ABSTRACT
This randomized trial conducted in France compared intramuscular (IM) and subcutaneous (SC) administration of two doses of a measles, mumps, rubella, and varicella (MMRV) combination vaccine (ProQuad®) administered one month apart to 405 children 12–18 months of age (NCT00402831). The 2-dose regimen of MMRV administered IM was shown to be as immunogenic as the 2-dose regimen administered SC for all antigens 6 weeks post-vaccination for the subjects who were initially seronegative for measles, mumps, rubella, or varicella (lower bounds of the two-sided 95% CIs for the difference in response rates for all antigens greater than −10% [range −2.1 for varicella to −3.0 for mumps]). The antibody response rates for all vaccine antigens 6 weeks after the second dose of MMRV were > 99% in both the IM and SC groups. Fewer subjects in the IM group experienced injection-site AEs compared with the SC group (17.8% and 28.6% post-dose 1, and 20.4% and 29.5% post-dose 2, respectively). From Day 0 to Day 4 post-dose 2, fewer subjects reported erythema and swelling in the IM group than in the SC group (15.4% and 27.0%, and 6.0% and 12.5%, respectively). In both groups, most injection-site AEs started during the first four days after vaccination; their intensity was mainly mild or ≤2.5 cm. The rates of fever were comparable between the two groups after each dose of MMRV. In conclusion, two doses of the MMRV vaccine were highly immunogenic and well tolerated when administered either SC or IM.
ClinicalTrials.gov Identifier: NCT00402831
Introduction
Immunization is recognized to be a highly cost-effective and life-saving intervention that can potentially eliminate vaccine-preventable diseases.Citation1 In 2012, the World Health Organization (WHO) set a target date for 2020 to eliminate measles and rubella.Citation2 The WHO also advocates routine childhood immunization against varicella in countries where the disease is an important public health and socioeconomic problem, where the vaccine is affordable, and where high (≥80%) and sustained vaccine coverage can be achieved.Citation3 Since the implementation of universal varicella vaccination in 1996 in the United States of America (USA), the incidence of varicella declined by 9 to 10-fold compared with the pre-vaccination period, with no evidence of waning effectiveness over time.Citation4–Citation7
To be an effective public health tool, vaccine coverage rates must be adequate. Pediatric combination vaccines are one approach that might improve coverage rates through the simultaneous administration of multiple antigens with one injection at the same visit.Citation8 Combination vaccines offer many advantages for implementing universal vaccination programs as they can simplify immunization schedules, which are very busy for infants in their first two years of life in many countries. They can also contribute to reducing parents’ and physicians’ concerns about the number of injections administered at each visit.
ProQuad® (measles, mumps, rubella, and varicella virus vaccine live, Merck & Co., Inc., Kenilworth, NJ, USA, henceforth referred to MMRV) is a vaccine that contains measles, mumps, rubella, and varicella viral antigens. The viral strains contained in the vaccine are the same as those used to manufacture the measles, mumps and rubella
(M-M-R®II or M-M-RVAXPRO®; Merck & Co., Inc., Kenilworth, NJ, USA, henceforth referred to as MMR) and varicella (VARIVAX®; Merck & Co., Inc., Kenilworth, NJ, USA) vaccines. A frozen formulation of MMRV was first approved in the USA in September 2005, and a refrigerator-stable formulation was approved in Europe in September 2006. The vaccine is indicated for active immunization for the prevention of measles, mumps, rubella, and varicella in children 12 months through 12 years of age. MMRV vaccine could facilitate the introduction of varicella vaccine into crowded national childhood immunization schedules, and thus, reduce the burden of varicella.Citation5,Citation7,Citation9 As of early 2018, approximately 30 million doses of MMRV were distributed worldwide.
The preferred route for vaccination varies according to national or local recommendations and physicians’ preferences, with some physicians preferring IM and others preferring SC injections.Citation10 Safety and immunogenicity of vaccines can vary according to the route and the site of injection, without affecting the level of clinical protection.Citation11,Citation12 Clinical trials have shown that MMRVAX PRO (known as M-M-RII in some countries) and VARIVAX were highly immunogenic and well tolerated when administered either SC or IM.Citation13–Citation17 According to the Summary of Product Characteristics for MMRV, the vaccine should be administered SC based on the route of injection assessed in pre-licensure immunogenicity and safety clinical trials. This study was designed to compare the immunogenicity and safety of two doses of MMRV when administered by the IM or the SC route to healthy children (NCT00402831), according to the vaccination schedule followed in the European Union when the study was conducted.
