ABSTRACT
Objectives: N. meningitidis carriage in Australia is poorly understood. This study aimed to estimate prevalence and risk factors for carriage of N. meningitidis in South Australian university students. We also sought to identify whether delayed freezing of oropharyngeal samples altered PCR positivity, cycle threshold, or culture positivity.
Methods: Oropharyngeal swabs were taken from first year university students and repeated after 3 months, with risk factor questionnaires completed at both visits. Specimens were subjected to real-time PCR screening for the presence of specific meningococcal DNA.
Results: The study enrolled 421 individuals, 259 returned at 3 months. At baseline, 56% of participants were female and 1.9% smokers. Carriage of N. meningitidis at baseline was 6.2% (95% CI, [4.2%, 8.9%]). Visiting a bar more than once a week (OR 9.07; [2.44, 33.72]) and intimate kissing (OR 4.37; [1.45, 13.14]) were associated with increased carriage. After imputing missing data, the point estimate for carriage at 3 months was 8.6% compared to 6.2% at baseline (OR 1.42; 0.91 to 2.20). Recovery of N. meningitidis on selective agar was significantly reduced in cryovials frozen at 48 hours compared to 6 hours (24/26, 92.3% vs. 14/26, 53.9%, p = 0.002).
Conclusion: Attending bars and engaging in intimate kissing is associated with oropharyngeal carriage in South Australian university students. Adolescent meningococcal vaccine programs should be implemented at school, prior to increased attendance at bars, intimate contact, and carriage acquisition. Delaying freezing of oropharyngeal specimens longer than 16 hours reduces yield of N. meningitidis by culture but not PCR detection.
Introduction
Neisseria meningitidis is a gram negative diplococcus that colonises the human pharyngeal mucosa.Citation1 Exposure to N. meningitidis is common, with pharyngeal carriage rates of approximately 10% in students aged between 15–19 years in the United Kingdom (UK).Citation2 On rare occasions, N. meningitidis passes through epithelial cells into the bloodstream causing rapid systemic meningococcal sepsis and potentially meningitis, usually within 10 days of exposure.Citation3
In South Australia, there are 2.2 cases of invasive meningococcal disease (IMD) per 100,000 people – one of the highest notification rates outside of the meningitis belt of sub-Saharan Africa.Citation4,Citation5 Serogroup B has remained the predominant group causing invasive disease in South Australia over the past decade.Citation6 The seasonal trend of invasive meningococcal disease in Australia usually peaks during winter and early spring (June to October).Citation7 It is not clear if seasonality impacts on meningococcal carriage prevalence.
In Australia, from 2003 to 2018 a single dose of Meningococcal C containing vaccine (MenCCV) was on the National Immunisation Program for all children at the age of 12 months.Citation8 On July 2018 this was replaced by a single dose of MenACWY vaccine. The meningococcal B vaccine (4CMenB) is licensed in Australia but is not funded in the National Immunisation Program. During 2017 and 2018, all year 10, 11, and 12 students in South Australia were offered 4CMenB vaccination as part of a cluster randomised trial to assess the impact of the 4CMenB vaccine on carriage.Citation9 Understanding the prevalence of N. meningitidis and risk factors for pharyngeal carriage are important precursors to the introduction of meningococcal vaccine programs.Citation4 Prevalence of carriage increases in adolescents, peaking at around 19 years of age.Citation10 Due to their high prevalence of N. meningitidis carriage, adolescents are considered an important group in the transmission of the bacteria. N. meningitidis carriage in Australia is poorly understood, primarily due to a dearth of carriage data being available. The only published data available reports a carriage rate of 1.7% from 294 private school boys in Queensland during 1989.Citation11
This study preceded and informed methods used in the cluster randomised trial assessing the impact of meningococcal B vaccine (4CMenB) on pharyngeal carriage of N. meningitidis. The larger trial is a population-based study involving metropolitan, rural, and remote high school students in South Australia. Over 34,000 students are to be immunised through their school immunisation providers, as well as have oropharyngeal swabs collected over a 3-month period.Citation9 Due to vast transport distances in the RCT (up to 1800km), we needed to establish the loss of isolate yield with different times to sample freezing.
