ABSTRACT
The burden of pneumococcal disease in adults is substantial from a social and economic point of view. This study assessed the cost-effectiveness of the 13-valent pneumococcal conjugate vaccine (PCV13) for the prevention of invasive pneumococcal disease and pneumococcal pneumonia in adults versus “no vaccination” and versus vaccination with the 23-valent pneumococcal polysaccharide vaccine (PPSV23). A Markov model was used to simulate three strategies: no vaccination, complete vaccination with PPSV23 and complete vaccination with PCV13. The comparison between strategies allowed the estimation of clinical and economic outcomes including incremental cost-effectiveness ratios (ICER) and incremental cost-utility ratios (ICUR). The model took into account the distributions of age, risk profile, vaccination status, type of immunization and time since vaccination in the population. A societal perspective was adopted and a lifetime horizon was considered. Different sources of data and assumptions were used to calibrate PPSV23 and PCV13 effectiveness. Inpatient costs were based on the 2013 diagnosis-related group (DRG) database for National Health Service (NHS) hospitals and expert opinion; NHS official tariffs were the main source for unitary costs. PCV13 shows ICURs of €17,746/QALY and €13,146/QALY versus “no vaccination” and vaccination with PPSV23, respectively. Results proved to be robust in univariate sensitivity analyses, where all ratios were below a €20,000 threshold, with the exception of the scenario with PCV13 effectiveness halved. In a probabilistic sensitivity analysis, 94% of simulations showed cost-effectiveness ratios lower than €20,000/QALY, in both strategies. It was found that PCV13 is a cost-effective strategy to prevent pneumococcal disease in adults in Portugal.
Introduction
Pneumococcal disease (PD) is caused by Streptococcus pneumoniae (S. pneumoniae or SP), a bacterium responsible for a large spectrum of infections that can be classified as invasive (e.g., septicemia and meningitis) or non-invasive (e.g., pneumonia without bacteremia or pleural effusion).Citation1 The burden of PD in adults is substantial, both from a societal and an economic point of view,Citation2 since PD is responsible for about 1.6 million annual deaths worldwide.Citation3
According to the Portuguese National Statistics Institute (PNSI), no major variation in the pneumonia mortality rate has been documented in recent years. Pneumonia was responsible for almost 6,000 deaths in 2013 in Portugal.Citation4 The pneumonia-related standardized mortality rate in Portugal is 25.1 deaths per 100,000 inhabitants, according to the last available report from the Directorate-General of Health.Citation5 Community-acquired pneumonia (CAP) is associated with significant health care resource consumption both in outpatient and especially in hospital settings.Citation6–Citation8 In Portugal, 93% of CAP-related hospitalizations occur in adults.Citation9 In addition to an increased risk of morbidity and mortality, pneumonia significantly decreases health-related quality of life. After a pneumonia episode, resuming daily activities and fully recovering global function may take four weeks or more.Citation10–Citation12
Despite the use of antibiotics, PD remains an important disease with a significant impact on adult morbidity and mortality, suggesting that vaccination is likely to be the only intervention with a significant impact on the incidence of pneumococcal pneumonia and invasive pneumococcal disease (IPD), as highlighted by the World Health Organization (WHO).Citation13 The 13-valent pneumococcal conjugate vaccine (PCV13) is indicated for the prevention of invasive disease, pneumonia and acute otitis media caused by S. pneumoniae. Except for otitis, the approved population includes all age groups.Citation14 In July 2015, PCV13 was included in the Portuguese National Immunization Program for infants born after January 1, 2015.Citation15 Two 2015 guidelines from the national Directorate-General of Health further define the risk groups for IPD in individuals under 18 years oldCitation16 and over 18 years oldCitation17 for which PCV13 is recommended (either with co-payment or free of charge). In immunocompetent adults, PCV13 vaccination is recommended for individuals with cerebrospinal fluid fistulas or cochlear implants, patients with some chronic diseases (e.g., diabetes, heart, respiratory, liver and renal diseases), pre-transplantation patients, and bone marrow donors.Citation17 In immunocompromised adults, PCV13 is recommended for individuals with asplenia or splenic dysfunction, active neoplastic disease, transplant recipients, primary immunodeficiency, human immunodeficiency virus (HIV) infection, nephrotic syndrome, iatrogenic immunosuppression, or Down syndrome.Citation17
In this context, it is relevant to estimate the cost-effectiveness of PCV13 for the prevention of IPD and pneumonia in adults, compared to “no vaccination” and to the other available vaccine (23-valent pneumococcal polysaccharide vaccine: PPSV23), in the Portuguese setting.
Results
In the base-case scenario considering the overall Portuguese adult population (18 years and older) over a lifetime horizon, PCV13 averted 5,712 and 5,597 deaths for PD when compared to “no vaccination” and PPSV23, respectively. The PCV13-attributable reduction of in-hospital PD episodes was 25,104 and 4,813 versus “no vaccination” and PPSV23, respectively.
PCV13 was associated with an increase in quality-adjusted life-years (QALYs) and life-years (LY) at an expense of higher costs versus “no vaccination” and PPSV23. Overall, the lifetime per-patient additional costs of the PCV13 vaccination strategy were €47 and €33 versus “no vaccination” and PPSV23, respectively. Cost-effectiveness ratios were higher versus “no vaccination” than versus PPSV23, both considering LY gained (LYG) (incremental cost-effectiveness ratio [ICER]: €11,082/LYG versus €8,151/LYG) and QALYs (incremental cost-utility ratio [ICUR]: €17,746/QALY versus €13,146/QALY) ().
