ABSTRACT
Influenza is a major public health burden, mainly prevented by vaccination. Recommendations on influenza vaccine composition are updated annually and constant benefit-risk monitoring is therefore needed. We conducted near-real-time enhanced passive surveillance (EPS) for the influenza vaccine, Fluarix Tetra, according to European Medicines Agency guidance, in 10 volunteer general practices in England using Fluarix Tetra as their principal influenza vaccine brand, from 1-Sep to 30-Nov-2016. The EPS method used a combination of routinely collected data from electronic health records (EHR) and a customized adverse events reporting card (AERC) distributed to participants vaccinated with Fluarix Tetra. For participants vaccinated with a different influenza vaccine, data were derived exclusively from the EHR. We reported weekly and cumulative incidence of pre-defined adverse events of interest (AEI) occurring within 7 days post-vaccination, adjusted for clustering effect. Of the 97,754 eligible participants, 19,334 (19.8%) received influenza vaccination, of whom 13,861 (71.7%) received Fluarix Tetra. A total of 1,049 participants receiving Fluarix Tetra reported AEIs; 703 (67%) used the AERC (adjusted cumulative incidence rate 4.96% [95% CI: 3.92−6.25]). Analysis by individual pre-specified AEI categories identified no safety signal for Fluarix Tetra. A total of 62 individuals reported an AEI with a known brand of non-GSK influenza vaccine and 54 with an unknown brand (adjusted cumulative incidence rate 2.59% [1.93−3.47] and 1.77% [1.42−2.20], respectively). In conclusion, the study identified no safety signal for Fluarix Tetra and showed that the AERC was a useful tool that complemented routine pharmacovigilance by allowing more comprehensive capture of AEIs.
Introduction
Influenza is a major public health burden resulting in significant clinical and economic impact each year. It is estimated that 290,000–650,000 seasonal influenza-associated respiratory deaths occur globally and between 15,000 and 70,000 deaths in the European Union and European Economic Area Member States each year.Citation1-Citation3 According to the World Health Organization (WHO), the most effective way to prevent the disease is vaccination, although other personal protective measures should also be adopted, like good hand and respiratory hygiene, avoiding contact with sick people and early self-isolation of those with influenza symptoms.Citation4 Due to frequent genetic and antigenic changes in influenza viruses, seasonal vaccines are regularly reformulated based on annual WHO recommendations on the composition of influenza virus vaccines.Citation5 Therefore, there is a need for constant benefit-risk monitoring of these vaccines.
Approaches to pharmacovigilance are constantly evolving to ensure that medicines and vaccines are safe and public confidence in them is maintained. A fall in public confidence can lead to public health issues; for example, the withdrawal of two batches of a specific brand of influenza vaccine in Italy during the 2014–2015 season resulted in a decline in influenza vaccine uptake.Citation6,Citation7 Furthermore, concerns about adverse events after vaccination with pandemic influenza A/H1N1 vaccine suggested that surveillance systems in place at the time may have been inadequate.Citation8
The role of the European Medicines Agency (EMA) includes supervision of pharmacovigilance activities by pharmaceutical companies.Citation9 The European regulatory requirement for manufacturers of seasonal influenza vaccines to conduct annual small scale clinical trials was withdrawn in 2015. Instead, the EMA has set out new guidance for influenza vaccine monitoring,Citation10 which should be seen in the context of the EMA’s wider approach to good pharmacovigilance practice.Citation11,Citation12 The goal of the new guidance is to be able to rapidly detect, in near-real-time early in the season, any significant increase in the frequency or severity of pre-defined adverse events of interest (AEIs).Citation10 The EMA has suggested three options for vaccine manufacturers to monitor AEIs following vaccination: (1) active surveillance, using existing methods of post authorization surveillance; (2) enhanced passive surveillance (EPS) in which vaccine usage is rapidly estimated and additional steps are taken to facilitate passive reporting of AEIs; and (3) data mining or other use of electronic health records (EHRs).Citation10-Citation12
General practice in England is an appropriate setting to implement EPS as specified by the EMA. It has a registration-based list system (one individual registered with one general practitioner [GP]) and has been highly computerized since 2004, with all key data coded.Citation13 Data extracted from these systems are widely used in research.Citation14 General practices are largely independent professional partnerships that make their own decision about which brand of influenza vaccine to purchase prior to the start of each influenza season. Practices administer influenza vaccines to recommended groups, starting in September of each year.Citation15 For the 2017−2018 influenza season, Public Health England reported uptake levels of 70% or higher in the 65 years and over age group, while an uptake of 50% was reported for individuals aged 6 months to under 65 years in clinical at-risk groups;Citation16 almost all individuals received the vaccine at a general practice, whilst approximately 10% were vaccinated at community pharmacies, school or work.Citation16
The present study was conducted to fulfil EMA pharmacovigilance requirements for Fluarix Tetra (GSK), an inactivated quadrivalent influenza vaccine. Our primary objective was to estimate on a weekly basis the crude and cumulative incidence rate of AEIs within 7 days following vaccination with a seasonal influenza vaccine. We employed an EPS method which used a combination of data routinely collected via EHR, together with data collected via an adverse events reporting card (AERC) to maximize the likelihood of capturing adverse events experienced by vaccinees receiving GSK’s Fluarix Tetra. Participants who received a non-GSK influenza vaccine were followed up using EHR systems only; they were not issued with an AERC. We have previously shown the feasibility of this method in general practice in England.Citation17,Citation18
Results
The total registered population of the 10 practices was 97,801 individuals. Of these, 97,754 had not opted out of data sharing, had a valid National Health Service (NHS) number, and their age and gender recorded in their EHR, and were therefore eligible to participate in the study.