Results
Disposition of trial participants
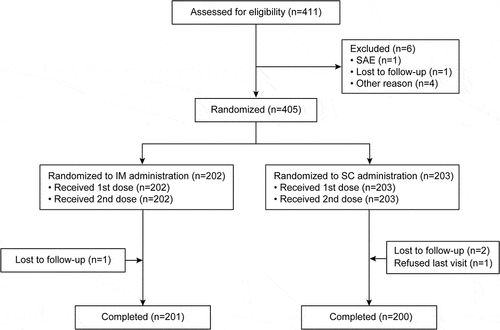
A total of 405 subjects were randomly allocated to either the IM group (202 subjects) or the SC group (203 subjects). All subjects received two doses of MMRV as planned in the protocol (). Four subjects withdrew from the study, one in the IM group (lost-to-follow-up) and three in the SC group (two lost-to-follow-up and one refused the last visit). In total, 178 (44.0%) subjects had at least one protocol deviation that excluded them from the PPS1 or PPS2 analyses (as defined in the Statistical Analyses section). The main reasons for excluding subjects from the analyses were either seropositivity to one of the 4 antigens at baseline or possible lack of appropriate randomization. Ninety-six (96) subjects were seropositive for one of the 4 antigens at baseline (3 [0.7%] for measles, 4 [1.0%] for mumps, 51 [12.6%] for rubella and 38 [9.4%] for varicella. These seropositivity rates are consistent with previous trials. For 73 subjects, it was unclear if the randomization procedure was followed and so these subjects were excluded from the primary analysis ().
Table 1. Characteristics of overall and antigen-specific per protocol set for primary analyses post dose 2 of MMRV (PPS2).
The gender and mean age at first vaccination of the subjects were similar between the IM and SC groups (48.0% and 53.7% males, and 13.7 [±1.4] and 13.7 [±1.5] months of age, respectively). The characteristics of the antigen-specific PPS2 for the primary analyses are summarized in . Overall, the baseline serostatus for measles, mumps, rubella, and varicella were similar between the two groups for PPS1 and PPS2. In the full analysis sets (FAS), baseline serostatus for measles, mumps, and varicella were similar between groups, but more subjects in the IM group were seropositive for rubella at baseline than in the SC group (15.8% and 9.4%, respectively).
Immunogenicity assessments
Primary immunogenicity analyses – antibody response rates post-dose 2
The antibody response rates (stratified by region) 6 weeks after the second dose of MMRV for the subjects who were initially seronegative for all vaccine antigens were >99% in both the IM and SC groups (). The 2-dose regimen of MMRV administered by the IM route was shown to be as immunogenic as the 2-dose regimen of MMRV administered by the SC route for all antigens at 6 weeks post-vaccination since the lower bounds of the two-sided 95% confidence intervals (CIs) for the difference in response rates for all antigens were greater than −10%. Therefore, the primary objective of the trial was met. Similar results were obtained for the analyses without stratification (results not shown) and the FAS ().
Table 2. Summary of non-inferiority analyses (stratified by region) of antibody response rates to measles, mumps, rubella, and varicella 6 weeks after the second dose of MMRV for participants initially seronegative to measles, mumps, rubella, and varicella – antigen specific per protocol set post-dose 2 (PPS2).
Table 3. Summary of non-inferiority analyses (stratified by region) of antibody response rates to measles, mumps, rubella, and varicella 6 weeks after the second dose of MMRV – Full Analysis Set (FAS).
Secondary immunogenicity analyses – antibody response rates post-dose 1 and GMTs post-dose 1 and 2
Four weeks after administration of the first dose of MMRV, the response rates for measles, mumps, rubella, and varicella in the antigen-specific PPS1 by the IM route were 100% (95% CI: 97.6; 100), 97.4% (95% CI: 93.4; 99.3), 98.4% (95% CI: 94.5; 99.8), and 98.6% (95% CI: 94.9; 99.8), respectively. In the SC group, the respective rates were 97.3% (95% CI: 93.2; 99.3), 91.3% (95% CI: 85.5; 95.3), 100% (95% CI: 97.3; 100), and 98.5% (95% CI: 94.8; 99.8). Similar results were observed for the FAS (results not shown).