Table 1. Results of base-case scenario.
Results for univariate sensitivity analyses showed some variation of cost-effectiveness ratios around the base-case results, although all ratios were below the €20,000 threshold with the exception of the scenario where PCV13 effectiveness was reduced by 50% (ICER €23,570/LYG and €18,650/LYG; ICUR: €37,515/QALY and €30,096/QALY; both versus “no vaccination” and PPSV23, respectively) ().
Table 2. Results of univariate sensitivity analyses.
Probabilistic sensitivity analysis (PSA) results were consistent with the deterministic results for the base-case scenario, since 94% of the simulations showed cost-effectiveness ratios lower than €20,000/QALY, in both comparisons.
PCV13 vaccination strategy outperforms “no vaccination” with at least a 50% probability if willingness-to pay (WTP) for an incremental QALY is above €15,000/QALY, with a 75% probability if WTP is above €17,000/QALY and in all simulations if the WTP is above €25,900/QALY. The PCV13 vaccination strategy outperforms the PPSV23 vaccination strategy with at least a 50% probability if the WTP is above €10,900/QALY, with a 75% probability if the WTP is above €12,600/QALY and in all simulations if the WTP is above €24,100/QALY.
Discussion
The results of this cost-effectiveness study comparing the PCV13 vaccination strategy versus “no vaccination” and PPSV23 show that PCV13 is a cost-effective option for the overall Portuguese adult population, with ICERs of €17,746/QALY and €13,146/QALY, respectively. These results proved robust to a variety of changes in the model parameters and assumptions. In almost all univariate analyses, the ICER was below €20,000/QALY. The robustness of these results is reinforced by PSA in which 94% of the simulations showed cost-effectiveness ratios lower than €20,000/QALY both in comparison to “no vaccination” and to vaccination with PPSV23.
This study has several limitations. First, the PCV13 effectiveness source used in the analysis (Community-Acquired Pneumonia Immunization Trial in Adults [CAPiTA])Citation20 included only immunocompetent adults aged 65 years or older. In our study, the analysis considered the overall adult population (aged 18 years and older) for which PCV13 is reimbursed in Portugal. Therefore, adjusted PCV13 effectiveness was estimated for other age groups considering the rate of change of PPSV23 effectiveness with age (see Supplementary Material, Table S2 for further detail). Although this assumption introduces uncertainty, it seems reasonable because PCV13 vaccination has proven to be effective in both childrenCitation21-Citation24 and the elderly, both in clinical trial Citation20 and real world settings.Citation25 PCV13 is likely to have similar efficacy in younger adults as immune responses in this population are comparable or better than in older adults.Citation26 In fact, in the case of PPSV23, effectiveness was also documented for the younger adult population for IPDCitation27 being even higher in this population (18 to 55 years). Furthermore, our results proved quite robust when PCV13 effectiveness was hypothetically halved in a univariate sensitivity analysis ().
Second, to the best of our knowledge, no head-to-head trial exists comparing PCV13 and PPSV23 efficacy in adult populations. Therefore, different sources and assumptions were used to calibrate PCV13 and PPSV23 effectiveness.
Third, the epidemiological calibration of the model was based on the diagnosis-related group (DRG) administrative database. It has been reported that the ICD coding scheme in DRGs may be inaccurate in the classification of patients with IPD.Citation28 Furthermore, ICD-9 codes have been associated with low to modest sensitivity for detecting CAP in hospital administrative databases. In particular, at least one quarter of pneumonia cases are undetected. Therefore, the true incidence of inpatient CAP has probably been underestimated in our study, although the incidence rates of hospitalised CAP reported in this study are similar to other European estimations from studies conducted in Denmark, Finland, France, Hungary, Poland and Norway.Citation29 Our results also proved robust when the incidence rate of inpatient pneumonia varied by ± 20% ().
Fourth, the analysis assumed an immunization rate of 100% among adults. Although this rate of immunization is impossible to achieve, this assumption has been used in previous studiesCitation28,Citation30,Citation31 and probably represents a conservative approach. The model only took into consideration the herd effects from the paediatric vaccination program and not the potential small benefits associated with herd protection externalities generated by adults’ vaccination,Citation32 although the pneumococcal carriage rate in adults is very low (<5% in CAPiTA study).Citation20 In this study, the inclusion of herd effect generated by children’s vaccination leads to a reduction in the incidence of IPD among the adult population, therefore reducing the maximum public health potential benefits of vaccinating adults. In fact, the cost-effectiveness ratio () is lower when herd effects from childhood vaccination are excluded from the analysis. The model assumes a linear link between impact and costs of the vaccination program. Therefore, providing that immunization rate is similar across all age groups, coverage does not affect the cost-effectiveness results. In a sensitivity analysis, we explored the effect of different uptake rates of vaccination among adults age groups by considering the immunization rate reported in Portugal in 2015–2016 for Influenza vaccination (50.1% in subjects ≥65 years and 5.0% in younger adults).Citation19 In comparison to the base-case scenario, the PCV13 ICER in this sensitivity analysis is lower versus both PPSV23 and no vaccination, due to the higher uptake rate among people which benefit the most from vaccination.