Influenza vaccine exposure
The study vaccination period covered 1 September to 30 November 2016 and the follow-up period captured AEIs from week 37 to week 48. A total of 19,334 out of 97,754 (19.8%) individuals were vaccinated against influenza ( and ). Most vaccinees (71.7%) received Fluarix Tetra (). Information on vaccination data (vaccine administration date and batch number) in the EHR was >99% complete for Fluarix Tetra and non-GSK influenza vaccine brands (Supplement Table 1). The non-GSK vaccines administered were LAIV manufactured by Astra Zeneca (quadrivalent) and inactivated vaccines manufactured by Sanofi Pasteur Europe (trivalent); Seqirus Vaccines Limited (trivalent); and Abbot Biologicals (trivalent).
Table 1. Summary of demographic characteristics by vaccine group.
Table 2. Summary of age category and risk group by vaccine group.
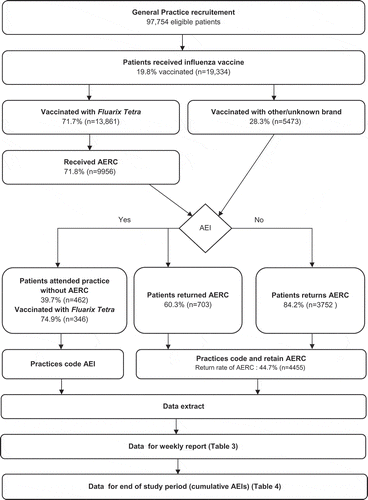
Figure 1. Flow diagram of study.
AEI: adverse events of interest; AERC: adverse events reporting card
Generally, GPs used Fluarix Tetra preferentially for older people: the mean age for people receiving Fluarix Tetra was 66 years versus 24 years for people receiving non-GSK brands (). Considering all age groups, the overall uptake of influenza vaccine was 19.8%. In the >65 years age group, 68.5% received any influenza vaccine, of whom 81.3% received Fluarix Tetra (). Among people with any high-risk condition, uptake of any influenza vaccine was 42.7%, of whom 75.2% received Fluarix Tetra (). Uptake of influenza vaccine was relatively low in pregnant women and children <4 years of age (24.1% and 18.3% overall, respectively; ).
Adverse events of interest reported for Fluarix Tetra
Of the 13,861 participants who were immunized with Fluarix Tetra, 9956 (71.8%) received an AERC, of whom 4455 (44.7%) returned it (note that individuals who were immunized with another brand were not given an AERC) (). A total of 1049 people receiving Fluarix Tetra reported AEIs overall; 67.0% (n = 703) of these were reported using the AERC.
Events reported for Fluarix Tetra via AERC
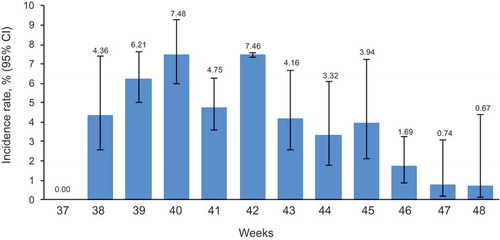
Weekly incidence rates via AERC were reported throughout the follow-up period (weeks 37 to 48). These varied from 0.67% (n = 2) at week 48 to 7.48% (n = 164) at week 40. Rates peaked between weeks 39−42, followed by a steady decrease throughout the remainder of the study (; Supplement Table 2).
Figure 2. Weekly incidence rate of any AEIs reported via AERC within 7 days post-vaccination in participants receiving Fluarix Tetra.
The figure shows the percentage of participants reporting an AEI at least once on the AERC estimated from logistic GEE models adjusted for clustering effect of general practices, with upper and lower limits of the 95% CI based on the robust variance estimate AEI: adverse event of interest; AERC: adverse event reporting card; 95% CI: 95% confidence interval; GEE: generalized estimating equation; LL: lower limit; UL: upper limit
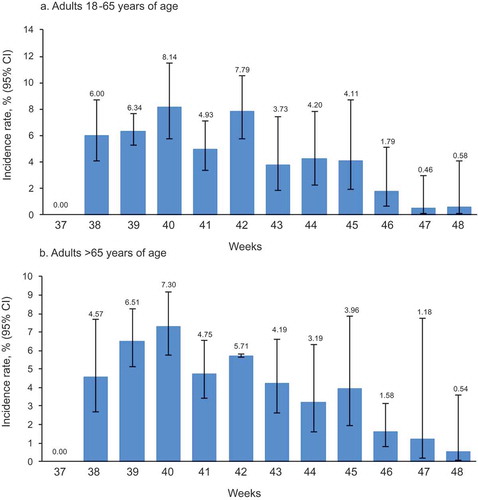
Across the follow-up period, AEIs were reported by one child in the 13−17 years age group, 238 adults aged 18−65 years, and 464 adults aged >65 years. Rates for adults aged 18–65 and over 65s peaked in weeks 39–42 and then declined (,).