The geometric mean titers (GMTs) for antibodies against measles, mumps, rubella, and varicella in subjects initially seronegative to the corresponding antigen were similar in the IM and SC groups post-dose 1 and post-dose 2 in the antigen-specific PPS (). The GMT for measles increased from post-dose 1 of MMRV and remained constant after dose 2; the GMTs for mumps and rubella increased slightly after dose 2. A booster effect was observed for varicella (>10-fold increase in GMT in comparison to levels achieved after the first dose of MMRV vaccine). Most of the subjects who were initially seropositive for one of the antigens achieved a 4-fold rise in titer post-dose 1 and post-dose 2 for the specific antigen. The GMTs post-dose1 and post-dose 2 were comparable in the FAS ().
Table 4. Summary of GMTs to measles, mumps, rubella, and varicella 4 weeks after the first dose and 6 weeks after the second dose of MMRV for participants initially seronegative to measles, mumps rubella and varicella – Antigen specific per protocol set post-dose 1 (PPS1) and post-dose 2 (PPS2).
Table 5. Summary of GMTs to measles, mumps, rubella and varicella 4 weeks after the first dose and 6 weeks after the second dose of MMRV – Full Analysis Set (FAS).
Safety and reactogenicity results
All 405 (100%) randomized subjects (202 in the IM group and 203 in the SC group) were included in the post-dose 1 safety analyses; 403 subjects (201 in the IM group and 202 in the SC group) were included in the post-dose 2 safety analyses.
Day 0 to day 28 post-dose 1
At least one injection-site or systemic adverse event (AE) was reported for 163 (80.7%) subjects in the IM group and 175 (86.2%) subjects in the SC group within 28 days following the first dose of MMRV (). During this period, about half of the subjects in each group reported at least one vaccine-related injection-site or systemic AE. Fewer subjects in the IM group experienced injection-site AEs compared with the SC group (17.8% and 28.6%, respectively). In both groups, most injection-site AEs started during the first four days after vaccination and their intensities were mainly mild or ≤2.5 cm. Injection-site erythema and injection-site swelling were less frequently reported in the IM group than in the SC group (5.0% and 14.3% and 1.0% and 3.9%, respectively). In contrast, injection-site pain was more frequent in the IM group (10.9%) than in the SC group (5.9%). There were no reports of injection-site rash of interest in the IM group and two (1.0%) reports of injection-site rash of interest in the SC group (one rubella-like rash and one varicella-like rash).
Table 6. Summary of injection site and systemic adverse events occurring from day 0 to day 28 post-dose 1 and post-dose 2 of MMRV.
The rates of systemic AEs within 28 days of dose 1 were similar between the groups (78.2% and 82.3% in the IM and SC groups, respectively). The rates of non-injection-site rashes of interest were also similar between the groups (). One subject (0.5%) in the IM group reported mumps/mumps-like illness; no mumps/mumps-like illness was reported in the SC group. The rates of vaccine-related fever within 28 days after the first dose were comparable for the two groups (35.6% in the IM group and 39.4% in the SC group). In addition, a body temperature ≥38.0°C (rectal or equivalent) was reported by 66.3% and 71.4% of subjects in the IM and SC groups, respectively. Of these, 15.6% and 18.1%, respectively, reported a temperature ≥39.4°C. Elevated temperature occurred mainly between Day 5 and Day 12 following the first dose in both groups.
There were two (1.0%) serious AEs (SAEs) reported in each group (viral gastroenteritis and viral tonsillitis in the IM group, and gastroenteritis and febrile convulsion in the SC group). None of the events were considered vaccine-related by the investigator. No deaths were reported. No AE led to withdrawal from the study following the first dose of MMRV.
Day 0 to day 28 post-dose 2
From Day 0 to Day 28 after the second dose, at least one injection-site or systemic AE was reported by 74.6% of subjects in the IM group and 72.0% of subjects in the SC group (). Fewer participants in the IM group reported at least one vaccine-related injection-site or systemic AE than in the SC group (34.8% and 42.5% of subjects, respectively). Similar to what was observed after the first dose, the imbalance in favor of the IM route was mainly related to two of the three solicited injection-site AEs from Day 0 and Day 4: erythema (15.4% vs. 27.0%, IM vs. SC group, respectively) and swelling (6.0% vs. 12.5%, IM vs. SC group, respectively); an equal number of subjects reported pain (10.0% in both groups). Only one subject reported an injection-site AE of severe intensity (pain, in the IM group).
