-specific immune responses induced by DC-based vaccines in clinical trials have been frequently observed.Citation5–Citation9 However, the antitumor efficacy of DC-based vaccines needs to be improved due to the suboptimal maturation of DCs with low level of IL-12.Citation7,Citation10
Adjuvants such as toll-like receptor agonists have been used to improve the immunogenicity of various new-types of vaccines, such as peptide/protein vaccines, DNA vaccines and DC-based vaccines.Citation11,Citation12 However, almost of adjuvants cannot be used for human vaccines due to their side effect. Only a few of adjuvants including aluminum salts, MF59, AS03, AF03 and AS04 have been licensed for human vaccines in the US and/or Europe.Citation11 Therefore, the development of safe and effective adjuvants for human use has been drawn the attentions from scientists.
Traditional Chinese medicine (TCM) has a long history in the treatment of human diseases. Accumulating evidence shows that TCM and its components have immunoregulatory effects, especially in the regulation of DC maturation.Citation13-Citation15 Therefore, TCM is a good candidate pool to search adjuvants. Pleurotus ferulae is a kind of TCM and has various biological activities such as anti-oxidant, anti-tumor, and anti-microbial.Citation16-Citation18 Our previous studies demonstrated that P. ferulae water extract promoted the maturation and IL-12 secretion of DCs and improved the antitumor effect of human papillomavirus (HPV) DC-based vaccine.Citation19,Citation20 Recently, P. ferulae polysaccharides (PFPS) were purified and characterized, which promoted DC maturation and cytokine production through TLR-4 signaling pathway.Citation21 Here, we investigated the effects of PFPS on the antitumor efficacy of HPV therapeutic DC-based vaccine in tumor mouse model induced by TC-1 cells.
Results
PFPS treated DCs pulsed with HPV peptides (HPV + PFPS + DCs) inhibited tumor growth
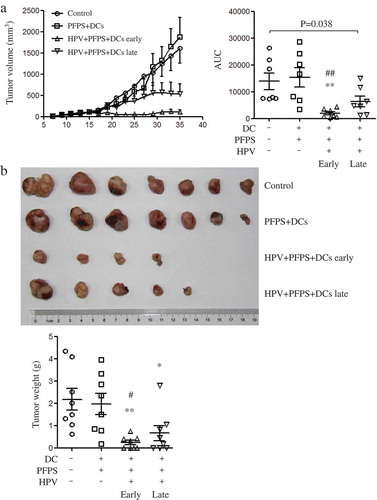
TC-1 tumor mouse model was used to detect the antitumor effect of HPV + PFPS + DCs. Based on our previous studies,Citation20,Citation22,Citation23 tumor volumes can reach 2000 mm3 in tumor mice around 30 days after TC-1 cell injection. Therefore, the one-week interval immunization was chosen. According to the time points of HPV + PFPS + DCs treatments, the strategies were designed as early therapy (HPV + PFPS+ DCs early) and late therapy (HPV + PFPS+ DCs late), in which tumor mice were treated with HPV + PFPS+ DCs on day 5 & 12 and day 12 & 19 after TC-1 cell injection, respectively. Tumor mice without treatment or injected with PFPS + DCs were named as control or PFPS + DCs, respectively. One mouse (1/8) with 3097 mm3 tumor was dead on day 27 in control group. One mouse (1/8) with 242 mm3 tumor was dead on day 17 in PFPS + DCs group. One mouse (1/8) with 1742 mm3 tumor was dead on day 31, one mouse was free of tumor and another one had very small tumor (6.8 mm3) in HPV + PFPS + DCs late group at the end of this experiment. The tumors of the three dead mice were isolated and weighted before the end of this experiment. All mice (8/8) were live and 4 mice were free of tumor in HPV + PFPS+ DCs early group at the end of this experiment. Compared to control and PFPS + DCs groups, HPV + PFPS + DCs early greatly inhibited tumor growth. HPV + PFPS+ DCs late also significantly suppressed tumor growth (). At the end of the experiment, tumor mice were sacrificed and tumors were isolated to take photo and weight (). Tumors were obtained from 7 mice in control group, 7 mice in PFPS + DCs group, 4 mice in HPV + PFPS + DCs early group and 5 mice in HPV + PFPS + DCs late group. Tumors in both HPV + PFPS + DCs early and late groups were smaller than that in control and PFPS + DCs groups. Tumor weight was also significantly decreased in both HPV + PFPS + DCs early and late groups.