Figure 3. Weekly incidence rate of any AEIs reported via AERC within 7 days post-vaccination according to age group in adult participants receiving Fluarix Tetra.
The figure shows the percentage of participants reporting an AEI at least once on the AERC estimated from logistic GEE models adjusted for clustering effect of general practices, with upper and lower limits of the 95% CI based on the robust variance estimate AEI: adverse event of interest; AERC: adverse event reporting card; 95% CI: 95% confidence interval; GEE: generalized estimating equation; LL: lower limit; UL: upper limit
The cumulative incidence rate of AEIs reported using the AERC for Fluarix Tetra during the follow-up period was 4.96% (95% confidence interval [CI]: 3.92−6.25) (Supplement Table 2). The cumulative incidence rates were 5.01% (95% CI: 3.77−6.62) in adults 18−65 years of age and 5.01% (95% CI: 4.02−6.23) in adults >65 years of age (Supplement Table 3).
Table 3. Weekly incidence rates of any AEI reported on the EHR or by AERC within 7 days post-vaccination by vaccine group.
Recruitment into the study by GP practices may have created a clustering effect, because participants in the same practice tend to share certain demographic and social characteristics. We therefore calculated an intra-cluster correlation coefficient (ICC) to measure the clustering effect between general practices. Importantly, the ICC was low (0.0053), indicating homogeneity in reporting rates of AEIs via AERC between practices.
Total events reported for Fluarix Tetra including EHR and AERC data
The patterns of reported AEIs for both EHR and AERC were similar to those reported using just AERC. The age of study participants reporting AEIs following vaccination with Fluarix Tetra using AERC and EHR data was distributed as follows: two children in the 6 months to 5 years group, three children in the 6−12 years group, one child in the 13−17 years group, 384 adults in the 18−65 years group, and 659 adults in the >65 years group.
The weekly incidence rate of AEIs during the follow-up period (weeks 37 to 48) reported via AERC and EHR for Fluarix Tetra varied from 3.11% (n = 14) at week 47 to 10.15% (n = 218) at week 40, peaking between weeks 39−42 (). The overall cumulative rate of AEIs for Fluarix Tetra during the follow-up period was 7.60% (95% CI: 6.43−8.96; n = 1,049) ().
Table 4. Cumulative incidence rates of any AEI reported on the EHR or by AERC within 7 days post- vaccination by vaccine group.
The ICC was 0.0033, indicating homogeneity in reporting rates of AEIs recorded via the EHR and AERC for Fluarix Tetra between general practices.
Adverse events of interest reported for non-GSK and unknown vaccine brands via EHR
Of note, participants who were immunized with non Fluarix Tetra vaccines were not provided with an AERC and therefore AEIs were captured using data routinely encoded in the EHR exclusively. A total of 62 individuals reported an AEI with a non-GSK influenza vaccine and 54 with an unknown brand of influenza vaccine. Weekly AEI incidence rates during the follow-up period ranged from 1.29% to 6.20% for non-GSK brands and from 0.70% to 2.85% for unknown vaccine brands (). The overall cumulative rate of AEIs during the follow-up period was 2.59% (95% CI: 1.93−3.47) for non-GSK brands, and 1.77% (95% CI: 1.42−2.20) for unknown vaccine brands (). The ICC was 0.0012 for non-GSK vaccine brands and −0.0006 for unknown brands.
Cumulative incidence rates of AEIs reported via the EHR or derived from the AERC according to pre-specified clinical category
The total cumulative rates of individual pre-specified AEIs during the follow-up period (weeks 37 to 48) reported for all vaccines are shown in . To evaluate any potential safety signal with Fluarix Tetra, the frequency of individual AEIs observed in the present EPS study was compared with the frequency of the AEIs included in the Fluarix Tetra Summary of Product Characteristics (SmPC).Citation19 All AEIs except headache occurred at a lower frequency in the present study than stated in the SmPC, and none occurred at a higher frequency (), confirming that no safety signal was detected for Fluarix Tetra in the EPS.
Table 5. Cumulative incidence rates of AEIs reported on the EHR or by AERC within the 7 days post-vaccination period by vaccine group according to EMA-specified clinical category (week 37–48).
Table 6. Observed frequency of AEIs for Fluarix Tetra in EPS (AERC and EHR data combined) compared with the frequency of solicited events included in the summary of product characteristics.
Hospital admission and deaths
A total of 12 participants were hospitalized within the 7 days post-vaccination period (nine participants vaccinated with Fluarix Tetra and three with an unknown vaccine brand). None were considered to be associated with vaccination based on their registered GP’s judgement. There were no deaths among study participants.