Non-injection site rashes were reported in 2.0% of the IM group and 4.0% of the SC group after dose 2. The most common rash reported was a rubella-like rash or varicella-like rash. The rates of non-injection-site rashes were similar between the groups ().
The rates of fever, including vaccine-related fever, during the 28-day period after the second dose of MMRV were comparable between the two groups. During this period, 56.1% of subjects in the IM group and 55.4% of subjects in the SC group experienced a body temperature ≥38.0°C (rectal or equivalent), including 14.3% and 15.9% who experienced a temperature ≥.4°C in each group, respectively. After the second dose, the distribution of days on which children experienced their highest body temperature throughout the 28-day follow-up period after dose 2 was uniform in both groups.
Two non-vaccine-related SAEs were reported after the second dose of MMRV. One subject in the IM group reported gastroenteritis occurring 25 days post-dose 2, and one subject in the SC group reported a digital distal amputation (due to trauma) occurring 37 days post-dose 2. No deaths were reported and no subjects were withdrawn from the study due to an AE following the second dose of MMRV.
Discussion
The results from this randomized trial showed that two doses of MMRV administered by the IM route are as immunogenic as two doses of MMRV administered by the SC route to healthy children aged 12 to 18 months who were initially seronegative for measles, mumps, rubella, and varicella. The non-inferiority of IM versus SC administration was also demonstrated in a previous clinical trial in children 12 to 18 months of age who concomitantly received MMR and varicella vaccines (M-M-RII and VARIVAX, both manufactured by Merck & Co., Inc., Kenilworth, NJ, USA) containing the same vaccine strains used in the MMRV vaccine evaluated in this study.Citation18 The serology data from this study showed a numerically higher seroconversion rate and GMT after the first dose in favor of the IM route for all antigens except for rubella. This difference did not persist after the second dose of MMRV. These results are consistent with those reported in previous studies with the same refrigerated formulation of MMRV administered to infants at 12 months of age, except for the mumps response rate which was numerically lower in this study (91.3% [95% CI: 85.5; 95.3]) than in two previous studies (97.7% [95% CI: 96.5; 98.6] and 99.5% [95% CI: 98.4; 99.9], respectively).Citation17,Citation19A likely explanation for this difference is the time at which the response rate was measured which was four weeks after the first dose in this trial compared with six weeks in the previous studies. The results are also in agreement with those of previous studies in which the antibody response rate for varicella was not affected by the baseline serostatus.Citation16,Citation17 The results are generally similar to those of another study in which a different MMRV vaccine was shown to be highly immunogenic when administered via the IM or SC route in the second year of life.Citation15
MMRV was well tolerated irrespective of the route of vaccine administration and the safety profile was consistent with previous studies of this vaccine.Citation13,Citation14 The safety profile was similar in both groups after the first and the second doses, except fewer participants reported injection-site AEs in the IM group after each dose, although they reported a higher rate of injection-site pain. Lower rates of injection-site AEs were also reported in a previous trial that compared IM and SC administration of VARIVAX concomitantly with a combination pediatric vaccine, and another trial that compared IM and SC administration of ZOSTAVAX (Zoster Vaccine Live, Merck & Co., Inc., Kenilworth, NJ, USA) in adults.Citation18,Citation20 Fewer injection-site AEs were also reported after IM administration of another MMRV vaccine, with slightly less injection-site swelling within three days after IM administration, although injection-site pain was similar for both routes of administration.Citation15 A meta-analysis of 10 trials comparing administration of a first dose of the licensed MMRV vaccines and concomitant administration of MMR and varicella vaccine (MMR + V) reported that MMRV vaccination resulted in significantly higher incidences of fever (RR = 1.19 [95% CI: 1.09; 1.31]) and rash (RR = 1.23 [95% CI: 1.06; 1.43]).Citation21 The second dose of MMRV was generally better tolerated than the first dose in both groups as previously reported.Citation13,Citation17
Based on the results of this study, the package circular for MMRV was updated to include the IM route of administration.Citation13 Since this study was conducted, several vaccine advisory groups, including the US ACIP and the German STIKO, have recommended or preferred separate administration of MMR and varicella vaccines for the first dose and the tetravalent MMRV vaccine for the second dose.Citation14,Citation22 These recommendations are based on data suggesting a small increased risk of febrile seizures with the first but not the second doses of MMRV compared with separate MMR and varicella vaccines.
