ABSTRACT
Influenza vaccines are a fundamental tool for preventing the disease and reducing its consequences, particularly in specific high-risk groups. In order to be licensed, influenza vaccines have to meet strict criteria established by European Medicines Agency. Although the licensure of influenza vaccines started 65 years ago, Hemagglutination Inhibition and Single Radial Hemolysis are the only serological assays that can ascertain correlates of protection. However, they present evident limitations. The present review focuses on the evaluation of cell-mediated immunity (CMI), which plays an important role in the host immune response in protecting against virus-related illness and in the establishment of long-term immunological memory. Although correlates of protection are not currently available for CMI, it would be advisable to investigate this kind of immunological response for the evaluation of next-generation vaccines.
Introduction
Influenza vaccines constitute the only available means of preventing influenza and its complications. Although influenza is a vaccine-preventable disease, it still constitutes a major health problem, accounting for about 3 to 5 million cases of severe illness and responsible for 290,000 up to 650,000 respiratory deaths per year.Citation1 Young children, pregnant women, immunocompromised subjects, subjects of any age with specific chronic medical conditions and the elderly have a higher risk for influenza-related co-morbidities; these may be life-threatening, requiring hospitalization, and even lead to death. In healthy children younger than 24 months of age, the risk of hospitalization is comparable to that of high-risk groups, or even higher. Specifically, children aged < 24 months run a significantly higher risk of being hospitalized than older children; in addition the youngest children have the greatest risk of hospitalization as a consequence of flu. Influenza-associated deaths in children often occur soon after symptom onset, mostly within 1 week. Wong et al.Citation2 found that the period between symptom onset and death was even shorter in previously healthy children than in children with high-risk medical conditions. Although no explanation for this observation is currently available, it has been hypothesized that abnormal immune regulation could underlie severe infection in certain previously healthy children.Citation3
Flu complications range from moderate (ear and sinus infections) to serious. The latter include pneumonia, myocarditis, encephalitis, myositis, rhabdomyolysis, multi-organ failure (such as respiratory and kidney failure) and sepsis. Flu also can make chronic health problems worse.Citation4
The elderly show reduced vaccine effectiveness as a result of immunosenescence. It is traditionally accepted that aging leads to a gradual decline of both innate and adaptive immune responses, thereby reducing the response towards infections and vaccines; today, however, immunosenescence is seen more as a remodeling of the immune system, causing an altered regulation of the various compartments. Indeed, while certain activities show a deterioration,Citation5 others are up-regulatedCitation6 or remain unchanged.Citation7 In addition to age, other factors influence the effectiveness of influenza vaccines: the antigen match between the circulating influenza strains and those strains contained in the vaccine itself, the vaccinee’s immunocompetence, and the antibody levels induced by previous infections or vaccinations.Citation8,Citation9
Criteria for influenza vaccine licensing
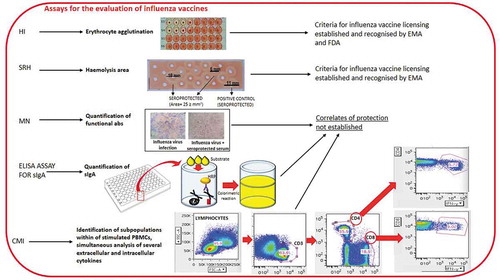
The evaluation of vaccine immunogenicity constitutes a critical aspect of vaccine marketing. In order to evaluate the host immune response to vaccines that provides protection, correlates of protection are used. Although the words “correlates” and “surrogates” are often used synonymously, their meanings are different. As specified by Plotkin,Citation10 “an immune function that is responsible for and statistically interrelated with protection is a correlate, while an immune response that is simply an easy measurement but not functional in protection is a surrogate”. In the case of influenza vaccines, correlates of protection for influenza are usually represented by serum antibody titers, which are mainly measured by means of the Hemagglutination Inhibition (HI) assay.Citation11 Indeed, antibodies can protect against influenza, as demonstrated by the fact that their parental or intranasal administration reduces infection rates in animal modelsCitation12,Citation13 and IgG trans-placental passage provides neonatal protection.Citation14,Citation15 Furthermore, in the human influenza challenge, treatment with an anti-M2e monoclonal antibody has proved effective and safe.Citation16
Several serological assays are commonly used to evaluate vaccine effectiveness; these include usually Single Radial Haemolysis (SRH), HI test and Virus Microneutralization (MN).
However, although the licensure of influenza vaccines began 65 years ago, HI and SRH are the only serological assays for the evaluation of humoral effectiveness that have correlates of protection established by the European Medicines Agency (EMA) which have to be met in order to obtain vaccine licensure. For this reason, they are considered the gold standard. Every year, vaccine manufacturers have to conduct clinical trials for the annual update of influenza vaccine composition. Specifically, pre- and post-vaccination serum samples are collected (approximately 21 days after the first blood draw) from 2 groups of at least 50 individuals aged 18–60 years and >60 years. Immunogenicity is assessed by means of three criteria identified by the Committee for Medicinal Products for Human Use (CHMP).
The proportion of vaccines that achieve an HI titer of 40 or SRH > 25 mm2 should be >70% in
18–60 year-olds and >60% in the over-60s. The seroconversion rate (SCR) (at least a 4-fold increase in titer) should be >40% in 18–60-year-olds and >30% in the over 60s. A mean geometric increase (ratio of pre- to post-vaccination) of >2.5 is required in 18–60-year-olds and >2 in over-60s. In the US, the same criteria are used by Food and Drug Administration (FDA), but the lower boundary of the 95% confidence interval (CI) has to be higher than or equal to that of the SCR and geometric mean titer (GMT) criteria.Citation17
At least one of the 3 criteria must be met by seasonal influenza vaccines, and all 3 criteria by pandemic influenza vaccines in order to be licensed.