Most previously published adult PCV13 cost-effectiveness studies assumed that the effectiveness of adult vaccination is similar to the paediatric effectiveness.Citation8,Citation30,Citation34–Citation37 The current study employs effectiveness estimates specific to the adult population, based on the published CAPiTA study,Citation20 which may increase the accuracy of the results hereby presented. Recent European cost-effectiveness studies of PCV13 vaccination in the adult population that used effectiveness data from the CAPiTA study present considerable variability in results.Citation39–Citation47 The heterogeneity of the results in published literature might be explained by differences in the comparators (PPSV23 or no vaccination), target age for vaccination, assumptions regarding resource use and costs, approaches followed to include the indirect effect of childhood vaccination in the models, dynamics of vaccination serotype coverage over time, calibration of the PPSV23 effectiveness and the incidence rate of IPD in adult population, in particular of hospitalized pneumonia.
The impact that some parametric options (calibration and modelling) have on the cost-effectiveness results of PCV13 in particular, and of immunization programs in general, highlights the need to be cautious in interpreting and extrapolating results of one particular study for other settings.
Materials and methods
Model structure
A cohort model with a Markov-type process (cycle length of one year) was used to estimate the clinical and economic outcomes of PCV13 vaccination, which include incremental cost-effectiveness and cost-utility ratios. The model starts from the overall Portuguese population aged 18 years or older and follows this cohort throughout the modelling horizon under three different strategies: no immunization, 100% immunization with PPSV23 and 100% immunization with PCV13 (“intervention strategy”). Results compare the intervention strategy with the other two alternative strategies. A lifetime modelling horizon was considered in base-case analyses for all cohorts. Benefits and costs were discounted at 5% as recommended by the National Authority of Medicines and Health Products (INFARMED) guidelines.Citation48 The model estimates the disease risk (incidence and fatality) based on population characteristics (distribution per age and risk profile). This model has been previously validated for other countries and used as a basis for publications by other authors.Citation42,Citation45 The economic model structure is presented in .
Figure 1. Economic model.
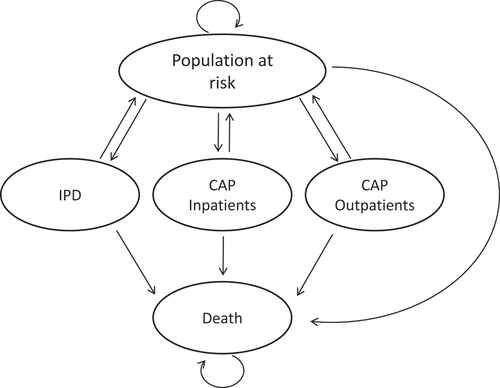
Expected outcomes were evaluated for each person in the model population on an annual basis, taking into consideration age, risk profile, vaccination status, type of immunization (PCV13 or PPSV23) and time since vaccination. Clinical results include the estimation in each scenario of IPD incidence (inpatient), number of hospitalizations and outpatient visits associated with pneumonia, mortality due to CAP or IPD, life-years and quality-adjusted life-years. Economic results presented include direct medical and indirect costs due to loss of productivity, and incremental cost-effectiveness and cost-utility ratios.
Demographic and epidemiological parameters
Estimates of the overall continental Portuguese population aged 18 years and older were obtained from National Statistics Office (INE) for 2013.Citation49 Population was grouped according to the level of risk for PD and/or related complications: 1) low-risk individuals are immunocompetent without any chronic medical conditions; 2) moderate-risk includes immunocompetent individuals with one or more chronic medical conditions, such as cardiovascular, liver, or pulmonary disease, or diabetes; 3) high-risk includes immunocompromised individuals as a result of splenic dysfunction, neoplasms (including Hodgkin’s disease, lymphoma), HIV, organ transplant, or chronic renal failure.Citation50
The proportion of individuals in each age group with low, moderate, or high risk was estimated using National Health Survey 2005/2006 microdata.Citation51 These were the most recent available data at the time of the analysis. The high-risk group prevalence was adjusted in order to include HIV prevalence estimates since these were not available in the National Health Survey. Retrieving data from official annual reports,Citation52,Citation53 HIV prevalence by sex and age was estimated considering year of diagnosis, mortality by year of diagnosis and patient age.
The incidence of IPD was estimated in two steps. First, the incidence of pneumococcal bacteremia, sepsis and meningitis were taken into account. Second, the serotype coverage of PCV13 and PPSV23 were factored in. In the case of pneumonia, the model was calibrated with the incidence for both inpatient and outpatient all-cause nonbacteremic pneumonia. This calibration is due to the fact that the model considers vaccine effectiveness rates against all-cause nonbacteremic pneumonia.
Pneumococcal bacteremia incidence was estimated from unpublished data. Since 1999, the Portuguese Group for the Study of Streptococcal Infections has monitored pneumococci causing invasive infections in Portugal. This is a laboratory-based surveillance system, in which 30 microbiology laboratories throughout Portugal are asked to identify all isolates responsible for IPD and to send them to a central laboratory for characterization.Citation54 For the purpose of our study, we had access to detailed data from 1,265 isolates responsible for adult IPD between 2009 and 2011, which allowed us to estimate Pneumococcal bacteremia incidence rates by age-group in 2011.