New methods for confirmation of N. meningitidis carriage are emerging, however the current gold standard remains plating oropharyngeal swabs directly on selective agar culture media.Citation12,Citation13 Logistically, direct plating of oropharyngeal swabs may not be possible in large population wide interventional vaccine studies.Citation13 Inserting swabs into cryopreservative broth and freezing the samples for processing later in batches has been the standard practice in pneumococcal carriage studies, and is now used in some meningococcal carriage studies.Citation14 However, the time from swab collection to freezing may be an important factor in the isolation of Neisseria species.Citation14 Saliva collection has been rarely used in meningococcal carriage studies, although this method is now being used by a group in the UK.Citation15,Citation16
Our study is the first to estimate carriage prevalence and risk factors of N. meningitidis in Australian university students. We sought to identify whether delayed freezing of oropharyngeal samples alters PorA PCR positivity, cycle threshold (Ct), or culture positivity of N. meningitidis. We also estimated the level of agreement between saliva samples and oropharyngeal swabs in detecting N. meningitidis carriage in adolescents, to inform if saliva testing is a useful replacement, or adjunct to oropharyngeal swabs.
Results
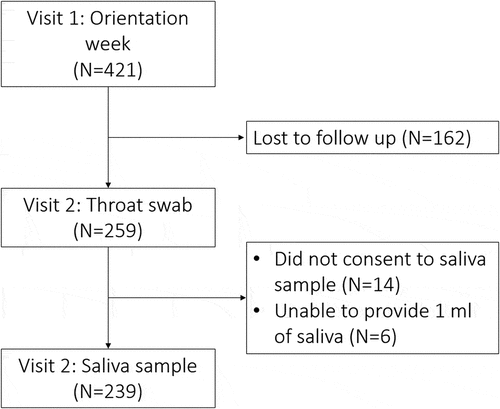
421 individual students were enrolled, mean age was 18.5 years (SD 1.4) (). There were 259 (61.5%) who returned after 3 months for a second swab (visit 2). A saliva sample was obtained from 239/259 (92.3%) of those that returned for visit 2 ().
Table 1. Characteristics of students at visit 1.
Carriage prevalence
Carriage prevalence of all groupable and non-groupable N. meningitidis from oropharyngeal swabs detected by porA rt-PCR at visit 1 was 6.2% (95% CI, 4.2% to 8.9%). No A, C, or X genogroups were identified (). Visit 1 carriage was higher in the students that did not return for their second swab compared to the group that returned (). Students who were lost to follow up at visit 2 were significantly more likely to have spent more nights at a pub or club in the week preceding visit 1 than those that were not lost to follow up (p < 0.001). Other characteristics were similar between participants that were lost to follow up and those that returned (Supplementary table). In participants returning at visit 2 the overall N. meningitidis carriage prevalence was 6.2% (95% CI, 3.8% to 9.9%) (). Accounting for missing data, the overall carriage prevalence at visit 2 among the 421 students who had a visit 1 oropharyngeal swab was estimated to be 8.6% (95% CI 5.0 to 12.2%). The logistic generalised estimating equation (GEE) found no evidence for a significant difference in the odds of carriage between the two visits (8.6% at visit 2 vs. 6.2% at visit 1, OR 1.42 (95% CI 0.91 to 2.20), p = 0.12). Of the students with swabs at both visits, 7 out of the 10 students who were PCR positive at visit 1 were positive again at visit 2. Three students who were PCR positive at visit 1 were negative at visit 2, all were group Y at visit 1. Nine students who were negative at visit 1 were positive at visit 2, 5 MenY, and 4 non-groupable.
Table 2. Proportion of Neisseria meningitidis pharyngeal carriage from oropharyngeal swab, at visit 1 (participants who were lost to follow up), visit 1 (returned for follow up swab), and combined total.
Table 3. Proportion of Neisseria meningitidis carriage at visit 2, in oropharyngeal swab, and saliva sample.