Figure 1. Tumor growth and tumor weight after HPV + PFPS + DCs early and late treatment.
After injection of TC-1 cells, tumor mice were immunized twice on days 5 and 12 in HPV + PFPS + DCs early and PFPS + DCs groups, or on days 12 and 19 in HPV + PFPS + DCs late group. (A) Tumor volumes (mean± SEM) were measured shown in the left panel. The area under curve (AUC) was calculated using Prism 5 and shown in right panel (mean± SEM). P value (Mann-Whitney test) is given. (B) Tumors were isolated and weighted at the end of this experiment. The tumor photo and weight (mean± SEM) are shown in upper and lower panels, respectively. *P < 0.05 and **P < 0.01 (ANOVA) compared to control group. #P < 0.05 and ##P < 0.01 (ANOVA) compared to PFPS + DCs group.
HPV + PFPS + DCs changed the profile of T cells, induced HPV-specific cellular responses and decreased myeloid-derived suppressor cells (MDSCs)
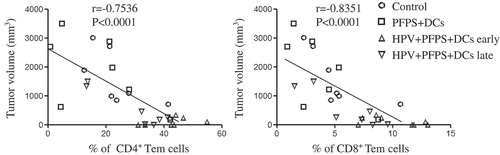
To investigate how HPV + PFPS + DCs suppressed tumor growth in vivo, the profile of T cells, HPV-specific cellular responses and the frequencies of MDSCs in spleens of tumor mice were analyzed by flow cytometry. We found that the frequencies of CD4+ and CD8+ T cells were significantly increased in both HPV + PFPS+ DCs early and late groups compared with control and PFPS + DCs groups (). The activation status of CD4+ and CD8+ T cells was further detected using CD44 and CD62L as markers to distinguish naïve (CD62L+CD44low), effector (Teff: CD62L−CD44low), effector memory (Tem: CD62L−CD44hi) and central memory (Tcm: CD62L+CD44hi) subsets.Citation24-Citation26 Compared to control group, the frequencies of CD4+ naïve T cells were significantly decreased in both HPV + PFPS + DCs early and late groups but the frequencies of CD4+ Tem cells in HPV + PFPS + DCs early group were significantly increased (). As shown in , the frequencies of CD8+ Tem and Tcm cells in HPV + PFPS + DCs early group were significantly increased compared to control group. We analyzed the correlations among the frequencies of CD4+ and CD8+ Tem cells with tumor volumes and found that the frequencies of CD4+ and CD8+ Tem cells were negatively correlated with tumor volumes (). There are no significant changes in the frequencies of CD4+ Teff and Tcm cells, and CD8+ naïve and Teff cells among all groups.
Figure 2. The frequencies of CD4+ and CD8+ T cells and their subsets in spleens of tumor mice.
Splenocytes were isolated from tumor mice at the end of this experiment to detect the frequencies (mean± SEM) of CD4+ (A) and CD8+ (B) T cells and their subsets by flow cytometry. The contour panels show the gating strategy. *P < 0.05 and **P < 0.01 (ANOVA) compared to control group. #P < 0.05, ##P < 0.01 and ###P < 0.001 (ANOVA) compared to PFPS + DCs group.
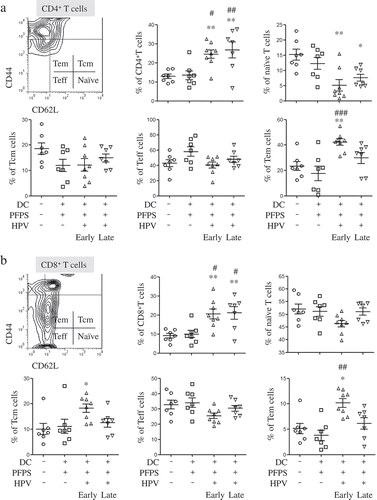
Figure 3. The correlation of CD4+ and CD8+ Tem cells with tumor volumes.
The nonparametric correlation was calculated by GraphPad Prism 5.