Pneumococcal meningitis, pneumococcal sepsis and overall inpatient pneumonia incidence rates were estimated using 2013 microdata from the DRG database that includes the discharges from Portuguese public hospitals.Citation55 Since a significant number of meningitis and sepsis episodes had no pathogen identified, the proportion of pneumococcal disease in the group of invasive infections with an unidentified pathogen was assumed to be the same as in episodes with an identified pathogen. Supplementary Table S1 presents the ICD-9 codes used to select the relevant episodes. The percentage of bacteremia and meningitis cases due to SP serotypes covered by the vaccine was estimated from Horácio et al. (2013)Citation56 for all IPD. It was assumed that the serotypes responsible for the meningitis and bacteremia were the same as the serotypes responsible for all IPD.
Due to lack of data, we assumed that outpatient pneumonia incidence rates for Portugal were proportional to inpatient rates multiplied by the ratio between the outpatient and inpatient pneumonia incidence rates found in the US population.Citation56
Portuguese general population mortality rates for all causes were calibrated using published mortality tables for 2011/2013Citation57 and adjusted for mortality due to meningitis, bacteremia and pneumonia, in order to avoid double counting. Pneumonia-specific mortality rates for 2013 were published by INE.Citation58 PD mortality rates were estimated from the 2013 DRG databaseCitation55 considering the episodes with a primary diagnosis of pneumococcal meningitis (ICD-9-CM 320.1), pneumococcal septicemia (ICD-9-CM 038.2), bacteremiaFootnote1 (ICD-9-CM 790.7) or pneumococcal pneumonia (ICD-9-CM 481).
Utility values by age group and sex were taken from Sisk et al. (2003)Citation59 and disutilities associated with each disease were the same as in Melegaro and Edmunds (2004).Citation60
describes the main demographic and epidemiological parameters used in the base-case scenario, as well as health-state utilities and yearly disutilities.
Table 3. Demographic and epidemiological parameters.
Effectiveness of PCV13 and PPSV23
Different sources of data and assumptions were used to calibrate effectiveness results for PPSV23 and PCV13. For both, effectiveness data were estimated according to age group, risk group and time since vaccination for each outcome (IPD and pneumonia).
For PPSV23, the model included data reported by Smith et al. (2008),Citation33 which estimated the cost-effectiveness of PPSV23 versus “no vaccination” among adults aged 50 years and over. In the Smith et al. study,Citation33 effectiveness of PPSV23 in preventing IPD was based on a Delphi panel of experts. The panel primarily relied on the results from Shapiro et al. (1991),Citation27 a large hospital-based case-control study evaluating PPSV23 effectiveness (Supplementary Material, Table S2).
Effectiveness of PPSV23 against all-cause nonbacteremic pneumonia was considered to be null, as assumed in other studies.Citation31,Citation33,Citation61–Citation67 The rate of decline in PPSV23 effectiveness against vaccine-type IPD over time was based on Smith et al. (2008),Citation33 and was applied beginning one year following vaccination.
For PCV13, effectiveness was based on the results of the per-protocol population of the CAPiTA studyCitation20 (Supplementary Material, Table S2). CAPiTACitation20 was a randomized, double-blind, placebo-controlled trial involving 84,496 adults aged 65 years or older, evaluating the efficacy of PCV13 in preventing first episodes of vaccine-type strains of pneumococcal CAP, nonbacteremic and non-invasive pneumococcal CAP, and IPD. Per-protocol population included all participants who met the criteria for the modified intention-to-treat population (episode of CAP or IPD with the onset of symptoms at least 14 days after vaccination), were eligible for the study (absence of immunocompromising conditions), received a vaccination and had no other major protocol violations.
Effectiveness of PCV13 against all-cause nonbacteremic pneumonia was estimated as the product of PCV13 efficacy in preventing vaccine-type nonbacteremic and non-invasive CAPCitation20 and the proportion of all-cause nonbacteremic pneumonia due to PCV13 serotypes (19.4%), as reported for the Spanish populationCitation67 (Supplementary Material, S3). It was assumed that PCV13 effectiveness in preventing IPD and pneumonia did not wane over the initial 5 years of the modelling horizon, based on the observation that PCV13 effectiveness appeared to be stable during the CAPiTA follow-up period (mean, 3.97 years).Citation20 Afterwards, it was assumed that the rate of PCV13 effectiveness decline was 50% of the PPSV23 effectiveness decline (Supplementary Material, Table S2 and Table S3).
Effectiveness of both vaccines was assumed to be the same irrespective of previous vaccination experience and no revaccination was assumed. Effectiveness of each vaccine (i.e., PPSV23 and PCV13) across vaccine-specific serotypes was assumed to be the same.
The model includes the herd effect due to widespread use in of PCV13 in young children. The herd effect on IPD is based on estimates by Miller et al. (2013) for England and Wales.Citation68 The elasticity of adult IPD incidence reduction took into account the difference in vaccine coverage between Portugal (61%)Citation69 and England/Wales (96%).Citation70 The indirect effect of PCV13 on all-cause nonbacteremic pneumonia is less well studied. In line with other authors,Citation71 it was assumed that for Portugal this effect would be proportional to the IPD herd effect, representing on average a 1% reduction in pneumonia incidence.