The strengths of this study include: 1) Reasonably large sample size; and 2) Antibody responses were assessed after each dose allowing for detection of a possible difference between the 2 routes of administration which could be masked if immunogenicity had only been assessed after dose 2. Limitations of the study include: 1) Although the study was randomized, it was open label and not placebo-controlled which could theoretically bias the safety assessment; 2) Immediate pain after injection was not assessed. While this is an interesting scientific question, the lack of a validated instrument to assess pain in this young age group limits the ability to address this question. 3) Cell mediated immunity (CMI) was not assessed. Evaluation of CMI could be another means to compare immune responses.
In conclusion, our results show that IM administration of MMRV in infants 12 to 18 months of age elicited similar antibody responses as those following SC administration, and the IM route was well tolerated with fewer injection-site AEs compared with SC administration. This confirms the IM route of administration as a valuable alternative route for administration of MMRV. Use of the IM route could facilitate vaccination with MMRV by physicians who prefer using the IM route in their routine vaccination practices. The millions of children vaccinated each year in routine immunization programs may benefit from the slightly better benefit/tolerability ratio demonstrated in this study. Furthermore, flexibility in using either route of administration without impact on vaccine safety or immunogenicity will diminish the number of vaccine delivery errors and the need to revaccinate children. Overall, these data could contribute to the improved performance of immunization programs against the four childhood diseases and also to the WHO’s measles and rubella elimination initiatives.Citation2,Citation23
Methods
Study design and participants
A phase III, open-label, randomized, comparative clinical trial of the immunogenicity and safety of MMRV administered by the IM or SC route to infants 12 to 18 months of age was performed at 33 sites in France. The primary objective was to demonstrate that a two-dose regimen of MMRV (one-month interval between doses) administered by the IM route to healthy children 12 to 18 months of age at the time of the first dose was as immunogenic as a two-dose regimen of MMRV administered by the SC route, i.e., was non-inferior in terms of antibody response rates to measles, mumps, rubella, and varicella at 6 weeks (~42 days) following the second dose. The secondary objectives were to describe: 1) the antibody response rates to measles, mumps, rubella, and varicella at one month (~30 days) following the first dose and at 6 weeks (~42 days) following the second dose of the vaccine; 2) the antibody titers to measles, mumps, rubella, and varicella at one month (~30 days) following the first dose and at 6 weeks (~42 days) following the second dose of the vaccine; and 3) the safety profile of two doses of MMRV both administered by the IM or the SC route.
Participants were healthy male and female subjects who had not previously been vaccinated against MMR or varicella and who had no clinical history of measles, mumps, rubella, varicella, and zoster. Subjects were excluded if they had previously received measles, mumps, rubella, or varicella vaccine separately or in any combination. They were also excluded if they had recent (≤30 days) exposure to measles, mumps, rubella, varicella, or zoster; recent (≤3 days) history of febrile illness or any severe chronic disease or medical condition likely to interfere with the trial assessments; known sensitivity or allergy to vaccine components; recent (≤30 days) high doses of systemic corticosteroid therapy or other immunosuppressive therapy; or receipt of any vaccine in the last 30 days or scheduled in the next 30 days.
The participants were randomized (stratified by center) to receive two doses of MMRV one month apart, either by the IM (Group 1) or the SC route (Group 2).
The trial was conducted in accordance with applicable national and local requirements and guidelines, the International Conference on Harmonisation, Good Clinical Practice standards, and the Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association, Declaration of Helsinki. Parents or legal guardians provided written, informed consent prior to inclusion in the trial.
Vaccine
MMRV is a lyophilized, live virus vaccine manufactured with recombinant human albumin for vaccination against measles, mumps, rubella, and varicella. After reconstitution, one dose (0.5 mL) contains a median cell culture infective dose (CCID50) of ≥103.0 for measles, ≥104.3 for mumps, ≥103.0 for rubella, and ≥103.99 plaque forming units of varicella virus. One needle was used to reconstitute the vaccine and another (size 16 mm 25G) was used to inject the vaccine. All doses were injected into the deltoid muscle of the upper arm, with the first dose in the right arm and the second dose in the left arm. For the IM injections, the needle was to be inserted perpendicular to the skin surface. For the SC injections, the needle was to be inserted at a 45° angle, taking care to deliver the vaccine only in the subcutaneous tissue.
