ABSTRACT
Purpose: To examine provider knowledge of HPV vaccination age guidelines in five countries.
Methods: A total of 151 providers of adolescent vaccinations in Argentina, Malaysia, South Africa, South Korea, and Spain were interviewed between October 2013 and April 2014. Univariate analyses compared providers’ understanding of recommended age groups for HPV vaccination to that of each country’s national guidelines.
Results: In three of five countries surveyed, most providers (97% South Africa, 95% Argentina, 87% Malaysia) included all nationally recommended ages in their target age group. However, a relatively large proportion of vaccinators in some countries (83% Malaysia, 55% Argentina) believed that HPV vaccination was recommended for women above age 26, far exceeding national guidelines, and beyond the maximum recommended age in the United States. National median minimum and maximum age recommendations cited by the respondents for HPV vaccination were 11 and 29 years in Argentina (national guideline: 11–14), 13 and 48 years in Malaysia (guideline 13–14), 8 and 14 years in South Africa (guideline 9–14), 10 and 20 years in South Korea (guideline 11–14), and 11 and 12 years in Spain (guideline 11–14). In all countries, a higher percentage of vaccinators included all nationally recommended ages for vaccination, as compared to providers who did not administer HPV vaccination.
Conclusions: Overall, a substantial proportion of providers incorrectly reported their country’s age guidelines for HPV vaccination, particularly the upper age limit. As provider recommendation is among the strongest predictors of successful vaccination uptake among adolescents, improved education and clarification of national guidelines for providers administering HPV vaccination is essential to optimize prevention of infection and associated disease.
Human papillomavirus (HPV) vaccines have been in use since 2006.Citation1 Nonavalent vaccination targets HPV types 6/11/16/18/31/33/45/52/58 which combined contribute to ~90% of cervical cancers, over 85% of anal, vulvar, vaginal, and head and neck cancers, and ~90% of genital warts.Citation2,Citation3 HPV vaccination functions as a prophylaxis against infection; therefore administration before natural HPV exposure, and 5–10 years prior to development of high-grade cervical intraepithelial neoplasia grades 2/3 are integral components of preventing cervical cancers and other HPV-associated diseases.Citation4,Citation5
Accordingly, the World Health Organization recommends HPV vaccination for girls between the ages of 9 and 14, with females ages 15 and older and males comprising secondary target populations.Citation6 A 2-dose schedule is recommended for girls and boys ages 9–14, with 3 doses recommended for ages 15 and above. In low and middle income countries, programs supported by Gavi focus on younger populations, vaccinating girls 9–14 years of age during the first year of implementation, and 9 year old girls exclusively in subsequent years.Citation7
National age guidelines for vaccination are a cornerstone of vaccination policy; standards which inform health providers of the most effective time to vaccinate children and adolescents.Citation8,Citation9 Though national and programmatic age recommendations for HPV vaccination exist, little global data have been published on providers’ knowledge of these guidelines, particularly outside of the U.S. Provider recommendation is one of the strongest predictors of HPV vaccination uptake among adolescentsCitation10,Citation11 and an incorrect understanding of the target age group by providers may lead to a less effective implementation of HPV vaccination programs in terms of both cost and disease prevention. This study was conducted among adolescent providers in Argentina, Malaysia, South Africa, South Korea and Spain to assess their knowledge of national HPV vaccination age guidelines.