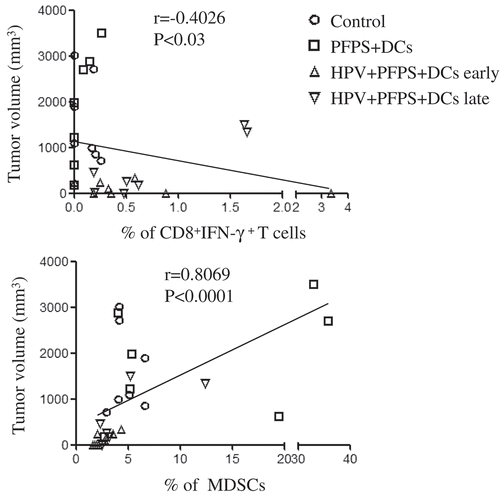
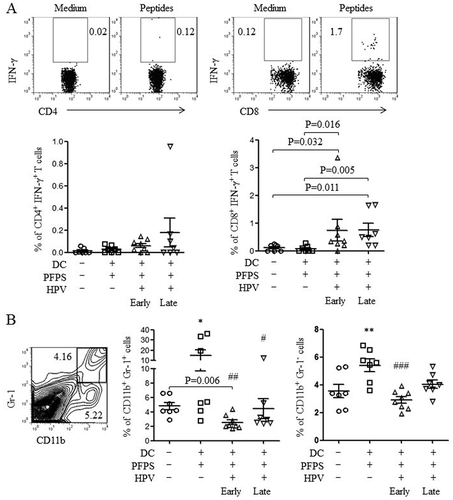
Next, we detected HPV-specific cellular responses in spleen of tumor mice upon HPV peptide treatment. HPV-specific CD4+ T cell responses were slightly increased in both HPV + PFPS + DCs early and late groups, whereas HPV-specific CD8+ T cell responses were significantly induced by HPV + PFPS+ DCs regardless of early or late treatment, which characterized by the increased frequencies of CD8+IFN-γ+ T cells (). The frequencies of HPV-specific CD8+IFN-γ+ T cells were negatively correlated with tumor volumes (). MDSCs are expended during cancer and inhibit antitumor immune responses.Citation27,Citation28 The frequencies of MDSCs (CD11b+Gr-1+) and macrophages (CD11b+Gr-1−) in spleens of tumor mice were analyzed by flow cytometry. HPV + PFPS + DCs early treatment significantly decreased the frequencies of MDSCs compared with control group but did not change the frequencies of CD11b+Gr-1− macrophages (). PFPS + DCs increased the frequencies of MDSCs and CD11b+Gr-1− macrophages, whereas HPV + PFPS + DCs late treatment did not change them. We observed that the frequencies of MDSCs were positively correlated with tumor volumes (). These results suggested that HPV + PFPS + DCs enhanced the activation status of CD4+ and CD8+ T cells, induced HPV-specific cellular responses and decreased the frequencies of MDSCs, which might mediate the inhibition of tumor growth.
Figure 4. HPV-specific cellular responses and the frequencies of MDSCs and macrophages.
Splenocytes were isolated from tumor mice at the end of this experiment. (A) Splenocytes were stimulated with HPV-16 E6 and E7 peptides overnight. HPV-specific cellular responses were analyzed by flow cytometry. The representative dot plots are shown in upper panels and the summary data (mean± SEM) of HPV-specific CD4+ and CD8+ T cells are shown in lower panels. P values (Mann-Whitney test) are given. (B) The frequencies (mean± SEM) of MDSCs (CD11b+Gr-1+) and macrophages (CD11b+Gr-1−) in spleens of tumor mice were detected by flow cytometry. The contour panel shows the gating strategy. P value (Mann-Whitney test) is given. *p < 0.05 and **p < 0.01 (ANOVA) compared to control group. #P < 0.05, ##P < 0.01 and ###P < 0.001 (ANOVA) compared to PFPS + DCs group.
