 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The epidemiology of pertussis—a vaccine-preventable respiratory infection typically caused by the bacterium Bordetella pertussis—remains puzzling. Indeed, the disease seems nowhere close to eradication and has even re-emerged in certain countries—such as the US—that have maintained high vaccination coverage. Because the dynamics of pertussis are shaped by past vaccination and natural infection rates, with the relevant timescale spanning decades, the interpretation of such unexpected trends is not straightforward. In this commentary, we propose that mathematical transmission models play an essential role in helping to interpret the data and in closing knowledge gaps in pertussis epidemiology. We submit that recent advances in statistical inference methods now allow us to estimate key parameters, such as the nature and duration of vaccinal immunity, which have to date been difficult to quantify. We illustrate these points with the results of a recent study based on data from Massachusetts (Domenech de Cellès, Magpantay, King, and Rohani, Sci. Transl. Med. 2018;10: eaaj1748. doi:10.1126/scitranslmed.aaj1748), in which we used such methods to elucidate the mechanisms underlying the ongoing resurgence of pertussis. In addition, we list a number of safety checks that can be used to critically assess mathematical models. Finally, we discuss the remaining uncertainties surrounding pertussis vaccines, in particular the acellular vaccines used for teenage booster immunizations.
The recent epidemiology of pertussis – an acute respiratory infection characterized by a prolonged coughCitation1 – cautions us against complacency about seemingly familiar infectious diseases. Historically, whooping cough was a prominent cause of mortality and morbidity in young children,Citation2 but the development of whole-cell pertussis vaccines in the 1930s marked a breakthrough that paved the way for routine pediatric immunization.Citation3 In the US, the roll-out of whole-cell vaccines led in a few decades to a substantial, typically >10-fold, decline in reported cases.Citation4 Since the mid-1970s, however, the disease has re-emerged, despite sustained high vaccine coverage.Citation4 The implementation of additional control measures, such as booster vaccination in adolescents,Citation5 appears to have had a modest impact on this growing burden.Citation6 These control difficulties emphasize the need to better understand the drivers of pertussis resurgence
The many complexities of pertussis epidemiology were noted by early investigators.Citation2 Age-specific incidence data in Massachusetts provide a case in point. As shown in ), the time series are noisy and apparently irregular, with several notable, but unevenly spaced, peaks. A robust feature, however, is the marked increase in cases in adolescents and adults, a shift typical of the recent epidemiology of pertussis in the US and other locations.Citation6,Citation13 From a public health perspective, interpreting such data is not straightforward. Focusing on a narrow time period, for example that embracing the large 2003 outbreak in adolescents (95% of whom had received 5 vaccine doses), one might conclude that vaccines are ineffective and confer only short-term protection. Looking at the entire time period, however, we see that other epidemics occurred in 1996 and 2000. The occurrence of multi-annual epidemic cycles is in fact a distinct feature of pertussis epidemiology that has persisted in the vaccine era.Citation14 The interval between peaks is well predicted by the time needed for the growing susceptible pool to reach a threshold.Citation15,Citation16 This example demonstrates the need to interpret recent pertussis data in an appropriately broad historical context, with the help of epidemiological theory to establish baselines and suggest the relevant timescales. Essentially, the dynamics of pertussis infections depend on the degree of susceptibility of the population, a complex quantity shaped both by the birth rate and by the level of immunity, which is a legacy of long-term vaccination and previous natural infection. Heterogeneities in rates of contact between individuals of different ages also play a key role in these dynamics,Citation17 since two equally susceptible age groups are expected to suffer different infection risks if their exposures to infection in other groups differ. In sum, interpreting pertussis data requires a thorough bookkeeping of the kinetics of infection and susceptible recruitment over the timescale of decades.
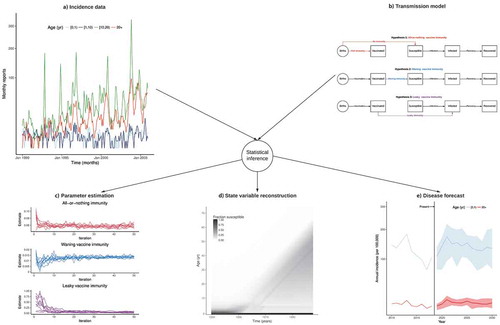
Figure 1. Confronting transmission models with incidence data to elucidate the epidemiology of pertussis.