Adolescent vaccination providers were recruited in Argentina, Malaysia, South Africa, South Korea, and Spain through non-probability convenience sampling between October 2013 and April 2014.Citation12 The main purpose of the study was to assess the acceptability of a two- versus three-dose vaccination schedule among health providers and mothers of adolescent girls, as previously described.Citation13 Participation was limited to providers who administered or oversaw provision of adolescent vaccines. Participants were recruited from databases of national professional organizations, national health insurance reviews and assessments, study staff contacts, websites, and lists from external consultants, and were contacted via mail, email, or phone. The target sample size was 30 providers per country, consistent with other studies of providers’ HPV vaccination perceptions.Citation14,Citation15
Participants were asked to specify the recommended age group for HPV vaccination within their respective countries: “for what age group is it recommended in [country name]?”, and to provide the dosage: “how many doses of HPV vaccine are needed?” Responses were classified as minimum and maximum recommended ages for HPV vaccination, and the number of doses required. While the study was ongoing, three doses were recommended internationally, and age recommendations varied according to specific country guidelines. Interviews were conducted face-to-face or over the phone by study staff trained in structured interviewing techniques. Informed consent was obtained from all study participants and study eligibility, consent, and survey documents were translated from English into local languages when necessary. In-country staff double-entered de-identified data into English language EpiData (EpiData Association, Odense, Denmark) forms. Data were cleaned and analyzed at the University of North Carolina (UNC), Chapel Hill. SAS 9.4 (SAS Institute Inc., Cary, NC) and R 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) software were used to compute all cross-tabulations, statistical graphics, and univariate analyses to compare provider’s HPV vaccination age and dosage recommendations to each country’s national guidelines. Study collaborators from each country received Institutional Review Board (IRB) approval from their respective institutions prior to data collection. UNC study staff received IRB approval for analysis of de-identified secondary data.
Of 353 providers contacted, 151 were interviewed from Argentina (n = 30), Malaysia (n = 30), South Africa (n = 31), South Korea (n = 30), and Spain (n = 30). One-third of providers practiced family medicine or were in general practice, 26% were obstetrician-gynecologists, and 22% were pediatricians. Most participants (80%) provided adolescent vaccinations in a clinic, and 40% in a hospital. The majority (70%) of providers administered HPV vaccination. Additional descriptive characteristics stratified by country have been reported previously.Citation12
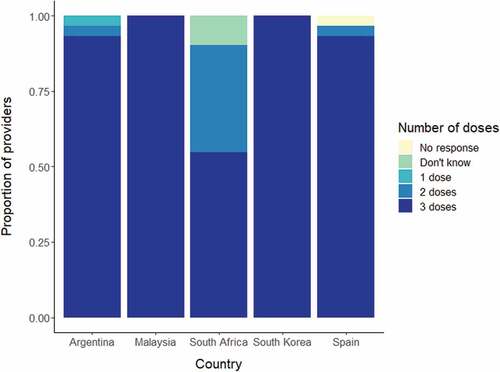
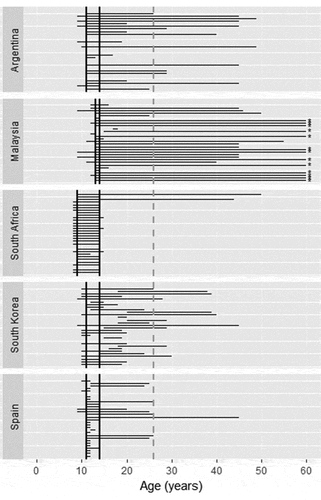
Median individually reported minimum and maximum recommended ages for HPV vaccination were 11 and 29 years in Argentina (national guideline reports: 11–14), 13 and 48 years in Malaysia (guideline: 13–14), 8 and 14 years in South Africa (guideline: 9–14), 10 and 20 years in South Korea (guideline: 11–14), and 11 and 12 years in Spain (guideline: 11–14) (, ). In three of five surveyed countries, most providers (97% South Africa, 95% Argentina, 87% Malaysia) included all nationally recommended ages for vaccination in their responses. However, a relatively large proportion of vaccinators in two countries (83% Malaysia, 55% Argentina) believed that HPV vaccination was recommended for women above age 26, far exceeding national guidelines, and beyond the maximum recommended age in the U.S.Citation16 In South Korea, 27% of vaccinators also recommended the HPV vaccine for women above age 26. In all countries, a higher percentage of HPV vaccinators included all nationally recommended ages, as compared to providers who did not administer HPV vaccination (Argentina: 95%, – ; Malaysia: 96%, 57%; South Africa: 100%, 94%; South Korea: 67%, 43%; Spain: 38%, 33%).