Discussion
The purpose of our study was to fulfil EMA pharmacovigilance requirements for Fluarix Tetra using an EPS method that collected data on pre-defined AEIs, using a combination of data routinely collected from EHRs and a reporting card system completed by vaccinees. We demonstrated that this method can be successfully implemented in a primary care setting.
The study identified no safety signal for Fluarix Tetra. The pattern of AEI reporting rates appeared similar from week to week. Compared with the Fluarix Tetra SmPC,Citation19 the reporting rates of individual AEIs were of similar or lower magnitude, and the cumulative end-of-season analysis revealed no unexpected events. Furthermore, none of the hospital admissions in the week following vaccination appeared to have a causal link with vaccination based on the data available at the weekly reviews and follow-up information requested. The overall incidence rate of AEIs for Fluarix Tetra was 4.96% (95% CI: 3.92−6.25) reported via AERC only and 7.60% (95% CI: 6.43−8.96) reported via the AERC or through data recorded in clinical consultations. The incidence rate for non-GSK influenza vaccines based on data recorded in clinical consultations was 2.59% (95% CI: 1.93−3.47). The rate of AEIs appeared to peak early in the season and decline over time. The reason for this is unclear, but we speculate that people who attend early in the season for their influenza vaccine are highly organized individuals who may be meticulous in reporting AEIs. However, we have no specific evidence to support this hypothesis.
Just under half (44.7%) of the Fluarix Tetra recipients who were issued with an AERC returned it. Two thirds of participants who reported an AEI with Fluarix Tetra did so via the AERC and one third at a GP consultation. It appears that the AERC was a useful tool that complemented routine pharmacovigilance, and allowed more comprehensive capture of AEIs experienced. An Australian study also described a trebling of AEI reports with Bacillus Calmette-Guérin vaccine following introduction of an EPS scheme, although the largest increase was observed among children.Citation20 The contribution of EPS in monitoring AEIs for other vaccines has been demonstrated in Australia and the Netherlands.Citation21,Citation22
Despite the demonstrated added value of combining routinely collected data from the EHR with data from the AERC, we cannot completely rule out potential under-reporting of AEIs in our study. In an Australian post-marketing surveillance study of healthcare professionals in which surveillance was conducted by short message service (SMS), one in eight (13.3%) recipients of influenza vaccine reported an AEI.Citation23 A study in the Netherlands that used three repeated web-based questionnaires, with response rates of 91%, 85%, and 78%, found an AEI rate of 38.5%.Citation24 A US study has evaluated adverse events using a system in which influenza vaccine recipients were asked to report via internet or telephone on a daily basis for 14 days post-vaccination regardless of whether an event was experienced or not; daily email reminders were sent and non-respondents were followed up by telephone.Citation25 The study found that 62% of people reported AEIs, though half were minor. Interestingly, minor AEIs peaked on the second day and more serious effects (e.g. hoarseness or wheezing, unusual weakness, dizziness) peaked on the fifth day post-vaccination.Citation25
The current study was sufficiently powered to detect very common (≥1/10), common (≥1/100 to <1/10) and uncommon (≥1/1,000 to <1/100) AEIs that are pre-defined by EMA, but was not designed to detect rare events (>1/10,000 and <1/1000) or very rare events (<1/10,000).Citation19 The ICC was low and the generalized estimating equations (GEE) adjusted percentages of AEIs were close to those reported from the unadjusted data, suggesting that the practices selected for this study were relatively similar in their approach to influenza vaccination and AEI reporting. Weekly analysis was more robust as vaccine exposure increased. Collecting data over successive seasons would provide information about year-on-year variation in rates of AEIs and may make it easier to detect any safety signal. Longitudinal data would be particularly helpful at the start of the season when it is hard to tell if sporadic reports represent any type of signal.
Our data are primarily applicable to adult vaccine recipients. In the UK, the live attenuated influenza vaccine nasal spray is used preferentially for children, and thus very few children received Fluarix Tetra and were issued with an AERC. Therefore, the data from our study do not fully represent all influenza vaccine recipients in England. A limitation of our study is that pneumococcus and shingles vaccines are commonly administered in UK primary care on the same day as the influenza vaccine, potentially leading to difficulties in distinguishing between events associated with one vaccine versus another. Co-administration of other vaccines during the study was expected to be limited, and therefore documentation of co-administration was not part of the study procedures. A high proportion of ethnicity data was missing. We could improve our EPS method by capturing on the EHR when a returned AERC indicated no AEI. Other options that could have been considered include using reminders to try to boost the return rate of the AERCs and offering an SMS or web-based system to report AEIs rather than a card-based system.
