ABSTRACT
Opioid use disorders (OUD) and fatal overdoses are a national emergency in the United States. Therapeutic vaccines offer a promising strategy to treat OUD and reduce the incidence of overdose. Immunization with opioid-based haptens conjugated to immunogenic carriers elicits opioid-specific antibodies that block opioid distribution to the brain and reduce opioid-induced behavior and toxicity in pre-clinical models. This study tested whether the efficacy of a lead oxycodone conjugate vaccine was improved by formulation in either aluminum hydroxide or the squalene-based oil-in-water emulsion MF59 adjuvant, which was recently FDA-approved for influenza vaccines in subjects 65+ years old. In adult BALB/c mice, alum formulation was more effective than MF59 at promoting the early expansion of hapten-specific B cells and the production of oxycodone-specific serum IgG antibodies, as well as blocking oxycodone distribution to the brain and oxycodone-induced motor activity. Alum was also more effective than MF59 at promoting early differentiation of peptide-specific MHCII-restricted CD4+ Tfh and GC-Tfh cells in adult C57Bl/6 mice immunized with a model peptide-protein conjugate. In contrast, alum and MF59 were equally effective in promoting hapten-specific B cells and peptide-specific MHCII-restricted CD4+ T cell differentiation in older C57Bl/6 mice. These data suggest that alum is a more effective adjuvant than MF59 for conjugate vaccines targeting synthetic small molecule haptens or peptide antigens in adult, but not aged, mice.
Introduction
Opioid use disorders (OUD) affect over 2 million people in the United States, and caused over 50,000 deaths in 2016. Treatment and other societal impacts of OUD cost the US an estimated $78 billion per year.Citation1 Therapeutic vaccines against OUD offer a viable treatment option that can be combined with current medications to improve clinical outcomes and potentially prevent fatal overdoses. The pre-clinical efficacy of vaccines against heroin and prescription opioids depends upon generating opioid-specific antibodies that selectively reduce opioid distribution to the brain, opioid-induced behavior, and toxicity associated with fatal overdoses in mice, rats and non-human primates.Citation2–Citation9 In immunized rats, greater vaccine efficacy correlated with higher individual opioid-specific serum IgG antibody titers.Citation2,Citation10 In immunized mice, vaccine efficacy correlated with the frequency of naïve and early activated hapten-specific B cells and carrier-specific T cells, as well as the formation of germinal centers (GC) in secondary lymphoid organs – an essential component of the adaptive immune response to vaccines.Citation11–Citation13 Vaccine efficacy against oxycodone required intact T cell signaling.Citation11,Citation14 Vaccine efficacy against oxycodone also correlated with specific patterns of oxycodone-specific serum IgG subclasses distribution, characterized by elevated IgG2a in wild-type mice immunized with an oxycodone vaccine combined with a neutralizing anti-IL-4 monoclonal antibody, or in IL-4 knockout mice immunized with the same oxycodone vaccine.Citation15 A better understanding of the immunological mechanisms underlying efficacy of addiction vaccines will provide a blueprint for development of more effective immunization strategies against opioids and other drugs of abuse.
In contrast to live attenuated vaccines, subunit or conjugate vaccines often require the use of an adjuvant to achieve optimal efficacy against the target antigen. Adjuvants enhance vaccine efficacy through recruitment of antigen presenting cells, and subsequent activation of antigen-specific T and B cells processes underlying generation of high affinity antibodies.Citation16–Citation19 Aluminum hydroxide (alum), the most commonly used vaccine adjuvant, promotes a Th2-polarized response in mice,Citation20 and a more balanced Th1 and Th2 response in humans.Citation21 Few alternatives to alum-based adjuvants have been approved by the FDA and the European Medical Agency (EMA), such as the TLR4 agonist monophosphoryl lipid A (MPLA) and the TLR9 agonist CpG,Citation13,Citation17 either formulated alone or in combination with alum. As novel adjuvants become available or approved, it is of interest to test them in combination with candidate vaccines to optimize their efficacy.