HPV + PFPS + DCs improved the survival of tumor mice and suppressed tumor recurrences
The above results showed that HPV + PFPS + DCs significantly inhibited the growth of tumors in TC-1 mouse model, especially the early treatment. Therefore, the survival of tumor mice was further measured after early treatment with HPV + PFPS + DCs. The strategy of treatment was shown in . Tumor volumes were measured from 9 days to 31 days due to tumor mice began to die on day 31 in control group. The growth of tumors was significantly suppressed in HPV + PFPS + DCs group compared with control group, which was consistent with the above experiment. At the end of this experiment (on day 63), all mice (8/8) died in control group and 3 mice (3/8) died in HPV + PFPS + DCs group (), suggesting that the survival of tumor mice was greatly improved by HPV + PFPS + DCs treatment. For the 5 survived mice in HPV + PFPS + DCs group, 2 mice had small tumors with the volumes of 903 and 1037 mm3, and 3 mice were free of tumor.
Figure 6. The survival of tumor mice and inhibition of tumor recurrence.
(A) The strategy of treatment. (B) The growth of tumors and survival of tumor mice. After HVP + PFPS+ DCs treatment, tumor volumes (mean± SEM) were measured and shown in left panel, the survival of tumor mice were monitored and shown in right panel. (C) The protective effect of HVP + PFPS+ DCs on tumor recurrences. 3 tumor free mice in HVP + PFPS + DCs group were re-challenged and 2 naïve mice were inoculated with TC-1 cells, then tumor growth was measured. The mean± SEM of tumor volumes was shown.
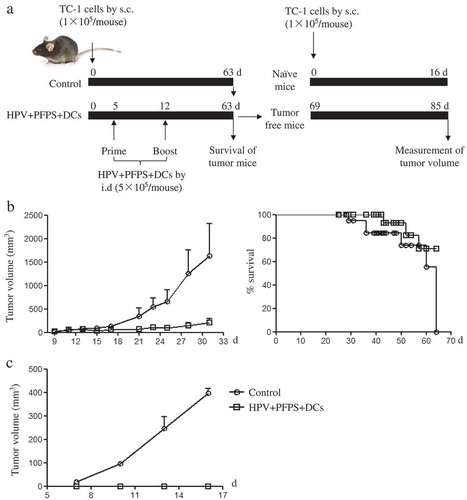
The re-challenge of tumor cells has been used to evaluate the effect of tumor vaccine on tumor recurrences.Citation29 To investigate whether HPV + PFPS + DCs can suppress the tumor recurrences, the 3 mice free of tumor were chosen from the 5 survived mice in HPV + PFPS + DCs group and re-challenged with TC-1 cells on day 69. At the same time, 2 naïve mice were challenged with TC-1 cells and served as control group. Tumors grew fast in control group but the tumor growth was completely suppressed in re-challenged tumor free mice (), suggesting that HPV + PFPS + DCs might induce memory immune responses. According to the above results ( and ), we speculated that the memory immune responses might be the HPV-specific CD8+ T cell responses.
Discussion
DC-based vaccines are potent vaccine platform against cancer due to the important role of DCs in immune system, but the clinical efficacy need to be improved. Lots of efforts have been focused on how to enhance DC maturation and IL-12 production.Citation30-Citation32 Our previous studies showed that the crude and purified polysaccharides from P. ferulae enhanced DC maturation and IL-12 production.Citation19,Citation21 In this study, we found that PFPS treated HPV DC-based vaccine inhibited tumor growth and recurrences, induced antigen-specific cellular responses, and improved the survival of tumor mice.
The survival rates of cancer patients are closely correlated with disease stages.Citation33 In developing countries, the survival rates were low due to the lack of early screening strategies, the poor treatment and clinical follow-up care.Citation34 Cervical cancers were mainly caused by HPV infection, especially high-risk types of HPV including HPV-16 and HPV-18,Citation35-Citation37 which were usually diagnosed at high disease stages in developing countries. Therefore, the antitumor effects of PFPS treated DCs pulsed with HPV peptides were investigated in both early and late therapeutic strategies. We found that HPV + PFPS + DCs showed powerfully antitumor effect in TC-1 tumor mouse model, which significantly suppressed tumor growth and reduced tumor weight in both early and late treatment groups compared with control group. Both HPV + PFPS + DCs early and late induced strong HPV-specific CD8+ T cell responses and increased the frequencies of CD4+ and CD8+ T cells in spleens of tumor mice. These results are similar with our previous study.Citation20 There are lack of effective strategies for the treatment of advanced cervical cancer. The HPV + PFPS + DCs showed the therapeutic effect on TC-1 tumor mice at late stage, which might provide a potential strategy for developing the therapeutic DC-based vaccine against cervical cancer.