Conclusions
Our EPS method combined routinely-collected data from the EHR with data from an AERC in people vaccinated with Fluarix Tetra to maximize the likelihood of capturing any AEI experienced. We succeeded in enrolling a substantial number of vaccinated people, exceeding the minimum sample size recommended by EMA (n = 1000),Citation10 as well as the anticipated number used in our sample size assumptions.Citation17 We demonstrated that our EPS method can be successfully implemented in a primary care setting. No safety signal for Fluarix Tetra was identified. Our EPS method combining routine data from the EHR with an AERC appears to be a useful way to improve capture of AEIs compared with classical passive surveillance alone. In conclusion, the study identified no safety signal during or at the end of the study that would impact public health or alter the benefit-risk profile of Fluarix Tetra. The study supports and confirms the safety profile of GSK’s Fluarix Tetra vaccines.
Methods
The study methods have been published previously in detailCitation17 and are summarized below. In summary, our EPS method was a combination of near-real-time passive surveillance of EHR systems, enhanced by use of an AERC in participants vaccinated with Fluarix Tetra; the method followed EMA guidance.Citation10 The study received a favourable ethics committee approval: Integrated Research Application System (IRAS) project ID: 211560; Research Ethics Committee (REC) reference: 16/NE/0271. All data were pseudonymized, as described previously.Citation17 Interim and final analyses were performed. Interim safety reports were assessed on a weekly basis by GSK’s safety core team to identify any potential safety concerns. The content and outcome of those investigations were communicated to the University of Surrey who could contact the participating sites for further insights as appropriate. We have previously reported an interim analysis of this study, where the AERCs were described as “orange cards” but refer to the same toolCitation18; the present paper reports the final analysis.
Setting and population
The sample size calculation has been reported previously.Citation17 Briefly, assuming that influenza vaccination uptake in the 2016−2017 season would be similar to the previous season, we estimated that approximately 70,000 individuals would be required to enroll approximately 5000 vaccinees. As the average practice size is 7034, we recruited 10 practices.
We enrolled 10 general practices that used GSK’s Fluarix Tetra as their principal brand of influenza vaccine, although other brands were also used. These practices were already part of the Royal College for General Practitioners Research and Surveillance Centre network. We picked the practices based on their principal vaccine brand, their representativeness of the general population and their willingness to participate. The practices were spread across England: three practices each in the North, Midlands and East, and South NHS regions, with the tenth practice in London, and encompassed conurbation, urban and rural areas. The practices used different EHR systems; seven used EMIS Webb, one used TPP SystmOne and one used INPS Vision. The process was the same for all clinical systems. Reimbursement to the practices followed National Institute of Health Research guidelines for industry sponsored studies. Before the start of the study, each participating GP was provided with induction training which covered the scope of the study and the method of achieving standardized encoding of the events reported. In addition, GPs were instructed on the need to prime vaccinees about the study and the importance of reporting any post-vaccination events.
The study vaccination period covered 1 September to 30 November 2016 and the follow-up period captured AEIs from week 37 to week 48. All individuals in an enrolled practice who received a seasonal influenza vaccination were eligible for the study providing they had a valid and pseudonymized NHS number, date of birth and gender recorded in the EHR. Individuals who received their vaccination outside of the practice (e.g. at a pharmacy) were included in the analysis. Any individual who was vaccinated with Fluarix Tetra outside the general practice did not receive an AERC. Individuals who had explicitly opted out of data-sharing were excluded. The study had no influence on which individuals were vaccinated or with which vaccine, although it was anticipated that national guidance would be followed.Citation26 These guidelines recommend that the following groups be vaccinated: people ≥65 years of age; people with a chronic medical condition; pregnant women; children 2, 3 or 4 years of age; and other individuals considered at risk or a close contact of people at risk.Citation26 However, any individual outside of the recommended groups could choose to be vaccinated.
Data collection
We utilized the database and weekly reporting system developed in 2015 for the Royal College of General Practitioners Research and Surveillance Centre, the primary care national surveillance system.Citation27,Citation28 We used two data sources: (1) practice EHR data (passive surveillance component) and (2) AERC completed by participants receiving Fluarix Tetra (enhanced component). The AERCs (the ‘orange card’) have been described previously.Citation17,Citation18 The cards were handed out at the routine vaccination and were returned either in person or by mail, ideally within 7 days post-vaccination, but no longer than 14 days. We performed a weekly pseudonymized data extract utilizing both data sources. Cross-sectional weekly reports were produced and published online.Citation29 A summary of the results is available on ClinicalTrials.gov (NCT02893878).
Vaccine exposure
The total number of people vaccinated was recorded and categorized as (1) vaccinated using GSK’s Fluarix Tetra; (2) vaccinated with another specified brand of influenza vaccine (recorded as ‘Non-GSK’); (3) vaccinated with an unknown brand of influenza vaccine. We reported the characteristics of the population exposed to influenza vaccine including age category at the time of immunization (6 months to 5 years, 6−12 years, 13−17 years, 18−65 years, >65 years); gender; ethnicity using UK Office of National Statistics categories and an ontological approach to maximize data captureCitation30; and a measure of socioeconomic status, the Index of Multiple Deprivation (IMD).Citation31 In addition, we reported vaccine exposure according to high-risk group specified by national guidance if applicable, and added a category “not at risk” where no obvious risk factor was recorded.
