ABSTRACT
Health Care Workers (HCWs) have an increased risk of contracting contagious disease, including mumps. In January 2017 the Italian National Vaccine Prevention Plan 2017–2019, recommended the administration of a dose of MMR vaccine (Measles-Mumps-Rubella) to the Health Care Workers (HCWs) that, working in a risky environment, did not carry out the complete vaccination cycle of MMR or that are seronegative for at least one of the three vaccine viruses. In October of the same year, the Advisory Committee on Immunization Practices (ACIP) recommended a third dose of a vaccine containing Mumps Virus for people previously vaccinated with 2 doses, belonging to a group or to a population at increased risk of acquire mumps in the event of an epidemic. We analyzed the clinical records and values of mumps-specific IgG antibodies of 3032 HCWs (mean age 32.80 ± 10.75 years), that underwent occupational health surveillance between January 1st 2017 and March 31th 2018. The HCWs were also screened for measles, rubella, mumps using serological methods. 13% (405) was seronegative for mumps, especially among HCWs between 18 and 36 years. We calculated the cost-effectiveness of two-doses and three-doses MMR vaccination. The cost of vaccination without screening was significantly more expensive (cost difference: 99 712 € and 184 996 €) both in case of two-dose and three-dose MMR vaccination respectively. Our study suggests that, in HCWs, the assessment of the mumps antibody titer before vaccination may be a useful complement to vaccination itself, because it is more accurate and cost-effective than direct immunization of unvaccinated subjects.
Introduction
Health Care Workers (HCWs) have an increased risk of contracting contagious disease, including mumps, due to their occupational exposure.Citation1,Citation2 Mumps is an infection caused by Paramyxovirus (MuV) that primarily affects the salivary glands and is characterized by painful inflammatory symptoms, such as parotitis and orchitis.Citation3 It’s generally a mild childhood disease, most often affecting children between five and nine years old. However, the MuV can infect adults as well and when it does, complications as meningitis (in up to 15% of cases), deafness, encephalitis and permanent neurological damage are more likely to happen.Citation3,Citation4
Mumps outbreaks have occurred primarily in populations in institutional settings with close contact or in close-knit communities.Citation4
Since the 1960s safe and effective vaccines against mumps have been available. The vaccine is most often incorporated into national immunization programs in a combined MMR vaccine. In countries where large-scale immunization against mumps has been implemented, the incidence of the disease has dropped dramatically.Citation1 Despite the public health efforts mumps is still circulating in Europe.Citation5 In 2016, 14,795 cases of mumps were reported to ECDC by 28 EU/EEA Member States. The notification rate was 3.4 cases per 100,000 population. Italy reported 675 cases of mumps in 2015 that corresponds to a notification rate of 1.1 cases per 100,000 population.Citation6
The current routine recommendation for 2 doses of MMR vaccine appears to be adequate for mumps control in the general population, but may be unable to prevent mumps outbreaks in prolonged, close-contact settings as in Hospital setting.Citation4
For this reason in October 2017 ACIP recommended a third dose of a mumps virus–containing vaccine for persons previously vaccinated with 2 doses who are identified by public health authorities as being part of a group or population at increased risk for acquiring mumps because of an outbreak.Citation4 This recommendation supplements the Italian Vaccine Prevention Plan 2017–2019 (Piano Nazionale di Prevenzione Vaccinale, PNPV), that recommends at least a dose of MMR vaccine to workers who operate in a risky environment, if they don’t have clinical records of 2 MMR doses, or are seronegative or have a presumably non-protective antibody titre for at least one of vaccine viruses.Citation7
In Italy, data on vaccination coverage among HCWs are not routinely available either at national or regional level.Citation8 A study performed in south-Italy in 2008 reported a total susceptibility rate of 9.3% for measles, mumps or rubella among HCWs.Citation9 In a survey carried out in an middle Italy hospital in 2016 the prevalence of whole MMR susceptibility was above 40% for any occupational or age category.Citation10 In other published studies the prevalence of susceptible subjects ranged 40–80%.Citation2,Citation11
Since the immunogenicity of mumps vaccine has reported to be lower than Measles and Rubella one, a considerable part of the HCWs could remain serologically susceptible despite a previous 2 dose MMR vaccination.Citation4,Citation12 In this regard, it should be noted that although there is consensus that high levels of circulating mumps antibodies are important in protection against mumps, no established cut-off level has been currently identifiedCitation5; therefore, regardless of the vaccine status, a subject exposed to MuV could be infected if the IgG titre isn’t high enough.
