ABSTRACT
In 2002, the WHO European Region was declared polio-free. Nonetheless global eradication has not yet been completed and the reintroduction from at risk areas is still possible. This seroprevalence study evaluated samples collected from each Italian region in the 12−50 years old age range to assess protection against Poliovirus (PV) 1, 2 and 3 among subjects immunised with different vaccination schedules. 1073 samples (50.5% females) were examined. WHO standardized microneutralization assay was used. Seroprotection rates were 92.9%, 96.2% and 83.4%, for PV1, PV2 and PV3, respectively. Geometric Mean Titres (GMTs) were higher for PV2 (52.8) and PV1 (41.1) than for PV3 (21.0). Increasing the age, a decreasing trend in seropositivity was observed, in particular for PV3. The 2017–2019 Italian National Immunisation Plan emphasises, as primary objective, the maintenance of the polio-free status and strongly validates the 2 + 1 schedule in the first year of life with IPV vaccine associated with the administration of booster doses at 6 years and during the adolescence. Surveillance system and high population immunity are crucial to ensure the maintenance of polio-free status.
Introduction
In the pre-vaccine era, poliovirus infection was the leading cause of permanent disability in children. Poliovirus (PV) is an enterovirus and can be divided into three different serotypes, namely poliovirus types 1, 2 and 3 (PV1, PV2, PV3). Most infected people do not have symptoms; transient manifestations are developed in about 25% of cases. The signs of meningeal irritation (non-paralytic polio) are exhibited by approximately 4% of cases. When the virus enters the central nervous system and replicates in motor neurons of the spinal cord, paralytic poliomyelitis occurs. It is a rare outcome but, depending on the degree and extent to which motor neurons are affected, temporary or permanent paralysis of the muscles may result. The typical clinical manifestation of paralytic poliomyelitis is acute flaccid paralysis (AFP) affecting the limbs, principally the legs, usually asymmetrically, while sensation remains intact. Persistent paralysis and resulting deformities are common sequelae.Citation1 Polioviruses are spread by faecal-to-oral and oral-to-oral transmission. Immunocompetent individuals generate humoral (circulating antibodies) and mucosal (secretory immunoglobulin A) immune responses. Detectable level of neutralizing antibodies in the blood is an excellent correlate of protection against paralytic disease. However, immunity is serotype-specific with no cross-protection between serotypes.Citation2
Great advancements in the disease eradication have been achieved by immunisation. In 1988, World Health Organisation (WHO) launched the Global Polio Eradication Initiative (GPEI) aimed to eradicate poliomyelitis by the year 2000.Citation3 The annual global burden of paralytic poliomyelitis was estimated to be greater than 350,000 cases, with wild poliovirus (WPV) transmission reported in more than 125 countries. Worldwide extensive use of live attenuated oral poliovirus vaccine (OPV) in mass vaccination campaigns and comprehensive routine immunisation services were coupled with attentive surveillance. A sharp decline of cases was obtained and, at the present, the countries with endemic polio are only three (Afghanistan, Nigeria and Pakistan).Citation4 Wild poliovirus type 2 (WPV2) was last detected in 1999 and global WPV2 eradication was declared in September 2015, while WPV3 has not been detected since 2012. In June 2002, Europe was classified polio-free. European Union countries that still included OPV in childhood immunisation schedule gradually switched to an inactivated polio vaccine (IPV) schedule, accordingly to the WHO strategy to limit the circulation of vaccine-derived poliovirus (VDPV) when the circulation of WPV had been ceased.
In Italy, mass immunisation with OPV started in 1964 and became compulsory in 1966 with Law n. 51 of 4 February 1966. Initially the schedule provided three doses of OPV at 3, 5 and 11 months of age and a booster dose at age of 3.Citation5 The last cases of paralytic diseases were diagnosed in 1982. Two cases of imported wild viruses were detected in not vaccinated children coming from Iran (1984) and India (1988).Citation6,Citation7 A sequential schedule composed of two doses of IPV (at 3 and 5 months) and two of OPV (at 11 months and 3 years of age) was employed since 1999. Since July 2002, a four doses IPV schedule was adopted.Citation8 The availability of a combined hexavalent vaccine for childhood containing also types 1, 2 and 3 inactivated polioviruses contributed to the achievement of coverage rates reaching and exceeding 95%.Citation5
Although PV2 is now an eradicated pathogen and WPV3 was last detected in 2012 in Nigeria, WPV1 remains endemic, at least in few countries. Then reinforcement of high levels of immunisation coverage is essential to prevent the re-introduction of poliovirus from other countries due to international travellers and migrant populations from conflict areas.Citation9 The spread of wild poliovirus from endemic areas to polio-free countries remains a potential risk, as vaccine induced immunity can wane and population sub-groups refuse vaccination.