Vaccine exposure data were either reported using a vaccine administration code, or through recording of vaccine brand and batch number. However, the vaccine brand was not consistently recorded when the vaccine was administered in a pharmacy, because recording of the brand is not required by the system that provides data about pharmacy administration.Citation32 We reported vaccine exposure data when either the date of administration or the batch number was available; data were considered complete if both administration date and batch number were available.
Adverse events
The AERC consisted of a structured list of pre-specified AEIs recommended by the EMA,Citation10 with the option for participants to record that no AEI or any adverse event occurred. Events were grouped into body systems: respiratory conditions (conjunctivitis, coryza, cough, epistaxis, hoarseness, nasal congestion, oropharyngeal pain, rhinorrhea, wheezing); musculoskeletal conditions (arthropathy, muscle aches/myalgia); neurologic conditions (Bell’s palsy, Guillain-Barre syndrome, headache, peripheral tremor, seizure/febrile convulsions); gastrointestinal conditions (decreased appetite, diarrhea, nausea, vomiting); general symptoms (drowsiness, fatigue, irritability, malaise); sensitivity/anaphylaxis (anaphylactic reactions, facial edema, hypersensitivity reactions); fever/pyrexia including actual temperatures recorded when available; local erythema; and rash.
Adverse events were recorded for 7 days post-vaccination. The practice recorded all returned AERCs. Any adverse events recorded on the AERC were entered on the participant’s EHR, but if no adverse event was recorded, this was not captured on the EHR. AEIs reported to the practice outside of the AERC were recorded on the EHR.
Hospital admissions and deaths
Hospital admissions were identified via our data extracts using a set of read codes. These varied in specificity and may not have given the reason for the admission. Therefore, the practice at which the participant was registered was contacted within a week from admission to determine the reason for the admission and any suspected causal link with vaccination in the professional judgement of the practice. Each practice was also required to provide the reason for any deaths and any suspected causal link with the vaccine. If a causal link was suspected, practices were encouraged to use the national reporting system “Yellow Card Scheme” in parallel to the reporting scheme described in this paper; in addition, if the event occurred after vaccination with Fluarix Tetra, the practice was required to report the event to GSK in parallel.
Study endpoints
The primary endpoint was the occurrence of AEIs within 7 days post-vaccination reported with the AERC in recipients of Fluarix Tetra. Data were stratified by age category (6 months−5 years, 6−12 years, 13−17 years, 18−65 years, >65 years). The secondary endpoint was the occurrence of AEIs within 7 days post-vaccination using data from the EHR (which includes data from the AERC), with data stratified by vaccine brand (Fluarix Tetra, non-GSK or unknown). Influenza vaccine exposure and return rate of the AERCs were analyzed as tertiary endpoints. As part of EMA pharmacovigilance requirements, the study also evaluated any potential safety signal with Fluarix Tetra by comparing the observed frequency of individual AEIs versus the frequency of solicited AEIs included in the Fluarix Tetra SmPC.Citation19 The occurrence of any unexpected event or an increase in frequency versus the SmPC could indicate a potential safety signal.
Statistical analysis
Weekly data were reported in the weekly vaccinated safety cohort which included all registered individuals who were eligible for the study and vaccinated during the week before the week of interest (reaching up to 7 days of follow-up post-vaccination during the week of interest). Cumulative data were reported in the cumulative vaccinated safety cohort which included all registered individuals who were eligible for the study and vaccinated at any point from study start-up to the week before the week of interest (i.e. cumulatively since the beginning of the study).
The number and proportion of individuals who received an influenza vaccine in the 10 general practices were calculated. We used descriptive statistics to report demographic characteristics. Recruitment into the study by GP practices may have created a clustering effect, because participants in the same practice are more likely to receive similar treatment for a given condition, and are likely to share similarities including geography, socioeconomic status, ethnic background, or age by virtue of the area in which they live. In the same way, GP practices that have chosen to work together are likely to share similarities. Similarities (or homogeneity) between participants in clusters reduce the variability of their responses, compared with that expected from a random sample. This clustering effect thus decreases the precision of the estimated rates (i.e. leading to a wider confidence interval around the estimated rates).
The incidence rates of AEI, their 95% CI and ICCs accounting for clustering effect were estimated from the logistic GEE models adjusted for clustering effect of GP practices with a robust variance estimate.Citation33 The exact CI for a proportion within a group, not accounting for clustering effect, was calculated using the method described by Clopper & Pearson.Citation34
For the weekly incidence rate of AEIs, the denominator was the number of participants in the weekly vaccinated safety cohort for the week of interest and the numerator was the corresponding number who reported an AEI within 7 days following vaccination with the seasonal influenza vaccine. For the cumulative incidence rate, the denominator was the number of participants in the cumulative vaccinated safety cohort up to the week of interest and the numerator was the corresponding number who reported an AEI within 7 days following vaccination with the seasonal influenza vaccine.