Current health care practice in Italy provide for two doses immunization of HCWs that can’t produce written documentation of previous vaccination. This strategy could not be enough protective in relation to the previous reported evidences, and considering the ACIP 2018 recommendation.
In our study we aimed to evaluate the impact of the ACIP recommendation on the mumps immunization practice among Italian HCWs. Moreover we analyze the total cost-effectiveness of the serological pre vaccine screening for mumps in this population.
Results
We included in our study the clinical records from 3032 HCWs (1015 male and 2017 female). The mean age was 32.80 ± 10.75 years (range18-74). Among examined HCWs, 13% tested negative whereas 87% showed a mumps IgG titre >11 AU/ml. The mean value of mumps IgG was 154.77 ± 113.39 AU/ml.
Main demographic characteristics of population in study are reported in .
Table 1. Demographic characteristics and mumps susceptibility of healthcare workers (n = 3032).
Regarding the vaccination history 2032/3032 (67%) HCWs failed to produce any written documentation, 728/3032 (24%) documented a two-dose vaccination, while 273/3032 (9%) exhibited only one-dose vaccination.
The immune status was not related to gender and country of birth after testing with multivariate analysis, while we observed an incremental OR between increasing age tertiles ().
Table 2. Association between gender, nationality, tertiles of age and low mumps IgG titre. Univariate and multivariate analysis.
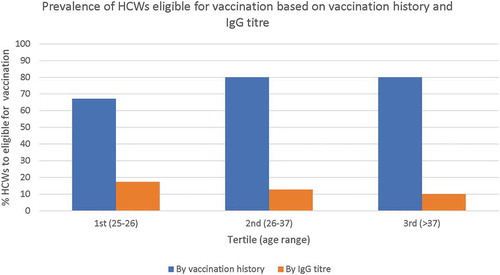
Definition of susceptible HCWs based on the evaluation of the vaccination history should have overestimated the percentage of seronegative subjects regardless the age class ().
Figure 1. HCWs (%) who need to be vaccinated with at least 1 dose of mumps vaccine, based on vaccination history and mumps IgG titre.
Regarding the cost analysis, mumps-specific IgG serological screening performed more cost-effective than the blind vaccination practice. Lacking pre-vaccination mumps IgG screening, 4337 or 7370 doses of MMR vaccine should have been administrated, in case of two-dose or three-dose vaccination respectively; the total cost should have been 147 891 € (2 doses schedule) or 251 317 € (3 doses schedule).
The pre-vaccination serological evaluation cost was 14 796 €; the administration of two or three doses in the 405 tested negative subjects should have costed 48 179 € or 66 321 €. The cost of vaccination without screening was significantly more expensive in both cases (cost difference: 99 712 € and 184 996 €).