Using CD44 and CD62L as markers, the activation status of CD4+ and CD8+ T cells was determined.Citation24-Citation26 We found the significantly negative correlations among the frequencies of CD4+ and CD8+ Tem cells with tumor volumes, indicating that the increased frequencies of activated T cells contributed the inhibition of tumor growth. We also found that HPV-specific CD8+ T cell responses were negatively correlated with tumor volumes, suggesting that HPV + PFPS + DCs induced antigen-specific cytotoxic responses promoted the control of tumor growth.
MDSCs and regulatory T cells (Tregs) were involved in the suppressive microenvironment induced by tumors, which facilitated tumor growth and inhibited immune responses.Citation27,Citation28,Citation38-Citation40 Consistently, we observed that the frequencies of MDSCs positively correlated with tumor volumes. Interestingly, the frequencies of MDSCs were significantly decreased in HPV + PFPS + DCs early group compared to control group, indicating that the decreased frequencies of MDSCs contributed the control of tumor growth and the generation of antitumor immune responses. Rossowska, et al.Citation41 reported that IL-12-transduced DCs enhanced the antitumor activity of DC-based vaccine through the reduction of MDSCs. PFPS treated DCs also produced high level of IL-12 that might be caused the decrease of MDSCs. We also observed that PFPS + DCs increased the frequencies of both MDSCs and macrophages (CD11b+Gr-1−) compared with control group. The tumor growth is similar between control and PFPS + DCs group, suggesting that the stimulatory and suppressive effects on tumor growth might be counteracted. In our previous study, we found that HPV DC-based vaccines significantly decreased the frequencies of Tregs.Citation20 However, the frequencies of Tregs were not significantly decreased by HPV + PFPS + DCs (data not shown). The different doses of DCs (1 × 106 VS 0.5 × 106) in these two studies might be caused the discrepancy. These results indicated that the promoted activation status of T cells, the induction of HPV-specific CD8+ T cell responses and the decreased frequencies of MDSCs were together contributed to the inhibition of tumor growth in HPV + PFPS + DCs early and late groups.
Although the tumor growth was significantly inhibited in HPV + PFPS + DCs late group, there was the room for the further improvement. The maturation of DCs is suppressed in tumor microenvironment,Citation42-Citation44 which dampened antitumor immune responses. Therefore, it is very important to enhance DC maturation in vivo using adjuvant to improve antigen presentation. Recently, Wang et al.Citation45 have reported that alum adjuvant only can suppress the established hepatocarcinoma in mouse model. In the future studies, the strategy of HPV + PFPS + DCs combined with PFPS as adjuvant in vivo will be investigated for the tumors with late stage.
In conclusion, PFPS treated HPV DC-based vaccine showed powerful antitumor effect in both early and late therapeutic strategies, suggesting that PFPS might be a good candidate adjuvant for DC-based vaccines.
Materials and methods
Animals
6–8 weeks old C57BL/6 female mice were bought from the Beijing laboratory animal research center (Beijing, China). Mice were housed in a temperature-controlled, light-cycled animal facility of Xinjiang University. All animal experiments were done under the guidelines of the Animal Care and Use Committee of College of Life Science and Technology, Xinjiang University.
Preparation of HPV DC-based vaccine
Bone marrow cells were collected from C57BL/6 mice and immature DCs were induced in the presence of GM-CSF (Peprotech) according to our previous description.Citation19 On day 7, immature DCs were collected and stimulated with 100 μg/ml of PFPS for 12 h, then 2 × 106/ml of PFPS treated-DCs were pulsed with HPV-16 E6 and E7 peptides (10 μg/ml for each peptide) including E643-57 (QLLRREVYDFAFRDL), E653-62 (AFRDLCIVYR), E711-20 (YMLDLQPETT), E744-62 (QAEPDRAHYNIVTFCCKCD) and E781-94 (DLLMGTLGIVCPIC). After 2 h, DCs were washed twice with PBS and resuspended in PBS at the concentration of 5 × 105 DCs/50 μl, named as HPV + PFPS + DCs. PFPS + DCs without HPV peptides were used as DC control.