There was a moderate level of agreement in the identification of N. meningitidis between oropharyngeal swabs and saliva sample collected from participants, Kappa = 0.62 (95% CI, 0.39 to 0.85). Among the subset of 239 participants, saliva collection identified an additional four participants with carriage; one group B, and 3 non-groupable (Supplemental table). These 3 non-groupable samples all had high Ct values (≥37 cycles) and isolates were unable to be cultured for whole genome sequencing, presumably because of very low numbers of organisms present. Of the 9 participants that had N. meningitidis detected in both swab and saliva samples, isolates were not recovered from 2 participants, 6 had isolates recovered from both their swab and saliva, and 1 from their swab only. The Ct values from the 9 participants that had N. meningitidis detected in both swab and saliva samples were similar (mean 33.3 vs 35.3 cycles, p = 0.30).
Association with N. meningitidis carriage
Attending two or more parties or bars in a week and kissing one or more persons in the last week were associated with increased N. meningitidis carriage in univariable analysis (). In multivariable analysis, going out to a bar or pub one night a week (OR 4.14 [95% CI, 1.06 to 16.15], p = 0.04), two or more nights a week (OR 9.26 [95% CI, 2.51 to 34.08], p = 0.001), and one or more persons kissed in the last week (OR 4.13 [95% CI, 1.63 to 10.45], p = 0.014) remained statistically significant. The number of participants with carriage (n = 26) was too small to assess an interaction effect between these variables or to include other predictors in the multivariable model.
Table 4. Univariable logistic regression of PCR positive adolescents at visit 1, with participant characteristics.
N. meningitidis identification after freezing at 6, 16, and 48 hours.
Recovery of N. meningitidis on selective agar was significantly reduced in the cryovials frozen at 48 hours following collection, compared with those frozen at 6 hours (24/26, 92.3% vs. 14/26, 53.9%, p = 0.002). There was minimal difference between samples frozen at 16 hours compared to 6 hours (24/26, 92.3% vs. 23/26, 88.5%, p = 0.56). PorA PCR Ct values in frozen samples were not statistically different when frozen at 6, or 16 hours compared to pre freezing PorA PCR. There was a small statistically significant increase in the Ct value at 48 hours compared to baseline (mean 31.9 vs 31.2 cycles, p = 0.01). Little difference was identified in the Ct values at 6 hours compared to baseline (mean 31.0 vs 31.2 cycles, p = 0.51), and 16 hours compared to baseline (mean 31.4 vs 31.2 cycles, p = 0.08).
Genotypic characterization of carriage
From the 55 specimens that were PorA PCR positive, N. meningitidis was grown from 46 samples. MLST analysis was performed on these isolates. The CC23 clonal complex was most common, followed by CC41/44 (). The most common sequence types were ST-23 (CC23 clonal complex) with 8 isolates (17%), followed by ST-1655 (CC23) with 5 isolates (11%), ST-35 (CC35) with 4 isolates (9%). The most common CC41/44 sequence type was ST-44 with 3 isolates (7%). Five of the isolates were non-groupable. There was 1 MenW CC 11 sequence type, and none of the predominant porA genotype (P1.7–2,4) that had caused serogroup B disease in South Australia.Citation17
Table 5. Genotypic characterization of recovered isolates from throat and saliva samples by meningotype and PubMLST finetyping analysis among participants.
Discussion
Overall rates of carriage of N. meningitidis in South Australian university student population were lower than anticipated. This is possibly related to low numbers of participants smoking in this cohort. The numbers of adolescents smoking was lower than rates reported in a 2016 annual survey for 15 to 29 year olds in South Australia of 10.5% (95% CI, 7.8% to 13.2%),Citation18 and lower than other recent carriage studies in similar age groups.Citation2,Citation19,Citation20
Some carriage studies have reported a sharp increase in the prevalence of pharyngeal N. meningitidis during the first year of university.Citation21,Citation22 We did not find a statistically significant increase in carriage between the start of university and the visit 2 swab after 3 months. Associations with carriage at visit 1 reflected those reported previously in overseas studies, with attending one or more parties or bars in the last week, and kissing one or more persons in the last week both independently associated with carriage.Citation2,Citation19,Citation20,Citation23 Of note, students that went out more than once a week and kissed one or more people had a carriage prevalence of 25%. The low numbers of participants who smoked, used e-cigarettes, or water pipes meant that little can be elucidated from these potential risk factors in this cohort.