This serosurvey for poliovirus antibodies aims to describe the immunity status to PV1, 2 and 3 using a microneutralization assay in Italian subjects immunised with different schedules. OPV, used until 2002, provides a long-term protection against paralytic disease through durable humoral and mucosal immunity. Differently, IPV has a better safety profile and is less able to counteract the spread of PV as compared to OPV, with the highest level of risk posed by the proximity of clustered un- or under-immunised groups to large population vaccinated using IPV-only scheme. The primary objective of the study is to assess seroprotection in Italian adolescents and adults. The secondary objective is to compare seroprotection levels according to gender, age group and geographical area of residence in order to unveil the distribution of unprotected subjects.
Results
The study population included 1,073 subjects (50.5% were females). The distribution according to gender, age and geographic area of residence is summarized in . Although no data on the vaccination status were available, as sera collection was carried on from January 2013 to December 2014 and IPV-only schedule was adopted for immunisation starting from July 2002, adolescents aged between 12 and 15 years of age corresponds to children who would have been immunised with the sequential schedule IPV+ OPV. Accordingly, the cohort of 16–50 years of age subjects would have been vaccinated with OPV. The sera were also selected according to different region of residence in order to reveal possible areas at risk.
Table 1. ‘Number of analysed samples stratified by age group, by gender and by Italian area of residence. *In italics the cohorts who would have been immunized with the sequential schedule IPV + OPV.
The percentage of positive samples and the levels of Geometric Mean Titres (GMT) by gender and poliovirus type are reported in . An antibody titre ≥ 1:8 was recorded in 92.9%, 96.2% and 83.4% of subjects for PV1, PV2 and PV3, respectively. Seroprevalence was similar in both gender. The percentage of females with protective titres was slightly higher than males for PV2 and PV3 without significant difference (p = 0.26 e p = 0.60 respectively), while seroprotection for PV1 was barely superior, but not statistically different (p = 0.11), in males. For PV1 and PV2, males exhibited higher values of GMT than females, while GMT for PV3 were higher in feminine gender. No statistically significant difference between gender for GMT was shown. A statistically significant difference () was found comparing PV1 versus PV2 (p < 0.001, OR 1.9; 95% CI 1.3–2.8), PV3 versus PV1 (p < 0.001, OR 2.6; 95% CI 1.9–3.5) and PV3 versus PV2 (p < 0.001, OR 5.0; 95% CI 3.5–7.1). The higher GMT was registered for PV2 (52.8) and the lowest for PV3 (21.0) with a statistically significant difference (p < 0.01). The GMT for PV1 (41.4) was significantly higher than PV3 (p < 0.001) and lower than PV2 (p < 0.05).
Table 2. Percentage of positive samples (titre ≥ 1:8) and Geometric Mean Titres (GMTs) by gender and poliovirus type.
Table 3. Comparison of seroprevalence and Geometric Mean Titres (GMTs) according to poliovirus type.
Seroprevalence rates and GMT for PV1 according to age are reported in . Seroprotection was elevated (above 90%) in adolescents and up to the 21–25 years old age group, and gradually decreased to 83.1% in the 36–40 years old age group. A similar trend was observed for GMT. Analyzing the trend in the different age groups there was a highly significant statistical difference (p < 0.005). In the percentage of positive samples and GMT for PV2 are depicted. Starting from a very high seroprevalence (98.7%) in twelve-year-old children, a decrease was shown in thirteen-year-old subjects and then the rate of positive samples remained above 95% until the 31–35 years old age group. The GMT showed an analogous trend, statistically decreasing with aging (p < 0.05). Seroprevalence for PV3 () displayed values above 95% in all age groups, with a maximum of 94.4% in thirteen-year-old children and a minimum of 71.2% in fourteen-year-old subjects. Higher levels of GMT were exhibited in the twelve- and thirteen-year-old individuals, showing a sharp decrease in the following age groups and remaining almost unchanged in adolescents and adults, without statistically significant difference.