Immunological assessment
Blood samples for immunological assessment were taken at Visits 1 and 2 before each injection and at the 3rd visit 42 days after the second dose of the vaccine. The serological testing was performed at Merck & Co., Inc. (Wayne, PA, USA) using appropriate enzyme-linked immunosorbent assays (ELISAs) for measles, mumps, and rubella, and the glycoprotein ELISA (gpELISA) for varicella-zoster virus.Citation24–Citation26
The primary immunogenicity criteria were the response rate for each antigen, defined as the percentage of participants who were seronegative for the specific antigen before the first injection (baseline) and who achieved the following antibody titers: measles antibody titer ≥255 mIU/mL (baseline < 255 mIU/mL), mumps antibody titer ≥10 ELISA Ab units/mL (baseline < 10 ELISA Ab units/mL), rubella antibody titer ≥10 IU/mL (baseline < 10 IU/mL), or varicella antibody titer ≥5 gpELISA units/mL (baseline < 1.25 gpELISA units/mL).
Safety assessment
Safety and tolerability were monitored after each dose of vaccine. The physician assessed any immediate AEs. The parent or legal guardian documented any AEs within the 28 days after each injection using a vaccination report card to record the size/intensity of solicited injection-site AEs from Day 0 to Day 4 (pain, erythema and swelling) and the following AEs from Days 0 to 28: axillary or rectal temperature (if the axillary temperature was ≥37.1°C); unsolicited injection-site AEs; measles-, rubella-, varicella- and zoster-like rashes, mumps and mumps-like illness, and systemic AEs. SAEs were monitored throughout the study.
Statistical analyses
The primary hypothesis was that 6 weeks (42 days) after the second dose of MMRV the response rates for each antigen in children in the IM group would not be inferior to those in children in the SC group. Non-inferiority for each antigen was achieved when the two-sided 95% CI around the difference in response rate between IM and SC administration (IM – SC) excluded a difference of 10% or more.
Under the assumption that 5% of subjects would have pre-vaccination detectable antibodies for measles, mumps, and rubella and 10% for varicella, and that 15% would be lost-to-follow-up or have a protocol deviation, it was estimated that 380 subjects (190 subjects randomized in each group) would provide 152 evaluable subjects in each group for the per protocol analyses for the measles, mumps, and rubella analyses, and 142 for the varicella analyses.Citation27 Assuming a response rate of 97% for the measles, mumps, and rubella antigens and 95% for the varicella antigen, it was calculated that 152 children per group would provide a power of 98.9% and 93.0% to detect non-inferiority of the IM route compared with the SC route for MMR and varicella, respectively, and an overall power of 90.1%.
Randomization was stratified by center but as the center recruitment was heterogeneous, ranging from 1 to 69 subjects, the statistical analyses were stratified by region (center’s data pooled based on their geographic location) to adjust for this heterogeneity.Citation28 The primary antibody response rate analyses, stratified by region, were performed on the antigen-specific PPS2 that included all randomized subjects who were seronegative for the corresponding antigen at baseline and who had valid pre- and post-dose 2 immunogenicity data for the specific antigen and no protocol violations. Sensitivity analyses without stratification by region were also performed. The secondary analyses of the antibody response rates were performed on the antigen-specific PPS1 that included all randomized subjects who were seronegative for the corresponding antigen at baseline and who had valid pre- and post-dose 1 immunogenicity data for the specific antigen and no protocol violations with and without stratification by region. Confirmatory analyses were performed on the FAS that included participants regardless of their initial serostatus or protocol deviations, who had received at least 1 dose of MMRV and who had immunogenicity data corresponding to post-dose 1 or post-dose 2, with and without stratification by region.
Descriptive analyses of the GMTs with their corresponding 95% CIs were performed for measles, mumps, rubella, and varicella within each group. In addition, descriptive analyses of antibody responses (GMT and 4-fold rise) to measles, mumps, rubella, and varicella at baseline after the first dose and the second dose of MMRV for subjects who were initially seropositive for measles, mumps, rubella, or varicella were performed on the FAS.
The safety and reactogenicity analyses were performed on the safety datasets for post-dose 1 and for post-dose 2. These datasets included all participants who had received at least 1 dose of MMRV and who had safety follow-up data at the corresponding time-point.
All statistical analyses were performed using SAS Version 9.1.3 (SAS Institute Inc, Cary, North Carolina, USA).