Our direct swab PCR method in the pilot study mirrored study processes for a large RCT.Citation9 In a UK study of over 2899 sample pairs, the sensitivity and specificity of direct swab PorA PCR compared to plating and then PCR of the isolate were 0.66 and 0.99 respectively.Citation13 The ability to grow isolates on selective agar were similar at 6 and 16 hours, however there was significant loss (46%) of isolates in samples frozen at 48 hours after collection. This was not reflected in the rt-PCR Ct values at 48 hours, suggesting a loss of viability of the bacteria if left more than 16 hours prior to freezing. Loss of isolates at 6 hours, 16 hours, and even 48 hours were less than previously reported in studies using an alternate transport medium.Citation15 STGG transport medium may be the primary reason for increased yields in this study.
Conflicting evidence currently exists regarding the suitability of saliva sampling in N. meningitidis carriage studies. Previous saliva methods have differed, with one study obtaining a saliva swab between the lower gums and lips,Citation24 and another using gargled 10 ml of sterile phosphate-buffered saline.Citation25 Using a swab for saliva collection resulted in overall carriage of 0.4% compared to 32.2% for nasopharyngeal swabs.Citation24 Higher detection was reported using mouth gargles, with N. meningitidis cultured in 10/89 (11%) compared to 13/89 (15%) in oropharyngeal swabs.Citation25 Our results suggest the saliva drool method of collection has the potential to provide a more accurate representation of carriage in adolescents, by increasing sensitivity when combined with oropharyngeal swabs. It does however come with additional costs, laboratory preparation time, and collection time. Saliva testing was of minimal additional value in this study. Higher detection of N. meningitidis in saliva than previously reported may have some implication for public health messaging, or contact tracing for the transfer of saliva through drink bottles and eating utensils.
In recent years P1.7–2,4 has been the predominant PorA type causing invasive disease in South Australia.Citation17 This is closely related to the NZ epidemic strain, but this PorA type was not identified from isolates in our pilot study. The majority of serogroup B isolates were comprised of other PorA-type from the CC 41/44, and all CC 23 were serogroup Y. Nevertheless, both CC 41/44 and CC 23 have previously been associated with disease.Citation26,Citation27 Previous comparisons of invasive disease sequence types to carrier types showed that ST-23 and ST-35 complexes were positively associated with non-invasive carriage.Citation28 Both the ST-23 and ST-35 complexes made up the higher proportion of isolates identified in our sample. It is not currently clear what impact the introduction of vaccines will have on inter-strain competition and carriage of non-disease causing meningococci.
The majority of carriage studies performed in university aged adolescents are cross-sectional analyses at a single point in time.Citation28 Longitudinal studies are essential for establishing a change in individuals. Longitudinal carriage studies that have been conducted generally report descriptive results of new acquisition, or carriage prevalence at each follow up period.Citation13,Citation19,Citation29–Citation31 Very few have attempted to statistically estimate the difference in carriage over a time period.Citation21 To estimate a statistically robust estimate of the difference in carriage prevalence in participants we used GEEs. This approach handles correlated data that arise from repeated measures of the same individuals over time. This approach was also used in a 2014 randomised control trial assessing the impact of MenACWY-CRM and 4CMenB on carriage longitudinally.Citation32
The primary motivation for an imputed analysis in this study was to account for differences in risk behaviours between returning students and those lost to follow-up. To illustrate, we found that students who spent one or more nights out at a club or pub in the last week at visit 1 (a) were more likely to be lost to follow-up, and (b) had higher carriage prevalence at both visits. Thus the loss of these students would be expected to lead to an underestimate of the carriage prevalence at visit 2 when restricting the analysis to just those students who returned. In accounting for these differences, the more realistic imputed analysis produces a higher carriage prevalence estimate at visit 2 (8.6%) compared to simply ignoring the missing data and restricting the analysis to returning students (6.2%). If loss to follow-up was related to unmeasured characteristics, and if these unmeasured characteristics were also predictive of carriage, then it is possible that our imputed estimate of carriage will be biased. Given that established predictors of carriage were included in the study questionnaire, however, we believe that the extent of this bias should be reasonably small.