Influenza vaccine formulations containing the squalene-based oil-in-water emulsion MF59 have been recently FDA-approved for use in patients over 65 years old. The MF59-containing formulation elicits protective antibody responses against influenza in patients ranging from 6 months to over 65 years of age.Citation22–Citation24 Pre-clinical studies have shown that MF59 elicits GC formation,Citation25 increases GC-Tfh differentiation,Citation26 and may be more effective than alum in mice.Citation25,Citation27,Citation28 These studies supported testing whether use of the newly-approved MF59 adjuvant increases the efficacy of a candidate vaccine against oxycodone, one of the most commonly abused prescription opioids in the United States, which consists of an oxycodone-based hapten (OXY) conjugated to the keyhole limpet hemocyanin (KLH) carrier protein (OXY-KLH).Citation2–Citation4 Additionally, because an MF59-containing vaccine is approved for use in patients over 65 years, we also considered whether the efficacy of MF59-adjuvanted vaccines may be age-dependent and whether adjuvant choice should take in account the age of the target population. For instance, individuals 55 and older accounted for an estimated 40% of opioid-related hospitalizations in 2015.Citation29
This study first tested whether the OXY-KLH vaccine formulated either in MF59 or in alum was effective in inducing oxycodone-specific IgG antibodies that blocked oxycodone distribution to the brain and oxycodone-induced motor activity in mice. The study further characterized hapten-specific B cell population subsets over time in BALB/c mice immunized with OXY-KLH formulated either in MF59 or alum. Then, the study focused on characterization of peptide-specific MHCII-restricted CD4+ T cell populations subsets over time in C57Bl/6 mice immunized with a model protein-peptide conjugate vaccine formulated in either alum or MF59. Finally, both hapten-specific B cell and peptide-specific MHCII-restricted CD4+ T cell population subsets were characterized in aged BALB/c and C57Bl/6 mice immunized with either OXY-KLH or the peptide-protein conjugate immunogen. In this study, a candidate vaccine for OUD was more effective when formulated in alum adjuvant rather than MF59. Furthermore, results suggest that alum-containing vaccine formulations are more effective than MF59-formulated vaccines for triggering early, but not late, GC formation and B and T cell differentiation in mice.
Results
OXY-KLH formulated in alum is more effective than MF59 for blocking oxycodone distribution to the brain and oxycodone-induced motor activity
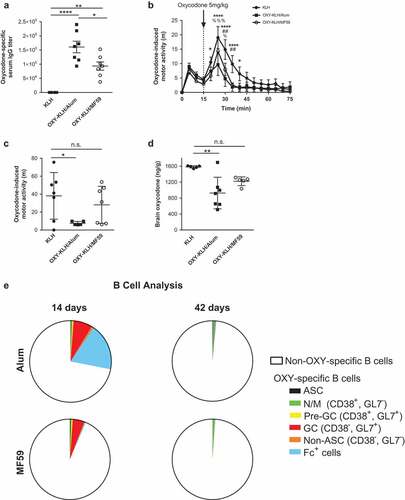
To determine whether formulation of an oxycodone vaccine in alum or MF59 adjuvants increased efficacy against oxycodone, mice were immunized with the candidate vaccine OXY-KLH formulated in either 2% alum or 50% MF59. These formulations were selected based on previous studies of OXY-KLH adsorbed on alum,Citation3,Citation11–Citation13 and per manufacturer recommended dosage in the case of MF59. Control mice were immunized with unconjugated KLH formulated in alum. Immunized mice were tested for oxycodone-specific serum IgG antibody titers and their efficacy in blocking oxycodone distribution to the brain, as well as oxycodone-induced locomotor activity, which is a behavioral effect associated with rewarding effects of opioids.
Oxycodone-specific serum IgG antibody titers were significantly higher in mice immunized with OXY-KLH formulated in alum as compared to OXY-KLH formulated in MF59, and both adjuvants showed a large increase in antibody titers as compared to the unconjugated KLH control (). Mice immunized with KLH, OXY-KLH plus alum, or OXY-KLH plus MF59 were subsequently challenged with 5 mg/kg oxycodone and monitored for changes in motor activity (). As anticipated, vaccination with OXY-KLH formulated with alum was effective at preventing oxycodone-induced motor activity compared to the KLH control group. By contrast, OXY-KLH formulated with MF59 was not effective in reducing oxycodone behavioral effects compared to KLH. In an independent experiment, immunized mice were challenged with 2.25 mg/kg oxycodone a week after the last vaccination to test for vaccine efficacy in blocking oxycodone distribution to the brain (). Mice immunized with OXY-KLH formulated in alum showed a statistically significant decrease in brain oxycodone concentration at 30 minutes post-injection, whereas mice immunized with OXY-KLH formulated in MF59 showed a smaller reduction in brain oxycodone concentration.
Figure 1. Behavioral, pharmacokinetic, and B cell comparison of alum and MF59. BALB/c mice were immunized with oxycodone vaccine OXY-KLH plus alum, OXY-KLH plus MF59, or a KLH control. (a) Mice were immunized on days 0, 14, 28, and 56, and OXY-specific IgG titers at 59 days were measured by ELISA (n = 7 per group). (b-c) Mice were immunized on days 0, 14 and 28, and motor activity induced by 5 mg/kg oxycodone was recorded over 60 minutes post-injection on day 45 (n = 7 per group). (c) Cumulative motor activity as total distance travelled during the first 15 minutes post-injection. (d) Mice were immunized on days 0, 14 and 28 and challenged with 2.25 mg/kg oxycodone on day 35, and oxycodone concentrations in brain 30 minutes after oxycodone administration were measured by GC-MS. (e) Mice were immunized on days 0, 14 and 28, and lymph nodes and spleens were collected at 14 or 42 days post-immunization (n = 5 per group). Total OXY-specific B cells populations were analyzed by flow cytometry. Data are mean±SEM. *p < 0.05, **p < 0.001, **** p < 0.0001.