Disclosure of potential conflicts of interest
No potential conflict of interest were disclosed. Cécile Eymin, Anne Fiquet, and Benoit Soubeyrand were employees of SPMSD; Barbara Kuter is employed by Merck
Acknowledgments
Although the sponsor formally reviewed a penultimate draft, the opinions expressed are those of the authorship and may not necessarily reflect those of the sponsor. All co-authors approved the final version of the manuscript. The authors take full responsibility for the content of this manuscript and thank MediCom Consult, Villeurbanne, France (supported by Sanofi Pasteur MSD) for medical writing and editorial assistance.
The authors would like to thank the subjects who participated in the trial, as well as the following investigators and their study-site personnel, for their contribution to the study: Yves Alembik (Strasbourg), Robert Amar (Rouen), Catherine Ambacher (Vence), Jean-Pierre Arsene (Le Havre), Pierre Azerad (Cannes), Fabien Barnier (Frejus), Sophie Barrois (Lyon), Gérard Beley (Essey les Nancy), Anne Bertrand (St Laurent du Var), Bernard Bohe (Cannes La Bocca), Jacky Bougle (Vence), Fabrice Bouillot (Essey les Nancy), Danièle Clavel (Lyon), François de Chabanolle (St Priest), Thiery David (Ecully), Michel Drai (St Raphael), Alexandre Gardea (Illkirch), Jean-Luc Gasnier (Les Sables d’Olonne), Nathalie Gelbert (Chambery), Kai Kassmann (Draguignan), Françoise Laine (Le Harve), Xavier Lanse (Le Havre), François Lecruit (Rouen), Luc Levesque (Le Havre), Raed Mardini (Oullins), Elisabeth Mothe (Le Havre), Michel Navel (Ancenis), Christian Petit (Draguignan), Françoise Rembert (Dunkerque), Philippe Renaux (Lyon), Evelyne Suaud (Darnetal), Jean-Michel Thiron (Rouen), Franck Thollot (Essey les Nancy), Phu My Tran (Nice).
The authors also would like to thank the Clinical staff at Sanofi Pasteur MSD for their contributions to the conduct of the study and review of the manuscript, MAPI Naxis for monitoring the study, and the laboratory staff at Merck & Co., Inc. (Wayne, PA, USA) for overseeing the immunoassays.
Additional information
Funding
References
- Andre FE, Booy R, Bock HL, Clemens J, Datta SK, John TJ, Lee BW, Lolekha S, Peltola H, Ruff TA, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 86;2008:140–146.
- World Health Organisation. Global measles and rubella strategic plan 2012 – 2020 2012 [ accessed 2016 Dec 6]. https://www.unicef.org/immunization/files/Measles_Rubella_StrategicPlan_2012_2020.pdf.
- World Health Organisation. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014;89:265–287.
- Baxter R, Ray P, Tran TN, Black S, Shinefield HR, Coplan PM, Lewis E, Fireman B, Saddier P. Long-term effectiveness of varicella vaccine: a 14-year, prospective cohort study. Pediatrics. 2013;131:e1389–96. doi:10.1542/peds.2012-3303.
- Baxter R, Tran TN, Ray P, Lewis E, Fireman B, Black S, Shinefield HR, Coplan PM, Saddier P. Impact of vaccination on the epidemiology of varicella: 1995-2009. Pediatrics. 2014;134:24–30. doi:10.1542/peds.2013-4251.
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–340. doi:10.1093/cid/ciq077.
- European Centre for Disease Prevention and Control. Varicella vaccination in the European Union 2015. http://ecdc.europa.eu/en/publications/Publications/Varicella-Guidance-2015.pdf.
- Ackerson BK, Sy LS, Yao JF, Cheetham CT, Jacobsen SJ. Impact of MMRV combination vaccine on childhood vaccination compliance. Am J Manag Care. 18;2012:e440–5.
- Scott LJ. Measles-mumps-rubella-varicella combination vaccine (ProQuad): a guide to its use in children in the. E.U. Paediatric Drug. 2015;17:167–174. doi:10.1007/s40272-015-0123-7.
- Herzog C. Influence of parenteral administration routes and additional factors on vaccine safety and immunogenicity: a review of recent literature. Expert Rev Vaccines. 2014;13:399–415. doi:10.1586/14760584.2014.883285.
- Ajana F, Sana C, Caulin E. Are there differences in immunogenicity and safety of vaccines according to the injection method?. Med Mal Infect. 2008;38:648–657. doi:10.1016/j.medmal.2008.09.012.
- Cook IF. Evidence based route of administration of vaccines. Hum Vaccin. 4;2008:67–73.