Resource use and costs
Inpatient costs by age group were estimated using 2013 DRG microdata.Citation55 Inpatient episode costs were the sum of hospitalization and follow-up costs occurring after hospital discharge. Portuguese NHS tariffs, in particular Order no. 20/2014, were used as the source of unitary costs. Due to the small number of meningitis episodes registered in the database, an average cost was used for all age groups.
Follow-up costs after hospitalization were estimated according to the opinion of a nationally representative panel of experts and by applying Order no. 20/2014 tariffs for unitary costs. Meningitis, bacteremia and sepsis follow-up consumption included two physician visits and two sets of blood tests,Footnote2 resulting in a cost estimate of €90.54 per episode. For pneumonia, experts considered that follow-up included one set of blood tests, with 20% of the patients having two physician visits, 30% of the patients measuring oximetry and all receiving a chest X-ray. Pneumonia follow-up costs were estimated at €98.2 per episode.
Pneumonia costs per outpatient episode were estimated at €137, including two physician visits and chest X-rays for all patients, emergency department admission for 70% of the patients and a set of blood tests for 10% of the patients. Prescription drugs were also included in the cost estimates.
Vaccine acquisition costs were taken from INFOMED (INFARMED drug database).Citation72 Value-added tax was excluded and an administration cost of €3.70 was considered.Footnote3 The overall cost per vaccine administration resulted in €15.59 and €59.46 for PPSV23 and PCV13, respectively. In the base-case scenario, vaccination was assumed to occur in the context of regular contacts with the health system that would have occurred anyway. Therefore, additional travel costs were not included in the base-case scenario.
The study adopted a societal perspective as recommended by INFARMED guidelines for economic evaluation.Citation48 The cost related to employees’ lost productivity was the only indirect cost included. The work cost per day was computed using the average wage in official labor market statisticsCitation73 and adjusted for the employment rate as reported in the employment survey of the 4th quarter of 2014.Citation74 Disease-attributable work-loss days were approximated as two times the average length of stay observed in the DRG database for inpatient episodes. For outpatient pneumonia episodes, 7 work-loss days were assumed. The overall direct and indirect costs per episode are presented by age and PD group ().
Table 4. Direct and indirect costs per episode by age and disease (including inpatient and outpatient costs of inpatient follow-up).
Sensitivity analysis
In order to assess the robustness of these results in relation to the assumptions made in the base-case scenario, both univariate analyses and PSAs were performed. In univariate analyses, the parameters more uncertain were varied, in particular, the herd effect size resulting from childhood vaccination, the incidence rate of pneumonia in inpatient and outpatient care, the effectiveness values of PCV13, the rate of adults’ immunization, and alternative discount rates. We further included a scenario where travel costs were considered for 50% of the vaccinated population ().
In the PSA, 5,000 simulations were run simultaneously varying several model parameters. Beta distributions were assumed for incidence rates, effectiveness rates and case-fatality rates. Lognormal distributions were used for costs, while utilities were considered uniformly distributed.
Conclusions
This study shows that adult vaccination (aged 18 years and older) in Portugal with PCV13 is cost-effective, producing health gains at costs below the usual acceptable willingness to pay threshold. The results are robust in all sensitivity analyses, with the exception of the scenario where effectiveness of PCV13 is very low. The PCV13 vaccination strategy is associated with a reduction of the pneumococcal disease burden, avoiding up to 5,712 deaths (compared with no vaccination) over the modelling horizon. These results strongly suggest that PCV13 should be added to the national therapeutic arsenal for preventing pneumococcal disease in adults.
Disclosure of potential conflicts of interest
The study was sponsored by Pfizer Portugal. Sponsoring was independent of the study outcome.
Mónica Inês holds Pfizer stock and/or stock options.
Supplemental Material
Download MS Word (25.7 KB)Acknowledgments
The authors express their gratitude to Professor Dr. Melo Cristino (Director of the Department of Clinical Pathology at Centro Hospitalar Lisboa Norte, EPE) for having provided access to further anonymous and detailed data regarding the results published by Horácio et al. (2013), which allowed us to estimate the incidence of pneumococcal bacteremia by age group.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
Notes
1. When estimating mortality rates, all bacteremia cases were considered due to the limited number of observations identified as pneumococcal bacteremia.
2. Complete blood count, blood glucose test, urea, creatinine, ionogram, serum AST and PCR.
3. Unit cost based on Order no. 20/2014.
References
- World Health Organization. Pneumococcal vaccines WHO position paper - 2012. Weekly epidemiological record No. 14. 2012; 87:129–144.
- Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine. 2010;28:4955–4960. doi:10.1016/j.vaccine.2010.05.030.
- World Health Organization. 23-valent pneumococcal polysaccharide vaccine WHO position paper. Weekly Epidemiological Record No. 42. 2008; 42:373–384.
- Instituto Nacional de Estatística, I.P. Estatísticas da saúde 2013. Edição 2015. Lisboa, Portugal: Instituto Nacional de Estatística, I.P.; 2015. [accessed 2016 Mar 1]. https://ine.pt.