OXY-KLH formulated in alum or MF59 triggers equivalent B cell expansion
To better understand the effect of alum and MF59 adjuvants on the adaptive immune response underlying vaccine efficacy, hapten-specific B cell population subsets were examined over time in mice immunized with OXY-KLH. To this end, analysis of OXY-specific B cells was performed by means of antigen-based magnetic enrichment paired with multiparameter flow cytometry.Citation11,Citation12 At 14 days post-immunization, mice immunized with OXY-KLH in alum showed a substantially larger number of Fc+ B cells as a proportion of total OXY-specific B cells (). However, at 42 days post-immunization, both the total number of oxycodone-specific B cells, and the proportions of ASC, naïve and memory B cells, GC B cells, and Fc+ cells, were not significantly different in the MF59 group as compared to alum. These data suggest that the early expansion of B cell subsets in mice immunized with alum may contribute to the greater vaccine efficacy.
Alum is more effective than MF59 in generating early Tfh and GC-Tfh cell responses to vaccination with a peptide-protein conjugate
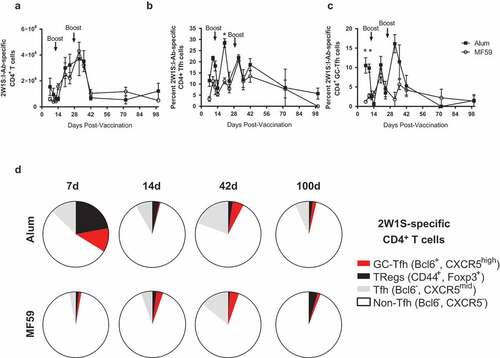
A 2W1S-based peptide:MHCII tetramer enrichment assay was used to assess expansion of 2W1S:I-Ab-specific CD4+ T cells as previously described.Citation30 Mice were immunized with 2W1S conjugated to SA-PE (2W1SSA-PE) formulated in alum or MF59 on days 0, 14, and 28, and lymph nodes and spleens were collected at intervals up to 100 days for analysis by flow cytometry.
During the initial immunization phase, the total number of 2W1S-Ab-specific CD4+ T cells increased in both groups, then decreased by 42 days (). While the total number of 2W1S:I-Ab-specific CD4+ T cells was similar in the alum-treated and MF59-treated groups, the frequencies of T follicular helper (Tfh) cells () and germinal center- Tfh (GC- Tfh) cells () were significantly higher in the alum-treated group at earlier time points. At 7 days after the first immunization, mice immunized with 2W1SSA-PE in alum showed larger populations of Tfh, GC-Tfh, and Treg cells as subsets of 2W1S:I-Ab-specific CD4+ T cells (). However, at 14 days the size of these T cell subsets was diminished, and beyond 14 days the T cell profiles did not differ between the alum and MF59 groups. These results suggest that vaccines formulated with alum induce a more robust early Tfh and GC-Tfh immune response, whereas alum and MF59 induce equivalent long-term T cell responses to conjugate vaccines.
Figure 2. Long-term flow cytometry analysis of 2W1S:I-Ab-specific CD4+ T cells using a peptide vaccine formulated with alum or MF59. Model immunogen phycoerythryin (PE) conjugated to streptavidin (SA), then conjugated to a model T cell peptide (2W1S) was used to track 2W1S:I-Ab-specific CD4+ T cells post-immunization. C57Bl/6 mice were immunized at days 0, 14 and 28, and cells were harvested at time points between 7 to 100 days (n = 3–6 per time point). (a) Total 2W1S:I-Ab-specific CD4+ T cells in the lymph nodes and spleen between 7 and 100 days post-immunization. (b) T follicular helper (Tfh) cells as percentage of 2W1S:I-Ab-specific CD4+ T cells. (c) GC-Tfh cells as a percent of 2W1S:I-Ab-specific CD4+ T cells. (d) Distribution of T cell subsets within the 2W1s:I-Ab-specific CD4+ T cell subsets at 7, 14, 42 and 100 days. Data are mean±SEM. *p < 0.05.