Discussion
The study is focused on the mumps serum immunity in a population of HCWs after the introduction of MMR vaccine. We found that 13% of HCWs was seronegative or had a low mumps IgG titre for mumps IgG titres; the higher percentage of not immune subjects was among workers aged 18–37 year, who are the largest part of our sample since the study included the medical student in population. Among HCWs born between 1980 and 1999, a time-period during which despite the vaccine availability the vaccination coverage rate was inadequate, we found the highest percentage of susceptible people. This data confirms low immunization coverage in this population and a probable natural immunization among older people. Published studies reported a similar rate of MMR coverage and an higher risk of infection in young adult than children.Citation13-Citation19
The mumps IgG titres has been seen decreasing over years after the vaccinationCitation12 and a two-dose MMR vaccination is considered not to be protective in the whole population. In 2017 CDC recommended the administration of a third dose of MMR vaccine in HCWs that may be exposed to a case of mumps, based on the consideration that the current routine recommendation for 2 doses of MMR vaccine appears to be sufficient for mumps control in the general population, but may be not adequate for preventing mumps outbreaks in prolonged, close-contact settings, even where coverage with 2 doses of MMR vaccine is high.Citation4
It is important to say that, although serology is commonly used as evidence of immunity according to vaccination guidelines,Citation7 the antibody titer is not necessarily correlated with protective immunity, in fact EIA mumps-specific IgG (commonly used for this purpose) doesn’t guarantee protection,Citation20 since both virus-neutralizing and nonneutralizing antibodies are measured in this test.Citation21
Recent studies shows that mumps antibody titres decline over time in people who have received 2 doses of MMR vaccineCitation21 and laboratory data indicate also poorer antibody quality (e.g., lower avidity antibodies) after either mumps vaccination and natural mumps infection or compared with the responses to other infections (rubella and measles).Citation4
The actual vaccine strategy in Italy recommends to administrate 2 doses MMR vaccination to HCWs who lack appropriate vaccine documentation.
In our population this strategy revealed to be inadequate to assess the eligibility for vaccination of HCWs since the greater part of them doesn’t exhibit any vaccine documentation.
In fact our study clearly shows that the strategy based on the written documentation of two-dose vaccination overestimated the real rate of susceptible operators in all cohort of birth (). Overestimation was over 50% even in the subjects born between 1991 and 1999 (1st tertile of our population), when the vaccination percentage of coverage in Italy was above 50%.
Moreover the strategy based on immunization history was non cost-effective; in fact we would had administered inappropriately almost 2000 doses of MMR vaccine to HCWs having a sufficiently high mumps IgG titre, misspending over 60,000 euros in the study period.
The main limit of our study was that we couldn’t test the correlation between vaccination history and serological immunization because of the lack of documentation records in most of the HCWs enrolled in the study. We couldn’t refer to information source other than own vaccination certificate since in Italy lacks a public accessible vaccination registry.
Moreover it may still be cost-effective to screen for seroconversion also to measles and rubella. Given the fact that measles and rubella have a correlate of protection that has actually been reasonably defined, it would be interesting, in the future, to include cost of screening for all three viruses.
Based on the results of our study, in HCWs, the assessment of the mumps antibody titer before vaccination may be a useful complement to vaccination itself, because it is more accurate and cost-effective then direct immunization of unvaccinated subjects, allowing a)the identification of people with very high antibody titers, who do not need to be vaccinated, and b) subjects having still a very low antibody titer in spite of a previous cycle of vaccination (complete or incomplete)..
Methods
This was a retrospective observational study, approved by the Ethical Committee for Research in Human Subjects of the Foundation PTV, Polyclinic Tor Vergata of Rome (PTV).
We retrospectively evaluated the mumps IgG titre among HCWs in a teaching hospital in Rome, who underwent the annual occupational medical visit from March 1st 2017 to March 31st 2018. Using ModuLab®, a Laboratory Information System for the complete management of the clinical pathology laboratory, based on the latest technologies available, it was possible to select all values of mumps-specific IgG antibodies. For each HCW the following data were collected: age, gender, vaccine status, IgG mumps antibodies titre, country of birth. The DiaSorin LIAISON® Measles IgG assay uses a chemiluminescence immunoassay (CLIA) technology, a variation of the standard enzyme immunoassay (EIA). The instrument automatically calculates mumps-specific IgG concentrations expressed in AU/ml and ranks the results. According to the manifacturers’ instructions values higher than 11 AU/ml were considered positive. Sensitivity and specificity are 98.5% (95% C.I.: 96.5–99.5%) and 98.2% (95% C.I.: 94.8–99.2%), respectively.Citation23 HCWs were classified into three age group after dividing them into tertiles.