Disclosure of potential conflicts of interest
Simon de Lusignan reports grants from the GSK group of companies for this and previous studies to assess the potential to detect European Medicines Agency listed possible brand-specific adverse events following immunisation in near real time. Simon de Lusignan has also been a member of Global Advisory Boards for Sanofi and Seqirus. Filipa Ferreira has nothing to disclose. Silvia Damaso declares she is employed by the GSK group of companies and holds shares in the GSK group of companies. Rachel Byford has nothing to disclose. Sameera Pathirannehelage has nothing to disclose. Anne Yeakey declares she is employed by the GSK group of companies, and holds shares in the GSK group of companies. Ivelina Yonova has nothing to disclose. Anne Schuind declares she is employed by the GSK group of companies, and holds shares in the GSK group of companies. Gael Dos Santos declares he is employed by the GSK group of companies, and holds shares in the GSK group of companies.
Authors’ contributions
The idea and contents of the article emerged from discussions among the authors, who have experience in epidemiology, vaccinology, and vaccine safety. Simon de Lusignan wrote the first draft; all authors contributed to subsequent revisions and approved the final version. The authors are solely responsible for final content and interpretation. The authors received no financial support or other form of compensation related to the development of the manuscript.
Trademark statement
Fluarix Tetra is a trademark of the GSK group of companies.
Supplemental Material
Download MS Word (33.5 KB)Acknowledgments
The authors thank the patients and practices who allowed pseudonymized data to be extracted from their general practice EHR system and who participated in this study. The authors also thank Frédéric Lin, Binutha Pereira, Vishvesh Shende, Stephanie Gilon, Helena Denise Guerra-Karekides, Karen Stuttard and Dominique Rosillon from GSK and Ana Correa from the University of Surrey for their contribution to this study. Finally, the authors thank Mary L Greenacre (An Sgriobhadair, UK, on behalf of GSK) for providing medical writing services, and Business & Decision Life Sciences platform (on behalf of GSK) for editorial assistance, manuscript coordination and writing support. Bruno Dumont coordinated the manuscript development and editorial support.
Supplementary material
Supplemental data for this article can be accessed accessed on the publisher’s website.
Additional information
Funding
References
- European Centre for Disease Prevention and Control. Factsheet about seasonal influenza; 2018 [accessed 2018 May]. https://ecdc.europa.eu/en/seasonal-influenza/facts/factsheet.
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–300. doi:10.1016/S0140-6736(17)33293-2.
- World Health Organization. Burden of disease; [accessed 2018 May]. http://www.who.int/influenza/surveillance_monitoring/bod/en/.
- World Health Organization. Fact sheets - Influenza (Seasonal); [accessed 2018 Sep]. http://www.who.int/news-room/fact-sheets/detail/influenza- (seasonal).
- World Health Organization. WHO recommendations on the composition of influenza virus vaccines; [accessed 2018 May]. http://www.who.int/influenza/vaccines/virus/recommendations/en/.
- Levi M, Sinisgalli E, Lorini C, Santomauro F, Chellini M, The BP. “Fluad case” in Italy: could it have been dealt differently? Hum Vacc Immunother. 2017;13(2):379–84. doi:10.1080/21645515.2017.1264738.
- Rosselli R, Martini M, Bragazzi NL, Watad A. The public health impact of the so-called “fluad effect” on the 2014/2015 influenza vaccination campaign in italy: ethical implications for health-care workers and health communication practitioners. Adv Exp Med Biol. 2017;973:125–34. doi:10.1007/5584_2017_39.
- Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ (Clinical Research Ed). 2011;343:d5956. doi:10.1136/bmj.d5956.
- Borg JJ, Aislaitner G, Pirozynski M, Mifsud S. Strengthening and rationalizing pharmacovigilance in the EU: where is Europe heading to? A review of the new EU legislation on pharmacovigilance. Drug Saf. 2011;34(3):187–97. doi:10.2165/11586620-000000000-00000.
- European Medicines Agency. Interim guidance on enhanced safety surveillance for seasonal influenza vaccines in the EU. London; 2014 [accessed 2018 Nov]. https://www.ema.europa.eu/documents/scientific-guideline/interim-guidance-enhanced-safety-surveillance-seasonal-influenza-vaccines-eu_en.pdf.
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP). Product- or Population-Specific Considerations I: Vaccines for prophylaxis against infectious diseases. EMA/488220/2012; 2013 [accessed 2018 Sep]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/12/WC500157839.pdf.
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP). Module VIII – Post-authorization safety studies (Rev 3). EMA/813938/2011 Rev 3; 2013 [accessed June 2018]. http://www.ema.europa.eu/docs/en_GB/document_library/_guideline/2012/06/WC500Scientific.pdf.
- de Lusignan S. Codes, classifications, terminologies and nomenclatures: definition, development and application in practice. Inform Prim Care. 2005;13:65–70.
- de Lusignan S, Hague N, van Vlymen J, Kumarapeli P. Routinely-collected general practice data are complex, but with systematic processing can be used for quality improvement and research. Inform Prim Care. 2006;14:59–66.
- NHS England. Enhanced Service Specification: Seasonal influenza and pneumococcal polysaccharide vaccination programme 2015/16; 2015.