(a) Age-specific monthly case reports of pertussis in Massachusetts during 1990–2005 (data from Ref. Citation7). (b) Schematic of three mathematical transmission models with three different assumptions on the nature of vaccine-derived immunity (all-or-nothing, waning, or leakyCitation7-Citation9). (c) Convergence plot of vaccine parameters (as defined in panel B) to their maximum likelihood estimates. (d) Model-based hindcasts of the fraction of individuals susceptible to pertussis infection, according to time (x-axis) and to age (y-axis). (e) Model-based forecasts of pertussis annual incidence in infants [0,1) yr and adults 20 yr. The figure illustrates how, via statistical inference methods,Citation10-Citation12 pertussis incidence data (panel A) can be confronted with transmission models to test different scientific hypotheses about the nature of vaccine immunity (panel B). Each fitted model leads to different parameter estimates and receives a different degree of support from the data (panel C). The best-fitting model (here the model with waning vaccine immunity) can then be used to infer quantities that are not directly observable (like the degree of susceptibility in the population, panel D) and to forecast the burden of disease (panel E). Panel D illustrates the end-of-honeymoon effect.Citation7 In the prevaccine era, cases are concentrated in young children who, upon recovery, develop long- lived immunity against reinfection, resulting in strong herd immunity in older individuals. The inception of mass vaccination leads to an overall reduction in transmission in those vaccinated and in the population at large. Hence, children who were not vaccinated (or in whom vaccinal protection did not initially take) are increasingly likely to reach adulthood having avoided natural infection. Concomitantly, older cohorts, with their long-lived immunity derived from natural infection during the prevaccine era, gradually die out. The result is the gradual buildup of susceptibles, which leads to a gradual resurgence.
Mathematical transmission models provide a formal and robust framework for this bookkeeping exercise and for contextualizing epidemiological data.Citation15,Citation18 Their key strength is in translating between processes that occur at different scales, from the disease’s natural history at the scale of the individual infection to population-scale epidemic dynamics. Mathematical models represent the epidemiological system under study by a simplified mathematical object, typically a set of equations that govern the dynamics of so-called state variables. These variables typically include numbers of individuals with similar infection or immune status. Other important ingredients of such models are parameters, i.e., fixed quantities that control the pace at which individuals transition between different states. Because different parameter values can lead to markedly different model behaviors and predictions, appropriate model parametrization is essential. Model parameters can be fixed according to external evidence, but sometimes their values are not known, or are known only imprecisely. Because of the lack of definite correlates of protection, for example, key parameters like the duration of infection- or vaccine-derived immunity are challenging to estimate for pertussis. In such cases, models can be confronted to time series of epidemiological records, allowing the latter to voice an opinion as to the most likely parameter values. In recent years, a range of robust statistical inference methods have been developed for this purpose.Citation10 Such methods allow one not only to estimate unknown parameters, but also to weigh the evidence for different biologically-motivated hypotheses, as expressed in the form of competing models.Citation19
In recent work,Citation7 we applied these techniques to dissect the epidemiology of pertussis in Massachusetts, where, as in other US states, a resurgence of pertussis has been observed since the mid-1970s () and Refs. Citation4, Citation20). To close current knowledge gaps in pertussis epidemiology,Citation21,Citation22 we formulated age-stratified transmission models that expressed a range of hypotheses about the nature and the degree of vaccinal immunityCitation8 and about the transmissibility and the observability of post-vaccine infections.Citation23,Citation24 A novel feature of our models was the inclusion of age-specific reporting rates to correct for observation biases caused by the use of highly sensitive serology to detect cases in adolescents and adults.Citation25 Using the aforementioned statistical methods, we confronted these models with age-specific incidence data to elucidate the drivers of pertussis resurgence. We found unambiguous evidence that pertussis vaccines confer imperfect, but quite slowly waning, immunity, a result that also held for DTaP vaccines, in opposition to widespread belief.Citation26,Citation27 How, then, to interpret the resurgence of pertussis, despite high vaccine coverage? We demonstrated that it resulted from a so-called “end-of-honeymoon” effect,Citation28 that is, a predictable consequence of incomplete historical coverage with an imperfect but highly effective vaccine (see legend of ). This result was based on the model-based reconstruction of the age-specific susceptibility profile over time ()). This further illustrates the usefulness of transmission models to infer quantities, such as susceptibility to pertussis infection, that cannot be directly observed.