- Direção-Geral da Saúde. Doenças respiratórias em números - 2015. Lisboa, Portugal: Direção-Geral da Saúde; 2016. [accessed 2016 Mar 1]. http://www2.portaldasaude.pt/NR/rdonlyres/150AC37D-BEB4-4F9E-8FC4-4ECEEBB39B40/0/i022215.pdf.
- Fleming NS, Ogola G, Ballard DJ. Implementing a standardized order set for community-acquired pneumonia: impact on mortality and cost. Jt Comm J Qual Patient Saf. 2009;35:414–421.
- Reyes S, Martinez R, Vallés JM, Cases E, Menendez R. Determinants of hospital costs in community-acquired pneumonia. Eur Respir J. 2008;31:1061–1067. doi:10.1183/09031936.00083107.
- Rozenbaum MH, van Hoek AJ, Fleming D, Trotter CL, Miller E, Edmunds WJ. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ. 2012;345:e6879. doi:10.1136/bmj.e6879.
- Araújo AT 10º Relatório Panorama das doenças respiratórias em Portugal Caminhos para o futuro. 2014/2015. [accessed 2016 Mar 1]. http://www.ondr.pt/10_Relatorio_ONDR.pdf.
- Brandenburg JA, Marrie TJ, Coley CM, Singer DE, Obrosky DS, Kapoor WN, Fine MJ. Clinical presentation, processes and outcomes of care for patients with pneumococcal pneumonia. J Gen Intern Med. 2000;15:638–646.
- Fine MJ, Stone RA, Singer DE, Coley CM, Marrie TJ, Lave JR, Hough LJ, Obrosky DS, Schulz R, Ricci EM, et al. Processes and outcomes of care for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team (PORT) cohort study. Arch Intern Med. 1999;159:970–980.
- Metlay JP, Fine MJ, Schulz R, Marrie TJ, Coley CM, Kapoor WN, Singer DE. Measuring symptomatic and functional recovery in patients with community-acquired pneumonia. J Gen Intern Med. 1997;12:423–430.
- World Health Organization (WHO). Pneumococcal vaccines WHO position paper. Weekly Epidemiological Record No. 23. 1999; 74:177–183.
- Summary of Product Characteristics of Prevenar 13. [accessed 2016 Mar 1]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf.
- Direção-Geral da Saúde (DGS). Programa Nacional de Vacinação. Introdução da vacina conjugada de 13 valências contra infeções por Streptococcus pneumoniae (Pn13). Norma nº 008/2015 de 01/06/2015 atualizada a 05/ 06/2015. Lisboa, Portugal: DGS; 2015.
- Direção-Geral da Saúde (DGS). Vacinação contra infeções por Streptococcus pneumoniae de grupos com risco acrescido para doença invasiva pneumocócica (DIP). Idade pediátrica (<18 anos de idade). Norma nº 012/2015 de 23/06/2015 atualizada a 06/ 11/2015. Lisboa, Portugal: DGS; 2015.
- Direção-Geral da Saúde (DGS). Vacinação contra infeções por Streptococcus pneumoniae de grupos com risco acrescido para doença invasiva pneumocócica (DIP). Adultos (≥18 anos de idade). Norma nº 011/2015 de 23/06/2015 atualizada a 06/ 11/2015. Lisboa, Portugal: DGS; 2015.
- Gouveia M, Ascenção R, Fiorentino F, Pascoal J, Costa J, Borges M. The cost and burden of schizophrenia in Portugal in 2015. Int J Clin Neurosci Ment Health. 2017;4:S13.
- Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA, IP). Vacinação antigripal da população portuguesa na época 2015/2016. Estudo na amostra ECOS. Lisboa, Portugal: Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA, IP); 2016.
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi:10.1056/NEJMoa1408544.
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195.
- Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Lee J, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–815. doi:10.1097/01.inf.0000027926.99356.4c.
- Pavia M, Bianco A, Nobile CGA, Marinelli P, Angelillo IF. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta-analysis. Pediatrics. 2009;123(6):e1103–e1110. doi:10.1542/peds.2008-3422.
- French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, Mwaiponya M, Zijlstra EE, Molyneux ME, Gilks CF. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010;362(9):812–822. doi:10.1056/NEJMoa0903029.
- McLaughlin JM, Jiang Q, Isturiz RE, Sings HL, Swerdlow DL, Gessner BD, Carrico RM, Peyrani P, Wiemken TL, Mattingly WA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for Community-Acquired Pneumonia in older US adults: A test-negative design. Clin Infect Dis. 2018 May 21. doi:10.1093/cid/ciy312.
- Isturiz RE, Hall-Murray C, McLaughlin JM, Snow V, Schmoele-Thoma B, Webber C, Thompson A, Scott DA. Pneumococcal conjugate vaccine use for the prevention of pneumococcal disease in adults <50 years of age. Expert Rev Vaccines. 2018 Jan;17(1):45–55. doi:10.1080/14760584.2018.1411196.
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med. 1991;21:1453–1460. doi:10.1056/NEJM199111213252101.
- Nichols MC, Bareta J, Coyle A, Landen M. Using hospital inpatient discharge data to supplement active surveillance for invasive pneumococcal disease: is the extract worth the exertion? Public Health Rep. 2016;131(3):404–410. doi:10.1177/003335491613100306.