Treatment of tumor model
TC-1 tumor cell line constitutively expressed HPV-16 E6 and E7,Citation46 was used to establish tumor mouse model to detect the antitumor effect of HPV + PFPS + DCs. TC-1 cells at log-phase growth were collected, washed and resuspended in PBS at a density of 1 × 106/ml. 1 × 105 TC-1 cells were subcutaneously injected into the right flank of C57BL/6 mice that were randomly divided into 4 groups (8 mice/group). Tumor mice were intradermally immunized twice with 5 × 105 HPV + PFPS + DCs at peri-tumoral sites at a one-week interval because the tumor mice without treatment will die around 30 days. For early therapy, tumor mice were immunized on day 5 & 12 (HPV + PFPS + DCs early). For late therapy, tumor mice were immunized on day 12 & 19 (HPV + PFPS + DCs late). Untreated group and PFPS + DCs treated group (on day 5 & 12) were named as control and PFPS + DCs, respectively. Tumors were measured every other day using calipers and tumor volumes were calculated according to the formula: tumor volume (mm3) = (length× width2)/2. After 35 days, mice were sacrificed and tumors were isolated to weight. Splenocytes were used to analyze the frequencies of macrophages, CD4+ and CD8+ T cells, and HPV-specific cellular responses by flow cytometry.
Flow cytometry
The splenocytes were used to analyze the frequencies of macrophages and T cells, and the subtypes of T cells. After washing with PBS, cells were stained with CD11b-PE and Gr-1-APC (Elabscience Biotechnology Co., Ltd, China) or CD4-FITC and CD8-PE (BD Biosciences) to analyze MDSCs, macrophages and T cells, respectively. For analysis of T cell subtypes, splenocytes were stained with CD4-FITC, CD44-PE and CD62L-APC or CD8-FITC, CD44-PE and CD62L-APC (BD Biosciences).
HPV-specific cellular responses were detected upon the treatment of HPV-16 E6 and E7 peptides in the presence of Golgi stop (monensin) (BD Biosciences) according to our previous description.Citation20 Briefly, IFN-γ production was detected by intracellular staining with IFN-γ-APC antibody (BD Biosciences) after surface staining with CD4-FITC and CD8-PE antibodies. After washing, all samples were collected on FACSCalibur (BD Biosciences) and analyzed by FlowJo platform (Tree Star, Inc., Ashland, OR).
The survival of tumor mice and tumor cell re-challenge
TC-1 tumor mice were randomly divided into 2 groups (8 mice/group). Tumor mice treated with HPV + PFPS + DCs twice on day 5 & 12 were also named as HPV + PFPS + DCs early group. The untreated tumor mice were served as control group. Tumors were measured every other day until day 31 due to tumor mice in control group began to die. The survival of tumor mice were monitored every day until all mice died in control group. To detect the effect of HPV + PFPS + DCs on tumor recurrences, tumor free mice (n = 3) in HPV + PFPS + DCs early group were re-challenged with 1 × 105 TC-1 cells on day 69. At the same time, 2 naïve mice were inoculated 1 × 105 TC-1 cells. Tumors were measured every other day.
Statistical analysis
Statistical analysis was done by one-way analysis of variance (ANOVA) or Mann-Whitney test. P < 0.05 was considered to be statistically significant.
Disclosure of potential conflicts of interest
The authors declare no competing financial interests.
Additional information
Funding
References
- Kalinski P. Dendritic cells in immunotherapy of established cancer: roles of signals 1, 2, 3 and 4. Curr Opin Investig Drugs. 10;2009:526–535.
- Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 260;1993:547–549.
- Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 154;1995:5071–5079.
- Carreno BM, Becker-Hapak M, Huang A, Chan M, Alyasiry A, Lie WR, Aft RL, Cornelius LA, Trinkaus KM, Linette GP. IL-12p70-producing patient DC vaccine elicits Tc1-polarized immunity. J Clin Invest. 2013;123:3383–3394. doi:10.1172/JCI68395.
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi:10.1038/nature06175.
- Rolinski J, Hus I. Breaking immunotolerance of tumors: A new perspective for dendritic cell therapy. J Immunotoxicol. 2014;11:311–318. doi:10.3109/1547691X.2013.865094.
- Hansen M, Met O, Svane IM, Andersen MH. Cellular based cancer vaccines: type 1 polarization of dendritic cells. Curr Med Chem. 2012;19:4239–4246.