MF59 is equivalent to alum for the vaccination of aged mice
Because an MF59-adjuvanted influenza vaccine is approved for use in elderly patients, we next performed flow cytometry analysis of antigen-specific B and T cells in mice at the end of their reproductive age (10 months old). Because the greatest difference between adjuvants was observed at 7–14 days after a single immunization, aged mice were immunized once with OXY-KLH formulated in either alum or MF59, and flow cytometry was performed 7 days post-immunization (). Whereas young mice (6–7 weeks old) immunized with OXY-KLH in alum showed a larger proportion of Fc+ B cells ()), no significant differences were observed among Fc+ cells or other subsets of OXY-specific B cells ())
Figure 3. Flow cytometry analysis of OXY-specific B cells and 2W1S:I-Ab-specific CD4+ T cells in elderly mice. Elderly C57Bl/6 mice (aged 9–10 months) were immunized with OXY-KLH or 2W1SSA-PE, and lymph nodes and spleens were collected and analyzed at 7 days post-immunization. (a) Proportion of OXY-specific B cells subsets in mice immunized with OXY-KLH formulated with alum or MF59 (n = 5 per group). (b) Proportion of T cell subsets in mice immunized with 2W1SSA-PE formulated with alum or MF59 (n = 5 per group).
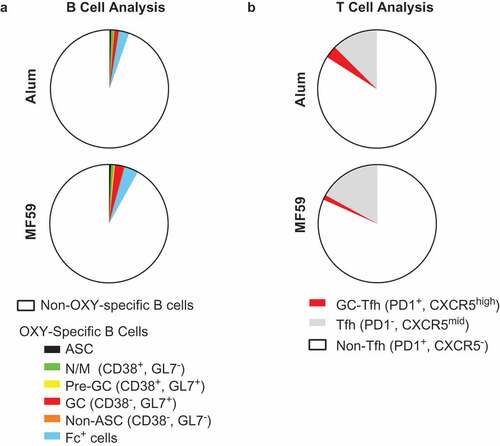
Peptide-specific T cells were next evaluated in aged mice immunized with 2W1SSA-PE formulated in either alum or MF59. No significant differences were observed in aged mice between the two adjuvants ()). Total Tfh and GC-Tfh cell numbers were equivalent between the two experimental groups (data not shown), and the total 2W1S:I-Ab-specific CD4+ T cell population showed a near-identical profile. This contrasted to the earlier time points post-immunization in young adult mice, where Tfh and GC-Tfh cell responses to alum were increased compared to MF59. Taken together, these experiments indicated that alum and MF59 adjuvants elicit similar B and T cell responses in aged mice.
Discussion
Adjuvants are important components of most vaccine formulations because of their role in boosting vaccine efficacy and tailoring immune responses. The FDA recently approved the use of the squalene-based oil-in-water emulsion MF59 in influenza vaccines administered to elderly patients. Our goal was to determine whether MF59 would provide a more effective adjuvant than alum for a candidate vaccine for OUD, and whether there were differences between the two adjuvants in eliciting antigen-specific B cells and CD4+ T cells in response to hapten- or peptide-protein conjugate vaccines.
We first tested whether formulating an oxycodone vaccine in either adjuvant would increase its efficacy in reducing oxycodone distribution to the brain and attenuating oxycodone-induced behavioral changes using the well-characterized locomotor activity test. We have previously shown that active immunization with the oxycodone vaccine OXY-KLH reduced oxycodone distribution to the brain in mice and rats,Citation2,Citation3 and prevented oxycodone-induced behavior and toxicity associated with overdose.Citation31 However, this is a first report of using oxycodone-induced motor activity to select a lead vaccine formulation. To date, OXY-KLH has been formulated in alum, Freund’s, MPLA, and alum mixed with MPLA.Citation13 Here, we hypothesized that MF59 would increase vaccine efficacy compared to the well-established adjuvant alum. Contrary to our expectations, oxycodone-specific serum IgG antibody titers were higher in mice immunized with OXY-KLH formulated in alum than mice immunized with OXY-KLH formulated in MF59. The OXY-KLH vaccine formulated in alum was also more effective in reducing oxycodone-induced motor activity in mice challenged with 5 mg/kg oxycodone. OXY-KLH in alum was also more effective at reducing oxycodone distribution to the brain in mice challenged with 2.25 mg/kg oxycodone. These doses (2.25–5 mg/kg) result in oxycodone plasma concentrations that are representative of the range found in patients with OUD (>50–150 ng/mL).Citation32,Citation33 Hence, a direct comparison of the oxycodone vaccine formulated in either MF59 or alum showed that alum is better at generating antibody levels that are effective in blocking clinically relevant doses of oxycodone.