Figure 1. Age specific PV1 seroprevalence and Geometric Mean Titers among Italian population, 2013–2014.
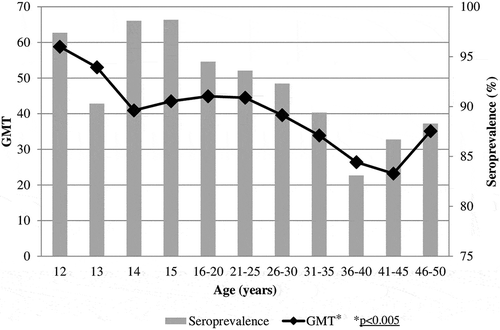
Figure 2. Age specific PV2 seroprevalence and Geometric Mean Titers among Italian population, 2013–2014.
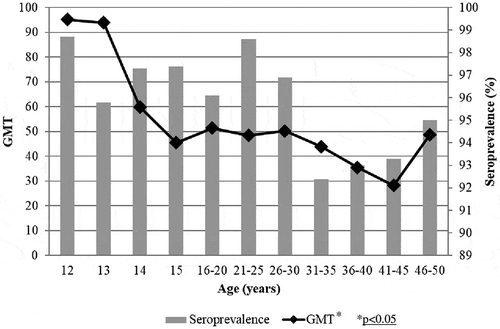
Figure 3. Age specific PV3 seroprevalence and Geometric Mean Titers among Italian population, 2013–2014.
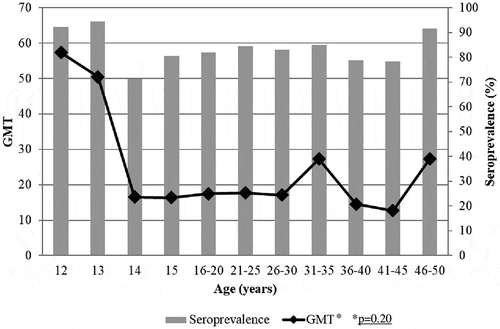
Assessing seroprotection and GMT according to geographical area of residence (North Italy, Central Italy, and South Italy), PV2 and PV3 showed the higher and the lowest rank of positive samples and antibodies levels in all areas, respectively. No statistically significant difference between the areas of the country was found.
Discussion
Eradication of wild Poliovirus still remains a strategic goal for Public Health, as the virus can disseminate from endemic areas to polio-free countries due to waning immunity and the decline of immunisation coverage rates. Epidemiological surveillance is essential to ensure and maintain the polio-free status.
The study revealed a seroprevalence rate of 92.9% for PV1 and 96.2% for PV2, while only 83.4% of tested samples was positive for PV3. Despite a good level of immunity against PV1 and PV2 in adults and in adolescents, at least starting from 14 years of age, the lowest rates of positivity were observed in twelve-year-old and thirteen-year-old children for these serotypes and in all ages for PV3. In addition, a decreasing trend with aging was noticed, with a statistically significant difference for PV1 and PV2, as seropositivity rates for PV3 were stable around 80% from adolescence to adult age, with a rise only in 46–50 year old subjects. A similar pattern was previously observed by Wallace et al., 2016 in a representative sample of non-institutionalised US population. High seroprevalence levels are expected in subjects that likely have recently completed the immunisation schedule.Citation10
Our results are also in accordance with the study of Reinheimer et al., 2012, that examined an European population.Citation11 In the period 2001–2010, the percentage of German individuals showing lack of immunity against PV3 significantly increased over years, reaching about 30%. Other authors already described the immunity level against PV3 as the lowest among all types of poliovirus.Citation12-Citation16 A lessened immunogenicity of PV3 when compared to PV1 and PV2 could explain this gap.Citation17
A recent review, analysing the risk of reintroduction of WPV in Europe assessed seroprotection in countries of European Union (EU) and European Economic Area (EEA). Although immunity rates were high in most countries, herd threshold was not reached, especially for PV3, in some nations.Citation18 The authors also draw attention to disparity in immunity levels within single countries, as found in Germany (formerly separated in East and West Germany) and in Italy, where different regional health authorities realised distinctive immunisation strategies. However, we found no difference, neither for seropositivity rates nor for GMT, according to geographical area in the samples we tested.