- Sabado RL, Bhardwaj N. Dendritic cell immunotherapy. Ann N Y Acad Sci. 2013;1284:31–45. doi:10.1111/nyas.12125.
- Koski GK, Koldovsky U, Xu S, Mick R, Sharma A, Fitzpatrick E, Weinstein S, Nisenbaum H, Levine BL, Fox K, et al. A novel dendritic cell-based immunization approach for the induction of durable Th1-polarized anti-HER-2/neu responses in women with early breast cancer. J Immunother. 2012;35:54–65. doi:10.1097/CJI.0b013e318235f512.
- Vacchelli E, Vitale I, Eggermont A, Fridman WH, Fucikova J, Cremer I, Galon J, Tartour E, Zitvogel L, Kroemer G, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2013;2:e25771. doi:10.4161/onci.25771.
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi:10.1038/nm.3409.
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi:10.3389/fimmu.2013.00114.
- Chen X, Yang L, Howard OM, Oppenheim JJ. Dendritic cells as a pharmacological target of traditional Chinese medicine. Cell Mol Immunol. 2006;3:401–410.
- Li J, Li J, Zhang F. The immunoregulatory effects of Chinese herbal medicine on the maturation and function of dendritic cells. J Ethnopharmacol. 2015;171:184–195. doi:10.1016/j.jep.2015.05.050.
- Li J, Zhang F, Li J. The immunoregulatory effects of traditional chinese medicine on treatment of asthma or asthmatic inflammation. Am J Chin Med. 2015;43:1059–1081. doi:10.1142/S0192415X15500615.
- Alam N, Yoon KN, Lee JS, Cho HJ, Lee TS. Consequence of the antioxidant activities and tyrosinase inhibitory effects of various extracts from the fruiting bodies of Pleurotus ferulae. Saudi J Biol Sci. 2012;19:111–118. doi:10.1016/j.sjbs.2011.11.004.
- Akyuz M, Kirbag S. Antimicrobial activity of Pleurotus eryngii var. ferulae grown on various agro-wastes. EurAsian J BioSc. 2009;3:58–63. doi:10.5053/ejobios.
- Choi D, Cha W, Kang S, Lee BR. Effect of Pleurotus ferulae extracts on viability of human lung cancer and cervical cancer cell lines. Biotechnol Bioprocess Eng. 2004;9:356–361. doi:10.1007/BF02933057.
- Li J, Wang X, Wang W, Luo J, Aipire A, Li J, Zhang F. Pleurotus ferulae water extract enhances the maturation and function of murine bone marrow-derived dendritic cells through TLR4 signaling pathway. Vaccine. 2015;33:1923–1933. doi:10.1016/j.vaccine.2015.02.063.
- Li J, Li J, Aipire A, Luo J, Yuan P, Zhang F. The combination of Pleurotus ferulae water extract and CpG-ODN enhances the immune responses and antitumor efficacy of HPV peptides pulsed dendritic cell-based vaccine. Vaccine. 2016;34:3568–3575. doi:10.1016/j.vaccine.2016.05.022.
- Li J, Yuan P, Wang X, Aipire A, Li M, Yang J, Tao H, Ying T, Fu C, Wei X, et al. Purification, characterization and bioactivities of polysaccharides from Pleurotus ferulae. Food Funct. 2017;8:1905–1914. doi:10.1039/c7fo00227k.
- Li J, Aipire A, Li J, Zhu H, Wang Y, Guo W, Li X, Yang J, Liu C. λ-Carrageenan improves the antitumor effect of dendritic cellbased vaccine. Oncotarget. 2017;8:29996–30007. doi:10.18632/oncotarget.15610.
- Aipire A, Li J, Yuan P, He J, Hu Y, Liu L, Feng X, Li Y, Zhang F, Yang J, et al. Glycyrrhiza uralensis water extract enhances dendritic cell maturation and antitumor efficacy of HPV dendritic cell-based vaccine. Sci Rep. 2017;7:43796. doi:10.1038/srep43796.
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi:10.1038/nature07486.
- Takai S, Sabzevari H, Farsaci B, Schlom J, Greiner JW. Distinct effects of saracatinib on memory CD8+ T cell differentiation. J Immunol. 2012;188:4323–4333. doi:10.4049/jimmunol.1101439.