It has been reported that MF59 increases B cell responses to immunization compared to alum.Citation25 In the context of active immunization against opioids, this study found that mice immunized with OXY-KLH in alum showed higher numbers of hapten-specific B cells and more pronounced GC B cell differentiation compared to MF59 (Supplemental ). These results are consistent with the enhanced production of oxycodone-specific serum IgG antibodies and greater vaccine efficacy against oxycodone when OXY-KLH was formulated in alum.
The finding that MF59 is not more effective than alum in promoting GC B cell differentiation differs from previous studies.Citation25,Citation26 However, study design varies with respect to antigen, immunization protocol, and immunological assays. For instance, previous studies found a significant effect of adjuvants on B cell responses in the lymph nodes closest to the injection site, while proximal lymph nodes and spleen showed no differences between groups.Citation25 In the current study, antigen-specific B cells were magnetically enriched from individual mouse samples consisting of pooled lymph nodes and spleen. While antigen-based magnetic enrichment may provide a sensitive method to detect scarce antigen-specific B cells in the total B cell repertoire, this method does not provide anatomical resolution. Future studies will benefit from integrating flow cytometric analysis of magnetically enriched antigen-specific B cells with immunohistochemistry assays to provide a more comprehensive analysis of vaccine responses.
Here, MF59 was found to promote Tfh cell responses, which is consistent with previous reports.Citation26 Antigen-specific CD4+ T cell population subsets were tracked over time in a model consisting of mice immunized with a peptide-protein conjugate paired with magnetic enrichment using 2W1S:I-Ab-MHCII tetramers and flow cytometry. Total 2W1S:I-Ab-specific CD4+ T cells were similar across both groups at all time points. The two adjuvant groups did not show significant differences in Tfh cells except at 20 days post-immunization, where the percentage of Tfh cells was higher in the alum than MF59 group. At earlier time points (days 7 and 14), mice immunized with 2W1SSA-PE formulated in alum showed higher GC-Tfh cells compared to formulations with MF59. To our knowledge, this is the first study that directly compares antigen-specific T cell responses to MF59 and a second vaccine formulation that includes a clinically-approved adjuvant. Since MF59 was not significantly better than alum in inducing adaptive immune responses against small molecule haptens and peptides conjugated to carrier proteins, perhaps use of MF59 should be limited to protein-based or live-attenuated vaccines.
Since MF59 was recently FDA-approved for influenza vaccines in patients 65+ years old,Citation34 MF59- and alum-adjuvanted vaccines were compared in 9–10 months aged mice to test for age-dependent differences in adaptive immune responses to these vaccine formulations. Analysis of antigen-specific B and T cell population subsets showed no differences in response to vaccines formulated in either alum or MF59 adjuvants. These data indicate that, in the context of peptide- and hapten-protein conjugate vaccines, MF59 and alum adjuvants were equally effective in mice. However, data from adult mice suggested that alum is more effective than MF59 to achieve optimal responses to opioid vaccines, and by extension other synthetic or conjugate vaccines.
These conclusions should be examined in light of the limitations of the study. First, while greater vaccine efficacy in the younger mice was found after three immunizations with OXY-KLH in alum, no statistically significant differences were found in the antigen-specific B or T cell populations between the two adjuvants at any time point later than 14 days after the first immunization. It was previously found that the frequency of early-activated hapten-specific B cell subsets, including GC B cells, correlated with subsequent vaccine efficacy against oxycodone and nicotine.Citation11 It is possible that adjuvants’ effect are limited to the early immune response to these vaccines, and that the early-activated antigen-specific B and T cell subsets shape and determine long-term vaccine efficacy against drugs of abuse. Alternatively, it is conceivable that at 35 days post-immunization and later, the immunological mechanisms that give rise to the pharmacological differences in vaccine efficacy are not detected by the methods used in this study. Additionally, while early immunological differences between the two adjuvants decreased in an age-dependent manner, later time points were not examined in aged mice, leaving the possibility that differences in response to the two adjuvants could arise under an extended immunization schedule. Still, these data show that the well-characterized alum adjuvant is equally or more effective than MF59 under several experimental conditions. In clinical trials of novel vaccines, it is beneficial to limit additional vaccine components to those that have regulatory approval and proven safety for use in humans. Based on these results, alum adjuvant is likely to be sufficiently effective for a potential clinical trial of OXY-KLH or other small molecule conjugate vaccines against opioids or other targets.
Materials and methods
Drugs and reagents
Oxycodone was obtained through the NIDA Drug Supply Program. Drug doses and concentrations are expressed as weight of the free base.
Mice
Male BALB/c and C57Bl/6 mice (Harlan Laboratories, 047 and 044 respectively, and Jackson Laboratories, 000651, 000664) were housed in a standard 12/12 hours light/dark cycle and fed ad libitum. Adult mice were 6–7 weeks old at arrival, while older mice were 9–10 months at arrival. Mouse studies were approved by the Minneapolis Medical Research Foundation and University of Minnesota Animal Care and Use Committees, and performed in accordance with the U.S. Department of Health and Human Services Guide for the Care and Use of Laboratory Animals, 8th edition.