- van de Garde EM, Oosterheert JJ, Bonten M, Kaplan RC, Leufkens HG. International classification of diseases codes showed modest sensitivity for detecting community-acquired pneumonia. J Clin Epidemiol. 2007 Aug;60(8):834–838. doi:10.1016/j.jclinepi.2006.10.018.
- Torres A, Cillóniz C, Blasi F, Chalmers JD, Gaillat J, Dartois N, Schmitt HJ, Welte T. Burden of pneumococcal community-acquired pneumonia in adults across Europe: A literature review. Respir Med. 2018 Apr;137:6–13. doi:10.1016/j.rmed.2018.02.007.
- Weycker D, Sato R, Strutton D, Edelsberg J, Atwood M, Jackson LA. Public health and economic impact of 13-valent pneumococcal conjugate vaccine in US adults aged ≥50 years. Vaccine. 2012 Aug 3;30(36):5437–5444. doi:10.1016/j.vaccine.2012.05.076.
- Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Hum Vaccin Immunother. 2013 Mar 1;9(3):699 –706.
- Edmunds WJ, Medley GF, Nokes DJ. Evaluating the cost-effectiveness of vaccination programmes: a dynamic perspective. Stat Med. 1999 Dec 15;18(23):3263–3282.
- Smith KJ, Zimmerman RK, Lin CJ, Nowalk MP, Ko FS, McEllistrem MC, Roberts MS. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine. 2008;26:1420–1431. doi:10.1016/j.vaccine.2008.01.007.
- Kuhlmann A, Theidel U, Pletz MW, von der Schulenburg JM. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Health Econ Rev. 2012;2:4. doi:10.1186/2191-1991-2-4.
- Martikainen JA, Soini EJ, Laine J, Ahman H, Postila V, Klemets P. Economic impact of 13-valent pneumococcal conjugate vaccine in Finnish adults ≥50 years with underlying chronic medical conditions. J Eval Clin Pract. 2014;20:333–341. doi:10.1111/jep.12131.
- Rozenbaum MH, Hak E, van der Werf TS, Postma MJ. Results of a cohort model analysis of the cost-effectiveness of routine immunization with 13-valent pneumococcal conjugate vaccine of those aged ⩾65 years in the Netherlands. Clin Ther. 2010;32:1517–1532. doi:10.1016/j.clinthera.2010.06.017.
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307:804–812. doi:10.1001/jama.2012.169.
- Jiang Y, Gauthier A, Annemans L, van der Linden M, Nicolas-Spony L, Bresse X. A public health and budget impact analysis of vaccinating at risk adults and the elderly against pneumococcal diseases in Germany. Expert Rev Pharmacoecon Outcomes Res. 2012;12:631–643. doi:10.1586/erp.12.55.
- Willem L, Blommaert A, Hanquet G, Thiry N, Bilcke J, Theeten H, Verhaegen J, Goossens H, Beutels P. Economic evaluation of pneumococcal vaccines for adults aged over 50 years in Belgium. Hum Vaccin Immunother. 2018 May 4;14(5):1218–1229. doi:10.1080/21645515.2018.1428507.
- van Hoek AJ, Miller E. Cost-effectiveness of vaccinating immunocompetent ≥65 year olds with the 13-valent pneumococcal conjugate vaccine in England. PLoS One. 2016 Feb 25;11(2):e0149540. doi:10.1371/journal.pone.0149540.
- Thorrington D, van Rossum L, Knol M, de Melker H, Rümke H, Hak E, van Hoek AJ. Impact and cost-effectiveness of different vaccination strategies to reduce the burden of pneumococcal disease among elderly in the Netherlands. PLoS One. 2018 Feb 9;13(2):e0192640. doi:10.1371/journal.pone.0192640.
- Mangen MJ, Rozenbaum MH, Huijts SM, van Werkhoven CH, Postma DF, Atwood M, van Deursen AM, van der Ende A, Grobbee DE, Sanders EA, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015 Nov;46(5):1407–1416. doi:10.1183/13993003.00325-2015.
- Kuchenbecker U, Chase D, Reichert A, Schiffner-Rohe J, Atwood M. Estimating the cost-effectiveness of a sequential pneumococcal vaccination program for adults in Germany. PLoS One. 2018 May 24;13(5):e0197905. doi:10.1371/journal.pone.0197905.
- Blommaert A, Bilcke J, Willem L, Verhaegen J, Goossens H, Beutels P. The cost-effectiveness of pneumococcal vaccination in healthy adults over 50: an exploration of influential factors for Belgium. Vaccine. 2016 Apr 19;34(18):2106–2112. doi:10.1016/j.vaccine.2016.03.003.
- Rodríguez González-Moro JM, Menéndez R, Campins M, Lwoff N, Oyagüez I, Echave M, Rejas J, Antoñanzas F. Cost effectiveness of the 13-valent pneumococcal conjugate vaccination program in chronic obstructive pulmonary disease patients aged 50+ years in Spain. Clin Drug Investig. 2016 Jan;36(1):41–53. doi:10.1007/s40261-015-0345-z.
- Falkenhorst G, Remschmidt C, Harder T, Wichmann O, Glodny S, Hummers-Pradier E, Ledig T, Bogdan C. Background paper to the updated pneumococcal vaccination recommendation for older adults in Germany. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2016;59(12):1623–1657. doi:10.1007/s00103-016-2466-9.