Although potentially powerful tools for unraveling pertussis epidemiology, mathematical models should be critically reviewed at each of the three steps of model development: model implementation, model estimation, and model validation. Regarding model implementation, the choice of a deterministic or stochastic model has important consequences for parameter estimation and model interpretation. Although deterministic models are commonly used because they are easy to implement and to fit to data,Citation29 mounting evidence indicates that fully stochastic models explain the dynamics of pertussis much better and in potentially very different ways.Citation7,Citation9,Citation30 Regarding model estimation, appropriate statistical inference methods are needed and now easily applicable via well-tested software packages.Citation10 In this respect, one should keep in mind the “curse of dimensionality”, whereby the volume of parameter space grows exponentially with the number of parameters to be estimated. This implies that even a large pre-specified random sample of parameter sets may be inadequate for proper exploration of the parameter space, a problem aggravated when – as is invariably the case in practice – parameters are correlated.Citation31,Citation32 During the model estimation step, it is also typical to carry out sensitivity analyses to assess the robustness of parameter estimates to realistic aspects of model misspecification. Regarding model validation, agreement between model and data requires careful assessment. Standard checks include the visual inspection of typical model simulations and the quantification of model-data agreement based on goodness-of-fit metrics (e.g., RCitation2 and its generalizations) or on more specific signatures (quantified using summary statistics) that capture important features of the data. When the data are sufficiently numerous, model predictions tested on data not used for parameter estimation (out-of-fit predictions) are indispensable as independent checks on the model’s predictive power and for diagnosis of over-fitting. Although not foolproof,Citation33 a careful examination of the elements of the above list can help establish confidence in scientific conclusions based on mathematical models.
Although our study provided a coherent and possibly unifying explanation for pertussis resurgence in the US, many questions remain. In particular, a more precise estimation of the duration of immunity conferred by DTaP is needed – though, as explained above, our results rule out very rapid waning. A similar estimate for Tdap will also prove valuable in quantifying the impact of the booster dose in teenagers, which was possibly masked by cohort effects due to the aging of the first DTaP-vaccinated birth cohorts.Citation6 We propose that applying the methods discussed here to more recent data should allow to pinpoint these quantities and help design future control strategies.
Disclosure of potential conflicts of interest
MDdC has received post-doctoral funding (through his host unit at the Institut Pasteur) from Pfizer, on a project related to the epidemiology of meningococcus and independent from this work. No potential conflict of interest was reported by the other authors. .
Additional information
Funding
References
- Edwards KM, Decker MD. Pertussis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines, chap. 23. 6th ed. Philadelphia (PA): Elsevier Saunders; 2013. p. 447–492.
- Gordon JE, Hood RI. Whooping cough and its epidemiological anomalies. Am J Med Sci. 1951;222:333–361.
- Shapiro-Shapin CG. Pearl Kendrick, Grace Eldering, and the pertussis vaccine. Emerg Infect Dis. 2010;16:1273–1278. doi:10.3201/eid1608.100288.
- Rohani P, Drake JM. The decline and resurgence of pertussis in the US. Epidemics. 2011;3:183–188. doi:10.1016/j.epidem.2011.10.001.
- Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, Mijalski CM, Tiwari T, Weston EJ, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55:1–34.
- Skoff TH, Martin SW. Impact of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccinations on reported pertussis cases among those 11 to 18 years of age in an era of waning pertussis immunity: A follow-up analysis. JAMA Pediatr. 2016;170:453–458. doi:10.1001/jamapediatrics.2015.3886.
- Domenech de Cellès M, Magpantay FMG, King AA, Rohani P. The impact of past vaccination coverage and immunity on pertussis resurgence. Sci Transl Med. 2018;10. doi:10.1126/scitranslmed.aao4496.
- Magpantay F, Riolo M, Domenech de Cellès M, King A, Rohani P. Epidemiological consequences of imperfect vaccines for immunizing infections. SIAM J Appl Math. 2014;74(6):1810–1830. doi:10.1137/140956695.
- Magpantay FMG, Domenech de Cellès M, Rohani P, King AA. Pertussis immunity and epidemiology: mode and duration of vaccine-induced immunity. Parasitology. 2016;143(7):835–849.
- King AA, Nguyen D, Ionides EL. Statistical inference for partially observed Markov processes via the R package pomp. J Stat Softw. 2016;69:1–43. doi:10.18637/jss.v069.i12.
- Ionides EL, Bretó C, King AA. Inference for nonlinear dynamical systems. Proc Natl Acad Sci USA. 2006;103:18438–18443. doi:10.1073/pnas.0603181103.