All subjects with incomplete clinical or serological records were excluded; HCW who tested positive for mumps-specific IgM antibodies were considered an incident case of mumps and were excluded from the analysis.
Concentrations of antibodies lower than Limit of Detection (LOD) have been replaced with LOD divided by two. Analyses were performed using STATA software (Version number 11). Univariate and multivariate analyses were used to investigate the factors related to the immunity of HCWs. Results were considered statistically significant a P value threshold of <0.05.
For the cost-effectiveness analysis, only the commercial cost of the CLIA and vaccines were included; we computed 4,88€ for each MMR test (CLIA) and 34,10 € for MMR vaccine (MMRvaxpro® Sanofi Pasteur MSD). The cost-effectiveness was calculated both for two-doses (Italian law recommendation) and three-doses (ACIP statement) MMR vaccination.
Abbreviations
| ECDC | = | European Centre for Disease Prevention and Control |
| CDC | = | Centre for Disease Prevention and Control |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
References
- World Health Organization, WHO. Immunization, vaccines and biologicals. http://www.who.int/immunization/diseases/mumps/en/
- Fortunato F, Tafuri S, Cozza V, Martinelli D, Prato R. Low vaccination coverage among italian healthcare workers in 2013. Hum Vaccin Immunother. 2015;11:133–39. doi:10.4161/hv.34415.
- Rubin S, Eckhaus M, Rennick LJ, Bamford CG, Duprex WP. Molecular biology, pathogenesis and pathology of mumps virus. J Pathol. 2015;235:242–52. doi:10.1002/path.4445.
- Marin M, Marlow M, Moore KL, Patel M. Recommendation of the advisory committee on immunization practices for use of a third dose of mumps virus-containing vaccine in persons at increased risk for mumps during an outbreak. MMWR Morb Mortal Wkly Rep. 2018;67:33–38. doi:10.15585/mmwr.mm6701a7.
- Eriksen J, Davidkin I, Kafatos G, Andrews N, Barbara C, Cohen D, Duks A, Griskevicius A, Johansen K, Bartha K, et al. Seroepidemiology of mumps in Europe (1996-2008): why do outbreaks occur in highly vaccinated populations?. Epidemiol Infect. 2013;141:651–66. doi:10.1017/S0950268812001136.
- Mumps. Annual epidemiological report on communicable diseases in Europe. Stockholm: ECDC, 2017.
- Ministero della Salute. Piano Nazionale Prevenzione Vaccinale. 2017-2019 (PNPV). http://www.salute.gov.it/imgs/C_17_pub-blicazioni_2571_allegato.pdf.
- Prato R, Tafuri S, Fortunato F, Martinelli D. Vaccination in healthcare workers: an Italian perspective. Expert Rev Vaccines. 2010;9:277–83. doi:10.1586/erv.10.11.
- Scatigna M, Fabiani L, Micolucci G, Santilli F, Mormile P, Giuliani AR. Attitudinal variables and a possible mediating mechanism for vaccination practice in health care workers of a local hospital in L’Aquila (Italy). Hum Vaccin Immunother. 2017;13:198–205. doi:10.1080/21645515.2016.1225638.
- Tafuri S, Martinelli D, Caputi G, Arbore A, Lopalco PL, Germinario C, Prato R. An audit of vaccination coverage among vaccination service workers in Puglia, Italy. Am J Infect Control. 2009;37:414–16. doi:10.1016/j.ajic.2008.10.030.
- Zamir CS, Schroeder H, Shoob H, Abramson N, Zentner G. Characteristics of a large mumps outbreak: clinical severity, complications and association with vaccination status of mumps outbreak cases. Hum Vaccin Immunother. 2015;11:1413–17. doi:10.1080/21645515.2015.1021522.