Table 1. Provider HPV vaccination age recommendations, stratified by country and HPV vaccinating status.
Figure 1. Provider-reported age recommendations for HPV vaccination.
* No maximum age recommendation indicated
Most providers (100% in Malaysia and South Korea; 93% in Argentina; 93% in Spain; and 55% in South Africa) reported that 3 doses of HPV vaccine are needed (). South Africa, which implemented a 2-dose school-based program in April 2014, after study completion, had the most variation in responses, with 35% of providers saying that 2 doses are needed, 55% that 3 doses are needed, and 10% who did not know.
Our findings suggest that among our small sample of providers within each country, knowledge of the recommended age group and dosage for HPV vaccination was not always consistent with national guidelines. The majority of providers in all five countries reported that 3 doses of HPV vaccination are needed, consistent with World Health Organization recommendations at the time of data collection.Citation17 Since that time, evidence that a 2 dose HPV vaccine schedule provides long lasting protection in adolescents 14 years and youngerCitation18,Citation19 has led to the regulatory approval of a 2-dose schedule for this age group in many countries. Reports of recommended age groups differed, with over half (55%-97%) of participating providers within all countries, barring Spain (38%), including all recommended vaccination ages. Likewise, a survey of 1,753 healthcare professionals in the U.S. found that 55% recommended HPV vaccination to the primary target population of adolescent females ages 11–12 years, and 16% of providers reported recommending HPV vaccination to females above age 26, exceeding national guidelines. In our study, a more substantial proportion of providers in three of five countries (34%-73%) reported maximum ages above 26 years. However, HPV vaccination should ideally be administered to younger age groups prior to age at HPV infection.Citation20,Citation21 Data from HPV vaccination studies have shown higher levels of neutralizing antibodies generated in younger adolescents aged 10–14Citation22 (10–15)Citation23 years post vaccination as compared with older female adolescents aged 15– 25Citation22 (16–23)Citation23 years. Program implementation has also provided evidence for vaccination of younger age groups; Australia’s HPV vaccination program demonstrated higher vaccine effectiveness for high-grade cervical precancer outcomes in younger women ≤14 years of age, with lower effectiveness among relatively older age groups who were more likely to be sexually active before receiving HPV vaccination.Citation24 These and similar findings have played a key role in informing national policy discussions and providing evidence for higher cost-effectiveness of vaccination policies directed at younger age groups.Citation25,Citation26
Our results are consistent with other research reporting that a notable proportion of health providers did not have a clear understanding of HPV vaccination age guidelines. In one survey of 254 health providers in the U.S., 25% incorrectly answered questions about recommended age groups to vaccinate girls and boys.Citation27 Qualitative interviews with 34 health providers in Minnesota and Washington suggested that providers did not feel an urgent need to vaccinate younger age groups, with 76% of providers in Washington not worried about completing all doses by age 12, and with 16 years of age being the earlier age at which providers expressed urgency to vaccinate.Citation28 Research has also underscored the importance of clearly communicating age guidelines for vaccination. A survey of 301 primary care physicians in the U.S. found that only 67% were likely to prescribe HPV vaccination to 11 and 12 year-olds, with a higher likelihood among those who agreed with a statement that HPV vaccine guidelines were clear.Citation29 Another study among 121 pediatricians, family practitioners, and internal medicine physicians serving minority populations in Brooklyn, N.Y. found that 45% reported that the ambiguity of national or local guidelines for HPV vaccination is a barrier to recommending HPV vaccination.Citation30