Every effort was made to limit attrition, however in longitudinal adolescent studies this is difficult to control. This is especially so in studies that don’t involve a treatment.Citation33 Other potential limitations with this study include the generalisability of results, with the low proportion of smokers in the study cohort suggesting an over-representation of health-conscious students. We did not conduct direct plating of oropharyngeal swabs and we compared the recovery of isolates in aliquots frozen at 16 and 48 hours with those frozen at 6 hours. It is possible that direct plating may have identified more meningococcal carriage than the direct swab PCR method and this requires further investigation in regards to isolate loss after freezing.Citation13 Regardless of the plating and testing methods used in this study, the sensitivity of swabbing is estimated to be between 60–83%.Citation34 The use of a standard operating procedure and training medical and nursing staff in swab technique should have maximised N. meningitidis detection.
Due to the carriage prevalence in this age group, adolescents are likely to be an important group to consider if meningococcal vaccines confer herd protection, although the effect of recombinant vaccines on carriage remains inconclusive.Citation32 In locations such as South Australia the direct impact of MenB vaccines on reducing invasive disease in adolescents means the introduction of a program is warranted, even without significant herd protection. University students with the highest risk of carriage may be the most likely to benefit from a MenB vaccination program. An adolescent immunisation program conducted in high schools would likely provide the best protection prior to increased mixing and behaviours associated with carriage.
Materials and methods
Visit 1
Recruitment occurred on The University of Adelaide campus during one week prior to commencement (orientation week, 20th to 24th of February 2017). First year university students aged 17 to 25 years were eligible to participate. Approximately 10,000 commencing students enrol at the University of Adelaide each year. Of the total university population, 27% are international students with 49% females and 51% males.Citation35 Oropharyngeal swabs from the posterior pharynx using a sweep from one tonsillar area to the other were collected by trained Registered Nurses and Medical Officers using sterile flocked swabs. The swabs were then placed into sterile vials containing 2 mL of skim milk, tryptone, glucose, and glycerol (STGG) transport medium (Thermo Scientific). These samples were kept in a cooler with ice bricks at temperatures between 8 and 14°C for up to 4 hours before being transferred to SA Pathology. A questionnaire adapted from the UK Meningococcal Carriage Group (UKMENCAR4) was completed by students to identify any associations with carriage.Citation23 Questions included age, gender, cigarette smoking, e-cigarette use, water pipe use, recent respiratory infection, antibiotic use, attending pubs or clubs, intimate kissing, household size, and ethnicity.
Ethics approval
This study was approved by the Women’s and Children’s Health Network Human Research Ethics Committee (HREC/16/WCHN/140) and all subjects provided written informed consent. Students received a $20 iTunes voucher on completion of swab collection to reimburse them for their time.
Visit 2
Students were contacted via email and telephone to return for a repeat oropharyngeal swab after 3 months. At least three attempts were made to contact students lost to follow up. These visits occurred between 30 May – 28 August 2017. For those that consented, 1ml of saliva was drooled into an empty sterile container and immediately syringed into a vial containing 1ml of STGG transport media. The oropharyngeal swabs and saliva samples were then stored and tested as described for visit 1.