Opioid-based hapten synthesis, peptides, and conjugates
An oxycodone-based hapten (OXY) containing a tetraglycine linker at the C6 position was synthesized from oxycodone as previously described.Citation11 For immunization studies, the OXY hapten was conjugated to KLH (Thermo Fisher, 77,600) by carbodiimide (EDAC) coupling chemistry and designated as OXY-KLH. For use as a coating antigen in ELISA, the OXY hapten was conjugated to OVA (Sigma, A5503-5G). For antigen-based magnetic enrichment of OXY-specific B cells, a biotinylated analog of the OXY hapten was conjugated to streptavidin bound to R-PE (SA-PE, Prozyme, PJRS25). A decoy reagent was prepared by conjugating the fluorescent dye Alexa-Fluor 647 (AF647, Thermo Fisher, A20173) to SA-PE. For immunization studies involving tracking of peptide-specific MHCII-restricted T cells (I-Ab), the biotinylated 2W1S peptide (EAWGALANWAVDSA) was conjugated to SA-PE.Citation11 For antigen-based magnetic enrichment, 2W1S peptide was conjugated either to APC or PE (2Wp:MHCII tetramers) as described.Citation30,Citation35,Citation36
Immunization
Mice were immunized s.c. with 75 μg of either OXY-KLH or unconjugated KLH as control. In studies focusing on analysis of 2W1S-specific T cell populations, mice were immunized s.c. with 25 μg of 2W1SSA-PE. All immunogens were adsorbed on 2% alum (Alhydrogel85, Brenntag Biosector, M0025312) or mixed with an equal volume of squalene-based oil-in-water emulsion AddaVax (MF59, Invivogen, vax-adx-10) to a final volume of 0.2 ml. These adjuvant doses were chosen based on our previously published optimal formulation of OXY-KLH in alum, Invivogen’s recommended use instructions, and existing studies using MF59.Citation25,Citation26,Citation37 Mice were immunized up to four times on days 0, 14, 28, and 56 as noted for each experiment.
Antibody analysis
Serum antibody analysis was performed by indirect ELISA in blood samples obtained by facial vein sampling. ELISA plates were coated with 5 ng/well of OXY-OVA conjugate or unconjugated OVA as control in 50 mM Na2CO3, pH 9.6 (Sigma, C3041-100CAP) and blocked with 1% gelatin. Mouse primary immune sera were bound to plates, and plates were washed and incubated with HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, 115–035-008) to determine oxycodone-specific serum IgG antibody titers as previously described.Citation3
Effect of immunization on oxycodone-induced motor activity
A behavioral study was performed to determine the extent to which the OXY-KLH vaccine formulated in either alum or MF59 prevented oxycodone-induced motor activity. Mice were immunized on days 0, 14, and 28, and locomotor sensitization was induced by two priming injections of oxycodone on days 41 and 43. Motor activity was recorded on day 45. Motor activity studies were conducted during the light cycle, and mice were first given 1 hour to habituate after transport to the behavioral room. Mice were injected with saline and placed in an open field (ENV-510S, Med Associates) for 15 minutes to establish a baseline. Then, mice were given 5 mg/kg oxycodone s.c. and their ambulatory activity was monitored for an additional 60 minutes. The entire apparatus was enclosed in a sound attenuating cubicle. Locomotor activity was monitored via infrared beam breaks and recorded by Activity Monitor 5.0 software (Med Associates).
Effect of immunization on oxycodone distribution
A pharmacokinetic study was performed to determine the extent to which the OXY-KLH vaccine formulated in either alum or MF59 prevented oxycodone distribution to the brain. Male BALB/c mice were immunized with OXY-KLH formulated in alum or MF59 on days 0, 14 and 28. On day 35 mice were given 2.25mg/kg oxycodone delivered s.c. and euthanized 30 mins post-injection via CO2 inhalation in IACUC-approved conditions. Animals were decapitated, trunk blood and brain were collected and oxycodone concentrations were analyzed via gas chromatography coupled to mass spectroscopy as previously described.Citation3
Analysis of antigen-specific B and T cell population subsets
Antigen-specific B and T cell populations were analyzed by antigen-based magnetic enrichment paired with multiparameter flow cytometry, as previously described.Citation11,Citation12 Briefly, lymph nodes and spleen were collected and disaggregated to a single-cell suspension before magnetic enrichment of antigen-specific B and T cells. Detailed procedures for B cell staining and flow cytometry, including gating strategies, has been previously reported. Supplemental material includes detailed procedures for T cell staining and gating strategies. Flow cytometry analysis was performed on a BD LSR II Fortessa using FACSDiva 7.0 software (BD Biosciences, 659528) and processed using FlowJo v10.1 (BD Biosciences).