- Ionides EL, Nguyen D, Atchadé Y, Stoev S, King AA. Inference for dynamic and latent variable models via iterated, perturbed Bayes maps. Proc Natl Acad Sci USA. 2015;112:719–724. doi:10.1073/pnas.1410597112.
- Bento AI, Riolo MA, Choi YH, King AA, Rohani P. Core pertussis transmission groups in England and Wales: A tale of two eras. Vaccine. 2018;36:1160–1166. doi:10.1016/j.vaccine.2018.01.046.
- Rohani P, Earn DJ, Grenfell BT. Opposite patterns of synchrony in sympatric disease metapopulations. Science. 1999;286:968–971.
- Keeling MJ, Rohani P. Modeling infectious diseases in humans and animals. Princeton (NJ): Princeton University Press; 2008. http://www.loc.gov/catdir/toc/fy0805/2006939548.html.
- Broutin H, Viboud C, Grenfell BT, Miller MA, Rohani P. Impact of vaccination and birth rate on the epidemiology of pertussis: a comparative study in 64 countries. Proc Biol Sci. 2010;277:3239–3245. doi:10.1098/rspb.2010.0994.
- Rohani P, Zhong X, King AA. Contact network structure explains the changing epidemiology of pertussis. Science. 2010;330:982–985. doi:10.1126/science.1194134.
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford: Oxford University Press; 1991. http://www.loc.gov/catdir/enhancements/fy0636/90014312-d.html.
- Lavine JS, Rohani P. Resolving pertussis immunity and vaccine effectiveness using incidence time series. Expert Rev Vaccines. 2012;11:1319–1329. doi:10.1586/erv.12.109.
- Lavine JS, King AA, Bjørnstad ON. Natural immune boosting in pertussis dynamics and the potential for long-term vaccine failure. Proc Natl Acad Sci USA. 2011;108:7259–7264. doi:10.1073/pnas.1014394108.
- Jackson DW, Rohani P. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol Infect. 2014;142:672–684. doi:10.1017/S0950268814000211.
- Domenech de Cellès M, Magpantay FMG, King AA, Rohani P. The pertussis enigma: reconciling epidemiology, immunology and evolution. Proc Biol Sci. 2016;283. doi:10.1098/rspb.2016.0343.
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA. 2014;111:787–792. doi:10.1073/pnas.1314688110.
- Domenech de Cellès M, Riolo MA, Magpantay FMG, Rohani P, King AA. Epidemiological evidence for herd immunity induced by acellular pertussis vaccines. Proc Natl Acad Sci USA. 2014;111:E716–E717. doi:10.1073/pnas.1323795111.
- Marchant CD, Loughlin AM, Lett SM, Todd CW, Wetterlow LH, Bicchieri R, Higham S, Etkind P, Silva E, Siber GR. Pertussis in Massachusetts, 1981-1991: incidence, serologic diagnosis, and vaccine effectiveness. J Infect Dis. 1994;169:1297–1305. doi:10.1093/infdis/169.6.1297.
- McGirr A, Fisman DN. Duration of pertussis immunity after dtap immunization: a meta-analysis. Pediatrics. 2015;135:331–343.
- Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367:1012–1019. doi:10.1056/NEJMoa1200850.
- McLean AR, Anderson RM. Measles in developing countries. Part II. The predicted impact of mass vaccination. Epidemiol Infect. 1988;100:419–442. doi:10.1017/S0950268800067170.
- Gambhir M, Clark TA, Cauchemez S, Tartof SY, Swerdlow DL, Ferguson NM, Salathé M. A change in vaccine efficacy and duration of protection explains recent rises in pertussis incidence in the United States. PLoS Comput Biol. 2015;11:e1004138. doi:10.1371/journal.pcbi.1004138.
- Rohani P, Keeling MJ, Grenfell BT. The interplay between determinism and stochasticity in childhood diseases. Am Nat. 2002;159:469–481. doi:10.1086/339467.
- Choi YH, Campbell H, Amirthalingam G, van Hoek AJ, Miller E. Investigating the pertussis resurgence in England and Wales, and options for future control. BMC Med. 2016;14:121. doi:10.1186/s12916-016-0665-8.
- Campbell PT, McCaw JM, McIntyre P, McVernon J. Defining long-term drivers of pertussis resurgence, and optimal vaccine control strategies. Vaccine. 2015;33:5794–5800. doi:10.1016/j.vaccine.2015.09.025.
- Basu S, Andrews J. Complexity in mathematical models of public health policies: a guide for consumers of models. PLoS Med. 2013;10:e1001540. doi:10.1371/journal.pmed.1001540.