- EMA. ProQuad Summary of Product Characteristics (SmPC). 2014. [ accessed 2014 Dec 5].http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000622/human_med_000997.jsp&mid=WC0b01ac058001d124.
- Klopfer SO, Stek JE, Petrecz M, Reisinger KS, Black SB, Goveia MG, Nicholson O, Gardner JL, Grosso AD, Brown ML, et al. Analysis of safety data in children after receiving two doses of ProQuad® (MMRV). Vaccine. 2014;32:7154–7160. doi:10.1016/j.vaccine.2014.08.067.
- Knuf M, Zepp F, Meyer CU, Habermehl P, Maurer L, Burow H-M, Behre U, Janssens M, Willems P, Bisanz H, et al. Safety, immunogenicity and immediate pain of intramuscular versus subcutaneous administration of a measles-mumps-rubella-varicella vaccine to children aged 11-21 months. Eur J Pediatr. 2010;169:925–933. doi:10.1007/s00431-010-1142-6.
- Silber JL, Chan IS, Wang WW, Matthews H, Kuter BJ. Immunogenicity of Oka/Merck varicella vaccine in children vaccinated at 12-14 months of age versus 15-23 months of age. Pediatr Infect Dis J. 2007;26:572–576. doi:10.1097/INF.0b013e318060d33d.
- Vesikari T, Becker T, Gajdos V, Fiquet A, Thomas S, Richard P, Baudin M. Immunogenicity and safety of a two-dose regimen of a combined measles, mumps, rubella and varicella live vaccine (ProQuad®) in infants from 9 months of age. Vaccine. 2012;30:3082–3089. doi:10.1016/j.vaccine.2012.02.062.
- Gillet Y, Habermehl P, Thomas S, Eymin C, Fiquet A. Immunogenicity and safety of concomitant administration of a measles, mumps and rubella vaccine (M-M-RVAX PRO) and a varicella vaccine (VARIVAX) by intramuscular or subcutaneous routes at separate injection sites: a randomised clinical trial. BMC Med. 2009;7:16. doi:10.1186/1741-7015-7-16.
- Bernstein HH, Eves K, Campbell K, Black SB, Twiggs JD, Reisinger KS, Conti RM, Flodmark C-E, Rombo L, Klopfer S, et al. Comparison of the safety and immunogenicity of a refrigerator-stable versus a frozen formulation of ProQuad (measles, mumps, rubella, and varicella virus vaccine live). Pediatrics. 2007;119:e1299–305. doi:10.1542/peds.2006-2283.
- Diez-Domingo J, Weinke T, de Lomas JG, Meyer CU, Bertrand I, Eymin C, Thomas S, Sadorge C. Comparison of intramuscular and subcutaneous administration of a herpes zoster live-attenuated vaccine in adults aged >/=50 years: A randomised non-inferiority clinical trial. Vaccine. 2015;33:789–795. doi:10.1016/j.vaccine.2014.12.024.
- Leung JH, Hirai HW, Tsoi KK. Immunogenicity and reactogenicity of tetravalent vaccine for measles, mumps, rubella and varicella (MMRV) in healthy children: a meta-analysis of randomized controlled trials. Expert Rev Vaccines. 2015;14:1149–1157. doi:10.1586/14760584.2015.1057572.
- Jacobsen SJ, Ackerson BK, Sy LS, Tran TN, Jones TL, Yao JF, Xie F, Cheetham TC, Saddier P. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine. 2009;27:4656–4661. doi:10.1016/j.vaccine.2009.05.056.
- World Health Organisation. Global vaccine action plan 2011-2020. 2013. [ accessed 2017 Jun 22]. http://www.who.int/immunization/global_vaccine_action_plan/en/.
- Hammond O, Wang Y, Green T, Antonello J, Kuhn R, Motley C, Stump P, Rich B, Chirmule N, Marchese RD. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol. 2006;78:1679–1687. doi:10.1002/jmv.20754.
- Shehab ZM, Brunell PA, Cobb E. Epidemiological standardization of a test for susceptibility to mumps. J Infect Dis. 149;1984:810–812.
- Johnson CD, Kumar ML, Whitwell JK, Staehle BO, Rome LP, Dinakar C, Hurni W, Nalin DR. Antibody persistence afer primary measles-mumps-rubella vaccine and response to a second dose given at four to six vs eleven to thirteen years. Pediatr Infect Dis J. 15;1996:687–692.
- Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med. 9;1990:1447–1454.
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 4;1985:213–226.