Encouraging health providers to target younger age groups for vaccination can be accomplished through existing communication channels. Messaging from professional organizations (i.e. American Academy of Family Physicians, American Academy of Pediatrics) influences providers to discuss and recommend HPV vaccination more often, and with greater strength.Citation31 The President’s Cancer Panel suggests similar avenues, recommending a CDC investigation into communication strategies with health professionals that emphasize the importance of universal vaccination of 11- and 12-year-olds in the U.S.Citation32 Word choice may also influence provider age recommendations; the Korea Centers for Disease Control & Prevention referred to the HPV vaccine as a “cervical cancer vaccine” at the time of introduction.Citation33 Emphasizing cancer prevention as opposed to protection against HPV could have possibly contributed to a relatively high proportion of providers recommending HPV vaccination to girls above age 26, although data are needed to support this argument. Examples of more intervention-based solutions include the use of the CDC’s Assessment, Feedback, Incentives, and eXchange program which offers consultation with an immunization specialist regarding current immunization rates and how to improve delivery practices through health providers.Citation34 This intervention has been shown to increase HPV vaccination coverage among adolescents ages 11–12 when delivered in-person or through a webinar format.Citation35
The primary limitation of our study stems from small sample sizes and use of convenience sampling. Although the number of individuals sampled is consistent with other research of provider perceptions of HPV vaccination,Citation14,Citation15 these groups are likely unrepresentative of all adolescent vaccination providers within each country. As such, the non-probability nature of the sampling method did not allow for deductions from our sample results to extend to the general population. Further, the quantitative structured interview was designed primarily to assess the acceptability of a two- versus three-dose vaccination schedule among health providers, which limited our ability to assess providers' knowledge on multiple levels; age and dosage variables are not perfect surrogates for providers’ knowledge of HPV vaccination guidelines. Additionally, while all study participants administered or oversaw the provision of adolescent vaccines, not all of them administered HPV vaccination, and a quarter were obstetrician-gynecologists, limiting the generalizability of our results to the overall cohort of providers of HPV vaccination in each country. Comparisons between individual countries should be performed with discretion due to differences in national HPV vaccination guidelines, vaccine availability, cultural context, and other unmeasured factors.
Taking into account these limitations, this study presents a unique compilation of provider data among five diverse locations, adding to prior research of HPV vaccination age recommendations in the U.S.Citation36 As provider recommendation is among the strongest predictors of successful vaccine uptake among adolescents,Citation13,Citation14 providers are well positioned to strongly recommend HPV vaccination for girls within the nationally recommended target age range. Programmatic interventions and ministries of health should provide clear policies on age recommendations for HPV vaccination at the national level. Assessing current provider knowledge of targeted age groups can inform ongoing national HPV vaccination policies for implementation; for instance, national vaccination policy recommendations using a rounded, easily remembered age such as 10 years, or a certain grade of school or birth year cohort.Citation29 Communicating these and comparable vaccination guidelines efficiently to providers will enable countries to most effectively protect key target populations against HPV infection and associated disease.
Disclosure of potential conflicts of interest
A. Mitch Dizon is a consultant for Teleflex. Karin Richter has been a paid speaker and has received travel support from Merck for meetings unrelated to this study. Silvia de Sanjose has received institutional grants from Merck for HPV related research. Jennifer S. Smith has received research grants, served on paid advisory boards, and/or been a paid speaker for GlaxoSmithKline and Merck over the past 5 years. The remaining authors have no conflicts of interest to disclose.
Acknowledgments
The authors wish to thank Sara B. Smith for her assistance with study administration.
Additional information
Funding
References
- 2006 approval letter - human papillomavirus quadrivalent (types 6, 11, 16, 18) vaccine, recombinant. Rockville (MD): Department of Health and Human Services; 2006.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi:10.1002/ijc.30716.
- Hartwig S, St Guily JL, Dominiak-Felden G, Alemany L, de Sanjosé S. Estimation of the overall burden of cancers, precancerous lesions, and genital warts attributable to 9-valent HPV vaccine types in women and men in Europe. Infect Agent Cancer. 2017;12(1):19. doi:10.1186/s13027-017-0129-6.