Laboratory
On receipt of samples at SA Pathology all specimens were subjected to real-time PCR (rt-PCR) screening for the presence of specific meningococcal DNA using porA gene detection prior to freezing. Samples with Ct values ≤40 were considered positive. Further rt-PCR analysis was used on porA positive specimens to determine which serogroup was detected (A, B, C, W, X, Y). Non-groupable genotype was defined as no amplification in any serogroup-specific rt-PCR assay. At visit 1, each sample was aliquoted into three cryovials and frozen at −80°C at three different time points (6, 16, and 48 hours) following collection time. No direct plating was performed. Samples were thawed after 2 weeks and prepared for automated extraction on the Roche MagnaPure system. Any samples yielding a porA positive rt-PCR prior to freezing were cultured for Neisseria species on selective agar (NIMM, Micro Neisseria Medium (VCTA), BioMerieux Australia) and incubated in CO2 at 35°C. Plates were kept for up to 72 hours and examined daily. Potential Neisseria species were identified by standard diagnostic laboratory bacteriological methods including oxidase reaction and confirmation of identity using Matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI ToF). Whole genome sequencing analysis of N. meningitidis isolates was also performed. All samples were sequenced on an Illumina NextSeq platform with Illumina NextSeq 500 mid-output (Illumina V2; 2 × 150 cycles) kit, targeting at least 50x genome coverage. Sequencing data were quality filtered using Trimmomatic (v0.36),Citation36 and de novo assembled using SPAdes (v3.11.1).Citation37 Multilocus sequence typing and N. meningitidis fine typing were performed using meningotype (v0.8.1-beta),Citation38 and on PUBMLST.Citation39
Statistical analysis
Using a conservative carriage prevalence estimate of 8% from UK data,Citation40 a sample size of 500 university students allowed for the estimation of carriage prevalence ± 2.4%, with precision defined as the half-width of a 95% confidence interval (CI).Citation2 In testing for a difference in carriage prevalence between visits, missing data on overall carriage at visit 2 was addressed using multiple imputation (m = 50 imputations). Multiple imputation was performed using chained equations, with a conditional logistic regression model used to impute carriage at visit 2. In addition to carriage at visit 1, additional auxiliary variables (times out at a party, pub, bar or nightclub in the last week and number of people kissed in the last week) were included in this logistic model to help satisfy a missing at random assumption and improve the prediction of missing values. Imputed datasets were analysed using logistic regression models, with generalised estimating equations (GEEs) used to account for repeated measurements within students. To identify risk factors for overall carriage at visit 1, univariable and multivariable logistic regression models were used. A linear mixed effects model was used to investigate if there was a difference in mean Ct values in samples after freezing at 6, 16, and 48 hours. Analyses were completed using Stata v.14.2.
Author contributions
HM, MM, and AL designed the study. LW, MT, and AL performed rt-PCR and LW culturing of specimens. LL and GR conducted WGS. MM, LW, LL, and MT acquired and entered data. MM and TS analysed the risk factors, PCR and culture data. LL and GR analysed WGS results. All named authors were involved in the interpretation of data, critically reviewing the content, and have approved the final version for publication.
Disclosure of potential conflicts of interest
HM is supported by a NHMRC CDF APP1084951 and is a member of the Australian Technical Advisory Group on Immunisation, Australian Government. HM is an investigator on vaccine trials sponsored by Industry (GSK, Novavax, and Pfizer). HM’s and MM’s institution receives funding for investigator led studies from Industry. HM and MM receive no personal payments from Industry. LL, GR, AL, MT, and RA report no conflict of interest.
Supplemental Material
Download ()Acknowledgments
VIRTU study team, Adelaide Health Technology Assessment team, SA Pathology, and The Women’s and Children’s Hospital Foundation. Dr Odile Harrison for helpful discussion regarding the analysis of genogroup typing.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Pizza M, Rappuoli R. Neisseria meningitidis: pathogenesis and immunity. Curr Opin Microbiol. 2015;23(Supplement C):68–72. doi:10.1016/j.mib.2014.11.006.
- MacLennan J, Maiden M, UK Meningococcal Carriage Group. UKMENCAR4: A meningococcal carriage study in 21,000 teenagers to understand changing meningococcal epidemiology and evaluate National vaccination policy. 20th International Pathogenic Neisseria Conference; 2016; Manchester, UK.
- Caugant DA, Maiden MC. Meningococcal carriage and disease-population biology and evolution. Vaccine. 2009;27(Suppl 2):B64–70. doi:10.1016/j.vaccine.2009.04.061.
- Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echaniz-Aviles G, Findlow J, Head C, et al. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–328. doi:10.1080/14760584.2017.1258308.
- Invasive Meningococcal Disease National Surveillance Report - With a focus on MenW - September. Australian Government, Department of Health; 2017 [ accessed 2018 Oct 16]. http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-meningococcal-W.htm.