- Coria LM, Ibanez AE, Pasquevich KA, Cobiello PL, Frank FM, Giambartolomei GH, Cassataro J. Brucella abortus Omp19 recombinant protein subcutaneously co-delivered with an antigen enhances antigen-specific T helper 1 memory responses and induces protection against parasite challenge. Vaccine. 2016;34:430–437. doi:10.1016/j.vaccine.2015.12.012.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi:10.1038/nri2506.
- Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi:10.1172/JCI31422.
- Sales NS, Silva JR, Aps LRMM, Silva MO, Porchia BFMM, Ferreira LCS, Diniz MO. In vivo electroporation enhances vaccine-mediated therapeutic control of human papilloma virus-associated tumors by the activation of multifunctional and effector memory CD8+ T cells. Vaccine. 2017;35:7240–7249. doi:10.1016/j.vaccine.2017.11.011.
- Zhang W, Yu X, Kwak M, Xu L, Zhang L, Yu Q, Jin JO. Maturation of dendritic cells by pullulan promotes anti-cancer effect. Oncotarget. 2016;7:44644–44659. doi:10.18632/oncotarget.10183.
- Zhang W, Okimura T, Xu L, Zhang L, Oda T, Kwak M, Yu Q, Jin J-O. Ascophyllan functions as an adjuvant to promote anti-cancer effect by dendritic cell activation. Oncotarget. 2016;7:19284–19298. doi:10.18632/oncotarget.8200.
- Vo MC, Lee HJ, Kim JS, Hoang MD, Choi NR, Rhee JH, Lakshmanan V-K, Shin S-J, Lee -J-J. Dendritic cell vaccination with a toll-like receptor agonist derived from mycobacteria enhances anti-tumor immunity. Oncotarget. 2015;6:33781–33790. doi:10.18632/oncotarget.5281.
- Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the cervix uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S43–103. doi:10.1016/S0020-7292(06)60030-1.
- Sankaranarayanan R, Swaminathan R, Jayant K, Brenner H. An overview of cancer survival in Africa, Asia, the Caribbean and Central America: the case for investment in cancer health services. France: IARC scientific publications; 2011. p. 257–291.
- Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 55;2002:244–265.
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802.
- Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S3/26–34. doi:10.1016/j.vaccine.2006.05.026.
- Kobayashi A, Weinberg V, Darragh T, Smith-McCune K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol. 2008;1:412–420. doi:10.1038/mi.2008.33.
- Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, Vatan L, Finlayson E, Huang E, Simeone D, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi:10.1158/0008-5472.CAN-08-3804.
- Shao B, Wei X, Luo M, Yu J, Tong A, Ma X, Ye T, Deng H, Sang Y, Liang X, et al. Inhibition of A20 expression in tumor microenvironment exerts anti-tumor effect through inducing myeloid-derived suppressor cells apoptosis. Sci Rep. 2015;5:16437. doi:10.1038/srep16437.
- Rossowska J, Pajtasz-Piasecka E, Anger N, Wojas-Turek J, Kicielinska J, Piasecki E, Duś D. Cyclophosphamide and IL-12-transduced DCs enhance the antitumor activity of tumor antigen-stimulated DCs and reduce Tregs and MDSCs number. J Immunother. 2014;37:427–439. doi:10.1097/CJI.0000000000000054.
- Orsini E, Guarini A, Chiaretti S, Mauro FR, Foa R. The circulating dendritic cell compartment in patients with chronic lymphocytic leukemia is severely defective and unable to stimulate an effective T-cell response. Cancer Res. 2003;63:4497–4506.
- Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–1472. doi:10.1038/sj.bjc.6601243.
- Troy AJ, Summers KL, Davidson PJ, Atkinson CH, Hart DN. Minimal recruitment and activation of dendritic cells within renal cell carcinoma. Clin Cancer Res. 1998;4:585–593.
- Wang B, Wang X, Wen Y, Fu J, Wang H, Ma Z, Shi Y, Wang B. Suppression of established hepatocarcinoma in adjuvant only immunotherapy: alum triggers anti-tumor CD8+ T cell response. Sci Rep. 2015;5:17695. doi:10.1038/srep17695.
- Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26.