- Castle PE, Fetterman B, Akhtar I, Husain M, Gold MA, Guido R, Glass AG, Kinney W. Age-appropriate use of human papillomavirus vaccines in the U.S. Gynecol Oncol. 2009;114(2):365–369. doi:10.1016/j.ygyno.2009.04.035.
- Hildesheim A, Herrero R. Human papillomavirus vaccine should be given before sexual debut for maximum benefit. J Infect Dis. 2007;196(10):1431–1432. doi:10.1086/522869.
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec. 2017;92(19):241–268.
- Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine. 2018;36(32Pt A):4761–4767. doi:10.1016/j.vaccine.2018.02.003.
- Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the advisory committee on immunization practices. Ann Intern Med. 2009;150(1):45–49. doi:10.7326/0003-4819-150-1-200901060-00009.
- Pickering LK, Orenstein WA. Development of pediatric vaccine recommendations and policies. Semin Pediatr Infect Dis. 2002;13(3):148–154. doi:10.1053/spid.2002.125857.
- Gamble HL, Klosky JL, Parra GR, Randolph ME. Factors influencing familial decision-making regarding human papillomavirus vaccination. J Pediatr Psychol. 2010;35(7):704–715. doi:10.1093/jpepsy/jsp108.
- Ylitalo KR, Lee H, Mehta NK. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US national immunization survey. Am J Public Health. 2013;103(1):164–169. doi:10.2105/AJPH.2011.300600.
- Vielot NA, Goldberg SK, Zimet G, Smith SB, McDonald MA, Ramos S, Morgan K, Joo Kim C, Richter KL, Peris M, et al. Acceptability of multipurpose human papillomavirus vaccines among providers and mothers of adolescent girls: A mixed-methods study in five countries. Papillomavirus Res. 2017;3:126–133. doi:10.1016/j.pvr.2017.04.001.
- Islam JY, Hoyt AM, Ramos S, Morgan K, Kim CJ, de Sanjose S, Butera N, Senkomago V, Richter KL, McDonald MA, et al. Acceptability of two- versus three-dose human papillomavirus vaccination schedule among providers and mothers of adolescent girls: a mixed-methods study in five countries. Cancer Causes Control. 2018;29:1115–1130. doi:10.1007/s10552-018-1085-1.
- Perkins RB, Clark JA. What affects human papillomavirus vaccination rates? A qualitative analysis of providers’ perceptions. Women’s Heal Issues. 2012;22(4):e379–e386. doi:10.1016/j.whi.2012.04.001.
- Javanbakht M, Stahlman S, Walker S, Gottlieb S, Markowitz L, Liddon N, Plant A, Guerry S. Provider perceptions of barriers and facilitators of HPV vaccination in a high-risk community. Vaccine. 2012;30:4511–4516. doi:10.1016/j.vaccine.2012.04.062.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination — updated recommendations of the advisory committee on immunization practices. Morb Mortal Wkly Rep. 2016;65(49):1405–1408. doi:10.15585/mmwr.mm6549a5.
- World Health Organization. Meeting of the strategic advisory group of experts on immunization, April 2014 – conclusions and recommendations. Wkly Epidemiol Rec. 2014;89(21):221–236.
- Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, Nene BM, Esmy PO, Joshi S, Poli URR, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17(1):67–77. doi:10.1016/S1470-2045(15)00414-3.
- Iversen O-E, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Nur Azurah AG, Fong SM, et al. Immunogenicity of the 9-valent HPV vaccine using 2-Dose regimens in girls and boys vs a 3-Dose regimen in women. JAMA. 2016;316(22):2411. doi:10.1001/jama.2016.17615.
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta‐analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–1799. doi:10.1086/657321.