This study represents an up-to-date survey on the presence and the levels of antibodies against different poliovirus serotypes in Italian population. In 2007, the assessment of immunity in a cohort of fertile women at the delivery showed low GMT, even if no woman was susceptible to infection.Citation19 A decrease of GMT was recorded in a survey on individuals living in the Veneto region (North Italy).Citation6 Children and adolescents (1–17 years old) exhibited significantly higher GMT levels than adults (over 65 years old), pointing out an age-related decline in antibody titres. Similarly, a reduction in neutralising antibodies levels according to age was found in university students whether Italian or from outside EU.Citation7 Seropositivity rates very close to 100% were observed in Apulian children from zero to 15 years old, with the lowest titres in infants in the first year of life who have not concluded immunisation and a decline of GMT associated with age, particularly for PV3 again.Citation5 As the risk of reintroduction of the virus is strongly reduced but still not completely eliminated, the low and decreasing levels of immunity in adolescents represent a matter of concern. A booster dose is strongly suggested in order to overcome the waning of seroprotection. In that respect, the 2017–2019 Italian National Immunisation Plan provides for the maintenance of polio-free status as main goal, suggesting, in addition to the IPV immunisation schedule with 3 doses at 3, 5 and 11 months, a first booster dose at age of 6 and a second one in adolescence (between 12 and 18 years).Citation20
Great improvements have been achieved in effective control of poliomyelitis by means of the development and the widespread use of the vaccine. Nonetheless, poliovirus remains endemic in three countries and many low-income countries still currently use OPV in their routine immunisation programmes. As a result, Italy for its geographical position and high rate of immigration is at risk for the re-introduction of the virus. Very recently an imported case of vaccine-associated paralytic poliomyelitis (VAPP) was described in a pediatric patient from Albania. The infant, affected by congenital agammaglobulinemia, was immunised with OPV according to local schedule. PV3 was isolated from stool samples and all precautions for the potential risk for virus transmission were taken. In immunodeficient individuals attenuated OPV-virus can replicate and persit for long time accumulating mutations that increase the possibility of reversion to neurovirulence and transmissibility. Therefore, they can act as a polio reservoir and infect unvaccinated and immunocompromised contacts.Citation21
The present study has some limitations. As it is based on a convenience sample, it is unknown if these results are generalizable to the current status of poliovirus immunity in the Italian population as a whole. Nevertheless, this study gives an indication on the rate of seropositivity/negativity according to age groups that can be expected in larger surveys. In addition, no data about vaccination history was available.
Besides, without sensitive seroprevalence assessment the existence of low-immunity pockets in the population cannot be excluded. Since the spread of PV can be efficiently prevented only when rates of susceptible people are closer to zero, the maintenance of sound surveillance and high population immunity, including through strengthened routine immunisation services, is crucial also in polio-free countries.
Materials and methods
Study design
A national observational seroprevalence study was conducted. Tested samples were collected in the period 2013–2014 for a previous survey on varicella antibodies prevalence.Citation22 Anonymous unlinked samples of residual sera from routine laboratory testing were provided by a reference laboratory from each Italian Regions and Autonomous Provinces. Samples from individuals known to have an immunosuppressive or acute infectious disease and those from individuals who had recently undergone a blood transfusion were excluded. Available sera were stored at −20°C. We selected samples of individuals between 12 and 50 years of age in order to compare immunisation with different schedules. As the subjects who received only IPV are considered at higher risk, the assessment started from 12 year of age (in mid-2002 the sequential schedule IPV-OPV was replaced by the full IPV one) towards the age of 50 (as, in Italy, OPV universal mass vaccination was implemented since 1964). Given the exploratory nature and the objectives of this observational study, rather than testing a formal hypothesis, an estimation approach through the provision of confidence intervals for the proportion of seronegativity was considered more appropriate than the power calculation in the traditional fashion. However, the sample size obtained from this estimation approach (1026 samples) was used to evaluate the power levels reached by assuming a difference of 10% in the seronegativity proportions between two age groups (<20 years and 40–50 years) with 608 and 114 samples respectively and permitting to obtain the appropriate power levels.
Ethical aspects
The study was approved by the Ethics Committee of the Province of Ferrara. Ethics Committee of the Istituto Superiore di Sanità (ISS) in compliance with European SeroEpidemiology Network approved the previous seroprevalence survey. Only data regarding gender and age were available.