- Marbaix S, Peetermans WE, Verhaegen J, Annemans L, Sato R, Mignon A, Atwood M, Weycker D. Cost-effectiveness of PCV13 vaccination in Belgian adults aged 65-84 years at elevated risk of pneumococcal infection. PLoS One. 2018 Jul 6;13(7):e0199427. doi:10.1371/journal.pone.0199427.
- Silva EA, Pinto CG, Sampaio C, Pereira J, Drummond M, Trindade R Orientações Metodológicas para Estudos de Avaliação Económica de Medicamentos. INFARMED; 1998 Nov.
- Instituto Nacional de Estatística (PNSI). População residente (N.º) por Local de residência (NUTS - 2013), Sexo e Grupo etário. Anual. Lisboa, Portugal: Instituto Nacional de Estatística; 2015. [accessed 2016 Mar 1]. https://www.ine.pt.
- Kyaw MH, Rose Jr. TM, Fry AM, Singleton JA, Moore Z, Zell ER, Whitney CG; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192:377–386. doi:10.1086/431521.
- Instituto Nacional de Estatística (PNSI) e Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA). Inquérito Nacional de Saúde (INS) 2005/2006 [microdados].
- Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA, IP). Infeção VIH/SIDA: a situação em Portugal a 31 de dezembro de 2012. Lisboa, Portugal: Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA, IP); 2013.
- Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA, IP). Infeção VIH/SIDA: a situação em Portugal a 31 de dezembro de 2013. Lisboa, Portugal: Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA, IP); 2014.
- Horácio AN, Diamantino-Miranda J, Aguiar SI, Ramirez M, Melo-Cristino J. Portuguese Group for the study of streptococcal infections. The majority of adult pneumococcal invasive infections in Portugal are still potentially vaccine preventable in spite of significant declines of serotypes 1 and 5. PLoS One. 2013;8:e73704. doi:10.1371/journal.pone.0073704.
- Administração Central do Sistema de Saúde, ACSS, I.P. 2014 [microdados].
- Data on file, Wyeth Research 2006.
- Instituto Nacional de Estatística (PNSI). Tábua Completa de Mortalidade para Portugal - 2011-2013. Lisboa, Portugal: Instituto Nacional de Estatística; [accessed 2016 Mar 1]. https://www.ine.pt.
- Instituto Nacional de Estatística (PNSI). Óbitos (N.º) por Local de residência (NUTS - 2013), Sexo, Grupo etário e Causa de morte (Lista OCDE adaptada); Anual 2013. [accessed 2016 Mar 1]. https://www.ine.pt.
- Sisk JE, Whang W, Butler JC, Sneller VP, Whitney CG. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann Intern Med. 2003;17:960–968. doi:10.7326/0003-4819-138-12-200306170-00007.
- Melegaro A, Edmunds WJ. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur J Epidemiol. 2004;19:353–363.
- Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31:6011–6021. doi:10.1016/j.vaccine.2013.10.024.
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2008;23:CD000422.
- Smith KJ, Wateska AR, Nowalk MP, Raymund M, Lee BY, Zimmerman RK. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in U.S. older adults. Am J Prev Med. 2013;44:373–381. doi:10.1016/j.amepre.2012.11.035.
- Evers SM, Ament AJ, Colombo GL, Konradsen HB, Reinert RR, Sauerland D, Wittrup-Jensen K, Loiseau C, Fedson DS. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 Western European countries. Eur J Clin Microbiol Infect Dis. 2007;26:531–540. doi:10.1007/s10096-007-0327-z.
- Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938–1943. doi:10.1001/archinte.167.18.1938.
- Fry AM, Zell ER, Schuchat A, Butler JC, Whitney CG. Comparing potential benefits of new pneumococcal vaccines with the current polysaccharide vaccine in the elderly. Vaccine. 2002;21:303–311.
- Menéndez R, Torres A, España PP, Pérez-Trallero E, López Hontangas JL, Marco F, Martínez de la Fuente AP, Marimón JM, Gimeno A, Cilloniz C, et al. Pneumococcal serotypes causing community-acquired pneumonia (CAP) among hospitalized adults in Spain using a new urinary antigen detection (UAD) test. The CAPA study. Eur Respir J. 2014;44:P1810. doi:10.1183/09031936.00003814.
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–768. doi:10.1016/S1473-3099(11)70090-1.
- Pfizer, Lda. Data on file, 2016.
- Public Health England. Quarterly vaccine coverage data tables. [accessed 2016 Mar 1]. https://www.gov.uk/government/publications/pneumococcal-polysaccharide-vaccine-ppv-vaccine-coverage-estimates.
- Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices (ACIP) - Summary Report. Atlanta (Georgia): Department of Health and Human Services, Centers for Disease Control and Prevention. February 26-27, 2014. [accessed 2016 Mar 1]. https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2014-02.pdf.
- INFARMED. INFOMED - base de dados de medicamentos de uso humano. [accessed 2016 Mar 1]. http://app7.infarmed.pt/infomed/inicio.php.
- Gabinete de Estratégia e Estudos (GEE). Boletim Estatístico - Maio de 2015. Lisboa, Portugal: Ministério da Economia, Gabinete de Estratégia e Estudos (GEE); [accessed 2016 Mar 1]. http://www.gee.min-economia.pt.