- National Notifiable Diseases Surveillance System, Invasive Meningococcal Disease Public Data Set 2009 to 2016. Australian Government, Department of Health. [ accessed 2017 Jan 4]. http://www9.health.gov.au/cda/source/pub_menin.cfm.
- Invasive Meningococcal Disease National Surveillance Report - With a focus on MenW - March. Australian Government, Department of Health; 2018. [ accessed 2018 Oct 16]. http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-meningococcal-W.htm.
- Australian Technical Advisory Group on Immunisation. The Australian Immunisation Handbook (updated June 2015). 10th ed. Canberra, Australia: Australian Government Department of Health; 2015.
- Marshall HS, McMillan M, Koehler A, Lawrence A, MacLennan JM, Maiden MCJ, Ramsay M, Ladhani SN, Trotter C, Borrow R, et al. B Part of It protocol: a cluster randomised controlled trial to assess the impact of 4CMenB vaccine on pharyngeal carriage of Neisseria meningitidis in adolescents. BMJ Open. 2018;8(7):e020988. doi:10.1136/bmjopen-2017-020988.
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–861. doi:10.1016/S1473-3099(10)70251-6.
- Ingram SB, Wilson BJ, Kemp RJ, Faoagali J. Neisseria meningitidis in a school population in Queensland. Med J Aust. 1990;152(6):332.
- Jordens JZ, Heckels JE. A novel porA-based real-time PCR for detection of meningococcal carriage. J Med Microbiol. 2005;54(Pt 5):463–466. doi:10.1099/jmm.0.45847-0.
- Jeppesen CA, Snape MD, Robinson H, Gossger N, John TM, Voysey M, Ladhani S, Okike IO, Oeser C, Kent A, et al. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect. 2015;71(1):43–52. doi:10.1016/j.jinf.2015.02.006.
- Finn A, Morales-Aza B, Sikora P, Giles J, Lethem R, Marlais M, Thors V, Pollard AJ, Faust S, Heath P, et al. Density distribution of pharyngeal carriage of meningococcus in healthy young adults: new approaches to studying the epidemiology of colonization and vaccine indirect effects. Pediatr Infect Dis J. 2016;35(10):1080–1085. doi:10.1097/INF.0000000000001237.
- Roberts J, Greenwood B, Stuart J. Sampling methods to detect carriage of Neisseria meningitidis; literature review. J Infect. 2009;58(2):103–107. doi:10.1016/j.jinf.2008.12.005.
- Finn A, Christensen H, Oliver J, Trotter C. Study to evaluate prevention of meningococcal carriage in teenagers (SPIT). Meningitis Research Foundation; 2016 [ accessed 2018 Jun 1]. https://www.meningitis.org/research-projects/study-to-evaluate-prevention-of-meningococcal.
- Lahra MM, Enriquez RP, National Neisseria N. Australian Meningococcal Surveillance Programme annual report, 2015. Commun Dis Intell Q Rep. 2016;40(4):E503–E511.
- Martin K, Bowden JCM. Key Smoking Statistics for SA - 2016. South Australian Health and Medical Research Institute (SAHMRI); 2017 [ accessed 2017 Feb 2]. https://www.sahmri.org/population-health-theme/resource-11/.
- van Ravenhorst MB, Bijlsma MW, van Houten MA, Struben VMD, Anderson AS, Eiden J, Hao L, Jansen KU, Jones H, Kitchin N, et al. Meningococcal carriage in Dutch adolescents and young adults; a cross-sectional and longitudinal cohort study. Clin Microbiol Infect. 2017;23(8):573 e571–573 e577. doi:10.1016/j.cmi.2017.02.008.
- Breakwell L, Whaley M, Khan UI, Bandy U, Alexander-Scott N, Dupont L, Vanner C, Chang HY, Vuong JT, Martin S, et al. Meningococcal carriage among a university student population - United States, 2015. Vaccine. 2018;36(1):29–35. doi:10.1016/j.vaccine.2017.11.040.
- Ala’aldeen DA, Oldfield NJ, Bidmos FA, Abouseada NM, Ahmed NW, Turner DP, Neal KR, Bayliss CD. Carriage of meningococci by university students, United Kingdom. Emerg Infect Dis. 2011;17(9):1762–1763. doi:10.3201/eid1709.101762.