- Public Health England. Seasonal influenza vaccine uptake in GP patients: winter season 2017 to 2018: final data for 1 September 2017 to 31 January 2018; 2018 [accessed 2018 Nov]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/710416/Seasonal_influenza_vaccine_uptake_in_GP_patients_winter_season_2017_to_2018.pdf.
- de Lusignan S, Dos Santos G, Correa A, Haguinet F, Yonova I, Lair F, Byford R, Ferreira F, Stuttard K, Chan T. Post-authorisation passive enhanced safety surveillance of seasonal influenza vaccines: protocol of a pilot study in England. BMJ Open. 2017;7:5. doi:10.1136/bmjopen-2016-015469.
- de Lusignan S, Dos Santos G, Byford R, Schuind A, Damaso S, Shende V, McGee C, Yonova I, Ferreira F. Enhanced safety surveillance of seasonal quadrivalent influenza vaccines in english primary care: interim analysis. Adv Ther. 2018;35(8):1199–214. doi:10.1007/s12325-018-0747-4.
- Electronic Medicines Compendium. GlaxoSmithKline UK. Fluarix Tetra Summary Product Characteristics; 2018 [accessed 2018 Nov]. https://www.medicines.org.uk/emc/product/3021/smpc.
- Clothier HJ, Crawford NW, Russell M, Kelly H, Buttery JP. Evaluation of ‘SAEFVIC’, A pharmacovigilance surveillance scheme for the spontaneous reporting of adverse events following immunisation in Victoria, Australia. Drug Saf. 2017;40(6):483–95. doi:10.1007/s40264-017-0520-7.
- Clothier HJ, Hosking L, Crawford NW, Russell M, Easton ML, Quinn JA, Buttery JP. Bacillus Calmette-Guerin (BCG) vaccine adverse events in Victoria, Australia: analysis of reports to an enhanced passive surveillance system. Drug Saf. 2015;38(1):79–86. doi:10.1007/s40264-014-0248-6.
- Kemmeren JM, van der Maas NAT, de Melker HE. Parental reports of adverse events following simultaneously given dT-IPV and MMR vaccines in healthy 9-year-old children. Eur J Pediatr. 2011;170(3):339–45. doi:10.1007/s00431-010-1294-4.
- Regan AK, Tracey L, Gibbs R. Post-marketing surveillance of adverse events following immunization with inactivated quadrivalent and trivalent influenza vaccine in health care providers in Western Australia. Vaccine. 2015;33(46):6149–51. doi:10.1016/j.vaccine.2015.10.005.
- van Balveren-Slingerland L, Kant A, Härmark L. Web-based intensive monitoring of adverse events following influenza vaccination in general practice. Vaccine. 2015;33(19):2283–88. doi:10.1016/j.vaccine.2015.03.014.
- Newes-Adeyi G, Greece J, Bozeman S, Walker DK, Lewis F, Gidudu J. Active surveillance for influenza vaccine adverse events: the integrated vaccine surveillance system. Vaccine. 2012;30(6):1050–55. doi:10.1016/j.vaccine.2011.12.041.
- Public Health England. Flu plan winter 2016/17; 2016 [accessed 2018 June]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/525967/Annual_flu_plan_2016_to_2017.pdf.
- Correa A, Hinton W, McGovern A, van Vlymen J, Yonova I, Jones S, de Lusignan S. Royal college of general practitioners research and surveillance centre (RCGP RSC) sentinel network: a cohort profile. BMJ Open. 2016;6:4. doi:10.1136/bmjopen-2016-011092.
- de Lusignan S, Correa A, Smith GE, Yonova I, Pebody R, Ferreira F, Elliot AJ, Fleming D. RCGP research and surveillance centre: 50 years’ surveillance of influenza, infections, and respiratory conditions. Br J Gen Pract. 2017;67(663):440–41. doi:10.3399/bjgp17X692645.
- Clinical Informatics and Health Outcomes Research Group. Post-authorisation passive enhanced safety surveillance of seasonal influenza vaccines: pilot study in England. Guildford (UK): University of Surrey; 2017. [accessed 2018 June]. http://www.ema.europa.eu/docs/en_GB/document_library/_guideline/2012/06/WC500Scientific.pdf.
- Tippu Z, Correa A, Liyanage H, Burleigh D, McGovern A, Van Vlymen J, Jones S, De Lusignan S. Ethnicity recording in primary care computerised medical record systems: an ontological approach. J Innov Health Inform. 2017;23(4):920. doi:10.14236/jhi.v23i4.920.
- Jordan H, Roderick P, Martin D. The index of multiple deprivation 2000 and accessibility effects on health. J Epidemiol Community Health. 2004;58:250–57.
- de Lusignan S, Hoghton M, Rafi I. Flu vaccination by pharmacists leads to suboptimal medical records. BMJ (Clinical Research Ed). 2017;359:j5084. doi:10.1136/bmj.j5084.
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi:10.1093/biomet/73.1.13.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of binomial. Biometrika. 1934;26:404–13. doi:10.1093/biomet/26.4.404.