Statistical analysis
Mean of oxycodone-specific serum IgG antibody titers, oxycodone concentrations, locomotor activity expressed as total ambulatory distance, and B and T cell numbers were compared by one-way ANOVA paired with Dunnett’s post hoc test for multiple comparisons and by Student’s t-test or Mann-Whitney test when analyzing two groups. Analyses were performed using Prism 7.0 (GraphPad).
Abbreviations
| germinal center | = | (GC) |
| T follicular helper | = | (Tfh) |
| antibody secreting cells | = | (ASC) |
| opioid use disorder | = | (OUD) |
| keyhole limpet hemocyanin | = | (KLH) |
| phycoerythrin | = | (PE) |
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Supplemental Material
Download ()Acknowledgments
Author Contributions: CR performed experiments and wrote the paper, SS helped perform flow cytometry, CA performed motor activity experiments and ELISA, CB helped write and edit the paper, DM provided reagents and wrote the paper, MP conceived the project and wrote the paper.
Supplementary material
Supplemental data for this article can be found on the publisher’s website.
Additional information
Funding
References
- Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54:901–906. doi:10.1097/MLR.0000000000000625.
- Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR. An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther. 2012;341:225–232. doi:10.1124/jpet.111.189506.
- Pravetoni M, Le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, Pentel PR. Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J Med Chem. 2013;56:915–923. doi:10.1021/jm3013745.
- Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG. Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One. 2014;9:e101807. doi:10.1371/journal.pone.0101807.
- Hwang CS, Smith LC, Natori Y, Ellis B, Zhou B, Janda KD. Efficacious vaccine against heroin contaminated with fentanyl. ACS Chem Neurosci. 2018. doi: 10.1021/acschemneuro.8b00079.
- Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, Janda KD. Development of a clinically viable heroin vaccine. J Am Chem Soc. 2017;139:8601–8611. doi:10.1021/jacs.7b03334.
- Raleigh MD, Laudenbach M, Baruffaldi F, Peterson SJ, Roslawski MJ, Birnbaum AK, Carroll FI, Runyon SP, Winston S, Pentel PR, et al. Opioid dose- and route-dependent efficacy of oxycodone and heroin vaccines in rats. J Pharmacol Exp Ther. 2018. doi: 10.1124/jpet.117.247049.
- Sulima A, Jalah R, Antoline JFG, Torres OB, Imler GH, Deschamps JR, Beck Z, Alving CR, Jacobson AE, Rice KC, et al. A stable heroin analogue that can serve as a vaccine hapten to induce antibodies that block the effects of heroin and its metabolites in rodents and that cross-react immunologically with related drugs of abuse. J Med Chem. 2018;61:329–343. doi:10.1021/acs.jmedchem.7b01427.
- Torres OB, Matyas GR, Rao M, Peachman KK, Jalah R, Beck Z, Michael NL, Rice KC, Jacobson AE, Alving CR. Heroin-HIV-1 (H2) vaccine: induction of dual immunologic effects with a heroin hapten-conjugate and an HIV-1 envelope V2 peptide with liposomal lipid A as an adjuvant. NPJ Vaccines. 2017;2:13. doi:10.1038/s41541-017-0013-9.
- Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK, Pentel PR. Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J Pharmacol Exp Ther. 2013;344:397–406. doi:10.1124/jpet.112.201194.
- Laudenbach M, Baruffaldi F, Vervacke JS, Distefano MD, Titcombe PJ, Mueller DL, et al. The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse. J Immunol. 2015;194:5926–5936. doi:10.4049/jimmunol.1500385.
- Taylor JJ, Laudenbach M, Tucker AM, Jenkins MK, Pravetoni M. Hapten-specific naive B cells are biomarkers of vaccine efficacy against drugs of abuse. J Immunol Methods. 2014;405:74–86. doi:10.1016/j.jim.2014.01.010.
- Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, Pentel PR. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One. 2014;9:e96547.
- Baruffaldi F, Kelcher AH, Laudenbach M, Gradinati V, Limkar A, Roslawski M, et al. Preclinical efficacy and characterization of candidate vaccines for treatment of opioid use disorders using clinically viable carrier proteins. Mol Pharm. 2018;15:4947–4962. doi:10.1021/acs.molpharmaceut.8b00592.
- Laudenbach M, Baruffaldi F, Robinson C, Carter P, Seelig D, Baehr C, Pravetoni M. Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Sci Rep. 2018;8:5508. doi:10.1038/s41598-018-23777-6.
- HogenEsch H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine. 2002;20:S34–9.
- Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi:10.1038/nrmicro1681.
- Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi:10.1084/jem.20052433.
- Vajdy M, Selby M, Medina-Selby A, Coit D, Hall J, Tandeske L, et al. Hepatitis C virus polyprotein vaccine formulations capable of inducing broad antibody and cellular immune responses. J Gen Virol. 2006;87:2253–2262. doi:10.1099/vir.0.81849-0.
- McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27:687–690. doi:10.1016/j.immuni.2007.11.003.
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi:10.1016/j.immuni.2010.10.002.
- Nakaya HI, Clutterbuck E, Kazmin D, Wang L, Cortese M, Bosinger SE, et al. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci U S A. 2016;113:1853–1858. doi:10.1073/pnas.1519690113.
- Block SL, Ruiz-Palacios GM, Guerrero ML, Beygo J, Sales V, Holmes SJ. Dose-range study of MF59-adjuvanted versus nonadjuvanted monovalent A/H1N1 pandemic influenza vaccine in six- to less than thirty-six-month-old children. Pediatr Infect Dis J. 2012;31:e92–8. doi:10.1097/INF.0b013e318257644f.
- Della CG, Nicolay U, Lindert K, Leroux-Roels G, Clement F, Castellino F, Galli C, Groth N, Levin Y, Del Giudice G. A dose-ranging study in older adults to compare the safety and immunogenicity profiles of MF59(R)-adjuvanted and non-adjuvanted seasonal influenza vaccines following intradermal and intramuscular administration. Hum Vaccin Immunother. 2014;10:1701–1710. doi:10.4161/hv.28618.
- Lofano G, Mancini F, Salvatore G, Cantisani R, Monaci E, Carrisi C, et al. Oil-in-water emulsion MF59 increases germinal center b cell differentiation and persistence in response to vaccination. J Immunol. 2015;195:1617–1627. doi:10.4049/jimmunol.1402604.
- Mastelic Gavillet B, Eberhardt CS, Auderset F, Castellino F, Seubert A, Tregoning JS, Lambert P-H, de Gregorio E, Del Giudice G, Siegrist C-A. MF59 mediates its B cell adjuvanticity by promoting T follicular helper cells and thus germinal center responses in adult and early life. J Immunol. 2015;194:4836–4845. doi:10.4049/jimmunol.1402071.
- Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A. 2008;105:10501–10506. doi:10.1073/pnas.0804699105.
- Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–5412. doi:10.4049/jimmunol.180.8.5402.
- 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes-United States. Centers for disease control and prevention. US Department of Health and Human Services; 2018. https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf
- Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi:10.1038/nprot.2009.9.
- Raleigh MD, Peterson SJ, Laudenbach M, Baruffaldi F, Carroll FI, Comer SD, Navarro HA, Langston TL, Runyon SP, Winston S, et al. Safety and efficacy of an oxycodone vaccine: addressing some of the unique considerations posed by opioid abuse. PLoS One. 2017;12:e0184876. doi:10.1371/journal.pone.0184876.
- Backonja M, Webster LR, Setnik B, Bass A, Sommerville KW, Matschke K, Malhotra BK, Wolfram G. Intravenous abuse potential study of oxycodone alone or in combination with naltrexone in nondependent recreational opioid users. Am J Drug Alcohol Abuse. 2016;42:539–549. doi:10.3109/00952990.2016.1167215.
- Harris SC, Perrino PJ, Smith I, Shram MJ, Colucci SV, Bartlett C, Sellers EM. Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse-deterrent controlled-release tablets in recreational opioid users. J Clin Pharmacol. 2013;54:468–477. doi:10.1002/jcph.235.
- FDA approves first seasonal influenza vaccine containing an adjuvant. U.S. food and drug administration, 2015 https://www.fda.gov/biologicsbloodvaccines/safetyavailability/vaccinesafety/ucm473989.htm:.
- Schmiel SE, Yang JA, Jenkins MK, Mueller DL. Cutting edge: adenosine A2a receptor signals inhibit germinal center T follicular helper cell differentiation during the primary response to vaccination. J Immunol. 2017;198:623–628. doi:10.4049/jimmunol.1601686.
- Yang JA, Tubo NJ, Gearhart MD, Bardwell VJ, Jenkins MK. Cutting edge: bcl6-interacting corepressor contributes to germinal center T follicular helper cell formation and B cell helper function. J Immunol. 2015;194:5604–5608. doi:10.4049/jimmunol.1500201.
- Wang C, Hart M, Chui C, Ajuogu A, Brian IJ, de Cassan SC, et al. Germinal center B cell and T follicular helper cell responses to viral vector and protein-in-adjuvant vaccines. J Immunol. 2016;197:1242–1251. doi:10.4049/jimmunol.1502472.