- Smith JS, Melendy A, Rana RK, Pimenta JM. Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Heal. 2008;43(4):S5.e1-S5.e62. doi:10.1016/j.jadohealth.2008.07.009.
- Pedersen C, Petaja T, Strauss G, Rumke HC, Poder A, Richardus JH, Spiessens B, Descamps D, Hardt K, Lehtinen M, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007;40(6):564–571. doi:10.1016/j.jadohealth.2007.02.015.
- Block SL, Nolan T, Sattler C, Barr E, Giacoletti KED, Marchant CD, Castellsague X, Rusche SA, Lukac S, Bryan JT, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (Types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118(5):2135–2145. doi:10.1542/peds.2006-0461.
- Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med. 2013;11(1):227. doi:10.1186/1741-7015-11-227.
- Chesson HW, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of human papillomavirus vaccination in the United States. Emerg Infect Dis. 2008;14(2):244–251. doi:10.3201/eid1402.070499.
- Kim JJ, Ortendahl J, Goldie SJ. Cost-effectiveness of human papillomavirus vaccination and cervical cancer screening in women older than 30 years in the United States. Ann Intern Med. 2009;151(8):538–545. doi:10.7326/0003-4819-151-8-200910200-00007.
- Warner EL, Ding Q, Pappas L, Bodson J, Fowler B, Mooney R, Kirchhoff AC, Kepka D. Health care providers’ knowledge of HPV vaccination, barriers, and strategies in a state with low HPV vaccine receipt: mixed-methods study. JMIR Cancer. 2017;3(2):e12. doi:10.2196/cancer.7345.
- Henrikson NB, Tuzzio L, Gilkey MB, McRee A-L. “You’re never really off time”: healthcare providers’ interpretations of optimal timing for HPV vaccination. Prev Med Reports. 2016;4:94–97. doi:10.1016/J.PMEDR.2016.05.002.
- Kulczycki A, Qu H, Shewchuk R. Primary care physicians’ adherence to guidelines and their likelihood to prescribe the human papillomavirus vaccine for 11- and 12-year-old girls. Women’s Hea Issues. 2016;26(1):34–39. doi:10.1016/j.whi.2015.07.012.
- Bruno DM, Wilson TE, Gany F, Aragones A. Identifying human papillomavirus vaccination practices among primary care providers of minority, low-income and immigrant patient populations. Vaccine. 2014;32(33):4149–4154. doi:10.1016/j.vaccine.2014.05.058.
- Hswen Y, Gilkey MB, Rimer BK, Brewer NT. Improving physician recommendations for human papillomavirus vaccination: the role of professional organizations. Sex Transm Dis. 2017;44(1):42–47. doi:10.1097/OLQ.0000000000000543.
- Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer. A report to the president of the United States from the president’s cancer panel. Bethesda (MD): National Cancer Institute; 2014.
- Cervical Cancer Vaccine Card News. Korea centers for disease control & prevention. 2016. [ accessed 2018 Nov 19]. https://nip.cdc.go.kr/irgd/manage.do?service=getAcbbsView&BBSSEQNUM=98&GRPID=nip&BRDCOD=form.
- AFIX (Assessment, Feedback, Incentives, and eXchange). [ accessed 2018 Oct 31]. https://www.cdc.gov/vaccines/programs/afix/index.html
- Gilkey MB, Dayton AM, Moss JL, Sparks AC, Grimshaw AH, Bowling JM, Brewer NT. Increasing provision of adolescent vaccines in primary care: a randomized controlled trial. Pediatrics. 2014;134(2):e346–53. doi:10.1542/peds.2013-4257.
- Berkowitz Z, Malone M, Rodriguez J, Saraiya M. Providers’ beliefs about the effectiveness of the HPV vaccine in preventing cancer and their recommended age groups for vaccination: findings from a provider survey, 2012. Prev Med (Baltim). 2015;81:405–411. doi:10.1016/j.ypmed.2015.10.007.