Microneutralization test
Samples with a volume of serum sufficient for the determination were tested in batches. A microneutralization assay was used for the assessment of the serum titres of Poliovirus types 1, 2 and 3 antibodies according to the WHO procedure.Citation23,Citation24
Briefly, sera were complement inactivated at 56 °C for 30 minutes and diluted from 1:8 to 1:1024. They were then placed in contact with a standard amount of 100 50% tissue culture infective doses (TCD50) of the three types of Sabin attenuated Poliovirus (type 1, L Sc2ab strain; type 2, P712ch2ab; type 3, Leon 12alb) in a multi-well culture plate. After a 3 hours incubation at 36 °C in a CO2 incubator, freshly trypsinized HEp-2 cells in suspension (approximately 1 × 10Citation5 to 2 × 10Citation5 per ml of human larynx epidermoid carcinoma cell line, HeLa derivative human) were added to each well containing the serum-virus mixture and the solution was incubated at 36 °C in a 5% CO2 incubator. The final test visually reading was obtained after 5 days. The 50% endpoint value was used as the serum titre. Titres of ≥1:8 were considered to be protective. Results were expressed in International Unit (IU) in accordance with the protocol.
Statistical analyses
All tests were conducted in duplicate. According to WHO recommendations, sera with a titre equal or above 1:8 were considered positive. When the results of the replicates were conflicting (one positive and one negative), the sample was considered negative and for the GMT a value of 3 was considered. When both results were positive with contrasting values, the sample was assumed as positive and the lower value was considered for GMT assessment. Seroprevalence was calculated as the percentage of positive samples (titre ≥ 1:8). Geometric Mean Titres (GMTs) were computed by log10 of reciprocal antibody titres.
Data were analysed using the χCitation2 test, Student’s t-test for unpaired data, Chi-squared test for trend, and 95% confidence intervals (95% CI) were calculated as appropriate. Analyses were performed using Epi Info™ 7 supplied by the Centers for Disease Control and Prevention (Atlanta, GA, USA). The statistical significance was set at p < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Funding for this study was provided by GlaxoSmithKline Biologicals SA [GSK study identifier: 201867]. GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this paper for factual accuracy but the authors are solely responsible for final content and interpretation.
Additional information
Funding
References
- World Health Organization (WHO). Polio vaccines: WHO position paper. Wkly Epidemiol Rec. 2016;91(12):145–168.
- Sutter RW, Pallansch MA, Sawyer LA, Cochi SL, Hadler SC. Defining surrogate serologic tests with respect to predicting protective vaccine efficacy: poliovirus vaccination. Ann N Y Acad Sci. 754;1995:289–299.
- Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine-live. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Philadelphia, PA: Elsevier-Saunders; 2017. p. 866–917.
- Global Polio Eradication Initiative. [ Accessed 2018 Feb 2]. http://polioeradication.org/where-we-work/polio-endemic-countries/
- Tafuri S, Prato R, Martinelli D, Calvario A, Bozzi A, Labianca M, Patti A, Lopalco PL, Germinario C. Serological survey on immunity status against polioviruses in children and adolescents living in a border region, Apulia (Southern Italy). BMC Infect Dis. 2008;8:.150. doi:10.1186/1471-2334-8-150.
- Majori S, Baldo V, Poli A, Riolfatti M, Alborino F, Bonello C, Frau S, Baldovin T, Dal Zotto A, Romano G, et al. Immunity to poliovirus among children and the elderly in north-east Italy. J Prev Med Hyg. 2006;47(1):12–15.
- Baldo V, Baldovin T, Cocchio S, Lazzari R, Saracino E, Bertoncello C, Buja A, Trevisan A. Seroepidemiology of polioviruses among university students in northern Italy. Clin Vaccine Immunol. 2012;19(8):1292–1295. doi:10.1128/CVI.00054-12.