- Neal KR, Nguyen-Van-Tam JS, Jeffrey N, Slack RC, Madeley RJ, Ait-Tahar K, Job K, Wale MC, Ala’Aldeen DA. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional study. BMJ. 2000;320(7238):846–849.
- MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MC, et al. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis. 2006;12(6):950–957.
- Orr HJ, Gray SJ, Macdonald M, Stuart JM. Saliva and meningococcal transmission. Emerg Infect Dis. 2003;9(10):1314–1315. doi:10.3201/eid0910.030344.
- Jordens JZ, Williams JN, Jones GR, Heckels JE. Detection of meningococcal carriage by culture and PCR of throat swabs and mouth gargles. J Clin Microbiol. 2002;40(1):75–79.
- Jounio U, Saukkoriipi A, Bratcher HB, Bloigu A, Juvonen R, Silvennoinen-Kassinen S, Peitso A, Harju T, Vainio O, Kuusi M, et al. Genotypic and phenotypic characterization of carriage and invasive disease isolates of Neisseria meningitidis in Finland. J Clin Microbiol. 2012;50(2):264–273. doi:10.1128/JCM.05385-11.
- Racloz VN, Luiz SJ. The elusive meningococcal meningitis serogroup: a systematic review of serogroup B epidemiology. BMC Infect Dis. 2010;10:175. doi:10.1186/1471-2334-10-175.
- Caugant DA, Tzanakaki G, Kriz P. Lessons from meningococcal carriage studies. FEMS Microbiol Rev. 2007;31(1):52–63. doi:10.1111/j.1574-6976.2006.00052.x.
- Bidmos FA, Neal KR, Oldfield NJ, Turner DP, Ala’Aldeen DA, Bayliss CD. Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J Clin Microbiol. 2011;49(2):506–512. doi:10.1128/JCM.01322-10.
- De Wals P, Gilquin C, De Maeyer S, Bouckaert A, Noel A, Lechat MF, Lafontaine A. Longitudinal study of asymptomatic meningococcal carriage in two Belgian populations of schoolchildren. J Infect. 1983;6(2):147–156.
- Jordens JZ, Williams JN, Jones GR, Christodoulides M, Heckels JE. Development of immunity to serogroup B meningococci during carriage of Neisseria meningitidis in a cohort of university students. Infect Immun. 2004;72(11):6503–6510. doi:10.1128/IAI.72.11.6503-6510.2004.
- Read RC, Baxter D, Chadwick DR, Faust SN, Finn A, Gordon SB, Heath PT, Lewis DJ, Pollard AJ, Turner DP, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384(9960):2123–2131. doi:10.1016/S0140-6736(14)60842-4.
- Tobler AL, Komro KA. Contemporary options for longitudinal follow-up: lessons learned from a cohort of urban adolescents. Eval Program Plann. 2011;34(2):87–96. doi:10.1016/j.evalprogplan.2010.12.002.
- Trotter CL, Gay NJ. Analysis of longitudinal bacterial carriage studies accounting for sensitivity of swabbing: an application to Neisseria meningitidis. Epidemiol Infect. 2003;130(2):201–205.
- The University of Adelaide, Planning & Analytics. The University of Adelaide; 2018 [ accessed 2018 Apr 10]. https://www.adelaide.edu.au/planning/statistics/.
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi:10.1093/bioinformatics/btu170.
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi:10.1089/cmb.2012.0021.
- Kwong JC, Gonçalves da Silva A, Stinear TP, Howden BP, Seemann T. Meningotype: in silico typing for Neisseria meningitidis; 2017 [ accessed 2018 Apr 11]. https://github.com/MDU-PHL/meningotype.
- Jolley KA, Maiden MC. BIGSdb: scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11(1):595. doi:10.1186/1471-2105-11-595.
- Fitzpatrick PE, Salmon RL, Hunter PR, Roberts RJ, Palmer SR. Risk factors for carriage of Neisseria meningitidis during an outbreak in Wales. Emerg Infect Dis. 2000;6(1):65–69. doi:10.3201/eid0601.000112.