- Kontio M, Jokinen S, Paunio M, Peltola H, Davidkin I. Waning antibody levels and avidity: implications for MMR vaccine-induced protection. Int J Infect Dis. 2012;206:1542–48. doi:10.1093/infdis/jis568.
- Cardemil CV, Dahl RM, James L, Wannemuehler K, Gary HE, Shah M, Marin M, Riley J, Feikin DR, Patel M, et al. Effectiveness of a third dose of MMR vaccine for mumps outbreak control. N Engl J Med. 2017;377:947–56. doi:10.1056/NEJMoa1703309.
- Greenland K, Whelan J, Fanoy E, Borgert M, Hulshof K, Yap K-B, Swaan C, Donker T, van Binnendijk R, de Melker H, et al. Mumps outbreak among vaccinated university students associated with a large party, the Netherlands, 2010. Vaccine. 2012;30:4676–80. doi:10.1016/j.vaccine.2012.04.083.
- Takla A, Böhmer MM, Klinc C, Kurz N, Schaffer A, Stich H, Stöcker P, Wichmann O, Koch J. Outbreak-related mumps vaccine effectiveness among a cohort of children and of young adults in Germany 2011. Hum Vaccin Immunother. 2014;10:140–45. doi:10.4161/hv.26642.
- Vygen S, Fischer A, Meurice L, Mounchetrou Njoya I, Gregoris M, Ndiaye B, Ghenassia A, Poujol I, Stahl JP, Antona D, et al. Waning immunity against mumps in vaccinated young adults, France 2013. Euro Surveill. 2016;21:30156. doi:10.2807/1560-7917.ES.2016.21.10.30156.
- Livingston KA, Rosen JB, Zucker JR, Zimmerman CM. Mumps vaccine effectiveness and risk factors for disease in households during an outbreak in New York City. Vaccine. 2014;32:369–74. doi:10.1016/j.vaccine.2013.11.021.
- Cohen C, White JM, Savage EJ, Glynn JR, Choi Y, Andrews N, Brown D, Ramsay ME. Vaccine effectiveness estimates, 2004–2005 mumps outbreak, England. Emerg Infect Dis. 2007;13:12–17. doi:10.3201/eid1301.060649.
- Coppeta L, Pietroiusti A, Lieto P, Ferraro M, Grelli S, Stillo M, Magrini A. Measles immunity in an Italian teaching hospital. Occup Med (Chic Ill). 2018. doi:10.1093/occmed/kqy132.
- Shirts BH, Welch RJ, Couturier MR. Seropositivity rates for measles, mumps, and rubella IgG and costs associated with testing and revaccination. Clin Vaccine Immunol. 2013;20:443–45. doi:10.1128/CVI.00503-12.
- Taddei C, Ceccherini V, Niccolai G, Porchia BR, Boccalini S, Levi M, Tiscione E, Santini MG, Baretti S, Bonanni P, et al. Attitude toward immunization and risk perception of measles, rubella, mumps, varicella, and pertussis in health care workers working in 6 hospitals of Florence, Italy 2011. Hum Vaccin Immunother. 2014;10:2612–22. doi:10.4161/21645515.2014.970879.
- Mauldin J, Carbone K, Hsu H, Yolken R, Rubin S. Mumps virus-specific antibody titers from pre-vaccine era sera: comparison of the plaque reduction neutralization assay and enzyme immunoassays. J Clin Microbiol. 2005;43(9):4847–51. doi:10.1128/JCM.43.9.4847-4851.2005.
- Haywood B, Patel M, Hurday S, Copping R, Webster D, Irish D, Haque T. Comparison of automated chemiluminescence immunoassays with capture enzyme immunoassays for the detection of measles and mumps IgM antibodies in serum. J Virol Methods. 2014;196:15–17. doi:10.1016/j.jviromet.2013.10.027.