- Conferenza permanente per i rapporti tra lo Stato, le Regioni e le Province Autonome di Trento e Bolzano. Accordo tra il Ministero della Salute, le Regioni e le Province Autonome di Trento e Bolzano sulle modifiche al calendario della vaccinazione antipoliomielitica; 2002 May 30. [Italian]. 10.1044/1059-0889(2002/er01)
- European Centre for Disease Prevention and Control. Wild-type poliovirus 1 transmission in Israel – what is the risk to the EU/EEA? Stockholm, Sweden: ECDC; 2013. [ Accessed 2018 Mar 2]. http://ecdc.europa.eu/en/publications/Publications/polio-risk-assessment-transmission-in-Israel.pdf
- Wallace GS, Curns AT, Weldon WC, Oberste MS. Seroprevalence of poliovirus antibodies in the United States population, 2009-2010. BMC Public Health. 2016;16:.721. doi:10.1186/s12889-016-3386-1.
- Reinheimer C, Friedrichs I, Rabenau HF, Doerr HW. Deficiency of immunity to poliovirus type 3: a lurking danger? BMC Infect Dis. 2012;12:.24. doi:10.1186/1471-2334-12-24.
- Frantzidou F, Diza E, Halkia D, Antoniadis A. A seroprevalence study of poliovirus antibody in the population of northern Greece. Clin Microbiol Infect. 2005;11(1):68–71. doi:10.1111/j.1469-0691.2004.00998.x.
- Pires de Miranda M, Carmo Gomes M, Rebelo de Andrade H. Seroprevalence of antibodies to poliovirus in individuals living in Portugal, 2002. Euro Surveill. 2007;12(6):E7–E8.
- van der Maas NA, Mollema L, Berbers GA, van Rooijen DM, van der Avoort HG, Conyn-Van Spaendonck MA, de Melker HE, van der Klis FR. Immunity against poliomyelitis in the Netherlands, assessed in 2006 to 2007: the importance of completing a vaccination series. Euro Surveill. 2014;19(7):20705. doi:10.2807/1560-7917.ES2014.19.7.20705.
- Martin N, Paterson BJ, Durrheim DN. Australia’s polio risk. Commun Dis Intell Q Rep. 2014;38(2):E107–13.
- Wallace GS, Pahud BA, Weldon WC, Curns AT, Oberste MS, Harrison CJ. Seroprevalence of poliovirus antibodies in the Kansas City metropolitan area, 2012-2013. Hum Vaccin Immunother. 2017;13(4):776–783. doi:10.1080/21645515.2016.1255386.
- Roivainen M, Montagnon B, Chalumeau H, Murray M, Wimmer E, Hovi T. Improved distribution of antigenic site specificity of poliovirus-neutralizing antibodies induced by a protease-cleaved immunogen in mice. J Virol. 1990;64(2):559–562.
- Nijsten D, Carrillo-Santisteve P, Miglietta A, Ruitenberg J, Lopalco PL. Is EU/EEA population protected from polio? Hum Vaccin Immunother. 2015;11(9):2123–2131. doi:10.1080/21645515.2015.1016673.
- Veronesi L, Affanni P, Verrotti Di Pianella C, Colucci ME, Tanzi ML. Immunity status against poliomyelitis in childbearing women in a province of northern Italy. A cross-sectional analysis. Ann Ig. 2013;25:427–433. doi:10.7416/ai.2013.1944.
- Ministero della Salute. Piano Nazionale della Prevenzione Vaccinale 2017-2019. G.U. Serie Generale, n. 41 del 18 febbraio 2017 [Italian].
- De Donno A, Kuhdari P, Guido M, Rota MC, Bella A, Brignole G, Lupi S, Idolo A, Stefanati A, Del Manso M, et al. Study Group on seroepidemiology. Has VZV epidemiology changed in Italy? Results of a seroprevalence study. Hum Vaccin Immunother. 2017;13(2):385–390. doi:10.1080/21645515.2017.1264828.
- Foiadelli T, Savasta S, Battistone A, Kota M, Passera C, Fiore S, Bino S, Amato C, Lozza A, Marseglia GL, et al. Nucleotide variation in Sabin type 3 poliovirus from an Albanian infant with agammaglobulinemia and vaccine associated poliomyelitis. BMC Infect Dis. 2016;16:277. doi:10.1186/s12879-016-1587-y.
- World Health Organization (WHO). Polio laboratory manual. 4th ed. Geneva, Switzerland: WHO/IVB/04.10; 2004.
- World Health Organization (WHO). Manual for the virological investigation of polio. Geneva, Switzerland: WHO/EPI/GEN/97.01; 1997.