Since different classes of antibodies are identified by the three serological assays, different degrees of correlation among them have been observed.Citation18
The SRH and HI tests recognize antibodies which bind the influenza virus and fix complementCitation19,Citation20 and viral HA, respectively, preventing the agglutination of erythrocytes caused by the influenza virus ().
The SRH assay is based on the measurement of the hemolysis areas,Citation20,Citation21 which correlates with influenza antibody concentration, since hemolysis is the outcome of antigen-antibody binding. The assay is specifically suitable for use in large-scale clinical trials, owing to its rapidity, reproducibility and reliability.Citation22,Citation23 The SRH assay has a higher sensitivity for influenza B strains.Citation24,Citation25
The HI assay is the gold-standard assay of antibody titers against HA, and is based on erythrocyte agglutination due to the ability of antibodies that specifically recognize HA to inhibit the binding of viral surface protein HA to sialic acid sites on the surface of red blood cells.Citation21 Avian (chicken or turkey) or mammalian (horse or guinea pig) erythrocytes are usually chosen for the assay. HI titers are quantified as the reciprocal of the highest serum dilution (titer) (1/dilution factor) that inhibits hemagglutination by binding with the virus.Citation26
HI also presents limitations, including low sensitivity for influenza B and avian viruses, unsuitability for LAIV evaluation, high inter-laboratory variability due to many factors and the absence of standardized protocols.Citation25 Regarding seasonal influenza A strains, SRH and HI show similar sensitivity.Citation24 A ≥ 25 mm2 zone is defined as a correlate of protection for SRH.Citation27 Concerning HI, an HI antibody titer ≥40 and a minimum 4-fold increase in antibody titer post vaccination are historically considered an immunological correlate of protection against infections caused by influenza viruses, and is associated with a 50% reduction in the risk of developing influenza. These data are based on a challenge study performed by the group of Hobson on adultsCitation27 and epidemiologic studies conducted mainly on young adults. [rev. inCitation17,Citation23,Citation28]. However, the biological role in establishing protection of titers higher than 1:40 remains to be elucidated. Indeed, in an analysis of 12 studies conducted on healthy adults, de Jong et al.Citation29 found that a mean titer of 28 was protective in 50% of the subjects. However, they reported protective titers ranging from 15 to 65, owing to differences in the studies. By contrast, a subsequent meta-analysis by Coudeville found that 17 was a median 50% protective titer.Citation30 In addition, application of the bayesian random-effects model developed by Coudeville to the data from Hobson’s study revealed that a titer of 29 provided a 50% level of protection. Regarding the correlation between HI titers and the establishment of clinical protection against influenza, the investigation revealed an increase in protection up to an HI titer of 150. Reaching HI titers higher than 150 did not provide a further significant protective ability; however, it is possible that HI titer > 160 must be reached in order to be protective both in children less than 6 years of age and in the elderly, since these two groups have an elevated risk of suffering influenza-related complications.Citation31 Concerning children, Black and colleagues found that 50% protection corresponded to an HAI titer of 110,Citation31 whereas few data are available on subjects aged over 65 years, to whom the protective titer of 40 is applied, as in younger adults.Citation32
In addition, over the last decade, an increasing proportion of circulating human influenza A(H3N2) viruses have exhibited lower levels of hemagglutination as a consequence of binding to HA.Citation33
The MN assay identifies functional antibodies that recognize the globular head of hemagglutinin (HA); these antibodies are able to halt receptor binding and internalization during membrane fusion, thus constituting the principal immune mediators toward influenza viruses.Citation34 Hence, the assay provides a true measure of the antibodies able to neutralize the ability of the virus to enter mammalian cells and replicate ().Citation35 In the MN assay, sensitive cells are inoculated with a mixture of viruses and serum (i.e. the serum samples tested) and the read-out is performed by microscopically observing cytopathic effects (CPEs). The neutralization titer (i.e. antibody titer of tested previously serum samples serially diluted two-fold) is defined as the serum dilution by means of which 50% of the wells are protected against a virus-induced CPE. CPE is evaluated by checking the 96-well plate under an optical microscope for the presence of local lesions in the cell monolayer, in terms of hole(s) in the cell monolayer, surrounded by destroyed cells, or complete destruction of the cell monolayer in the well.Citation11 However, the assay is labor-intensive and displays poor reproducibility among laboratories.Citation36,Citation37 Currently, no threshold for protection against influenza has yet been established for the MN test.Citation23
All the above-mentioned assays measure antibody titers in the peripheral blood, but they do not evaluate the establishment of local mucosal immunity.
Evaluating local mucosal immunity is particularly important with regard to the efficacy of live attenuated influenza vaccines (LAIVs). Although these vaccines entered the market in 2013, we still do not have specific knowledge of the immunological mechanisms they induce, nor do they have correlates of protection.Citation38 A study conducted by GorseCitation39 on older, chronically ill adults found that LAIVs, but not trivalent inactivated vaccines (TIVs), were able to induce heterosubtypic immunity in terms of both humoral and cellular immune responses. Comparison of the immune response in children and adults immunized with LAIVs or TIVs, revealed that both vaccines were effective; however, significant differences emerged in B-cell and antibody responses elicited by LAIVs or TIVs in the two groups.Citation40,Citation41
Specifically, inactivated influenza vaccine (IIV) proved more effective in adults, whereas LAIVs provided higher protection in children,Citation42 although the immune mechanisms underlying these differences have not yet been clarified.Citation41 With particular regard to influenza virus-specific HI, only a small increase in serum HI responses was observed in adults immunized with LAIV, and these responses were considerably lower than those induced by IIV. Both LAIV and IIV similarly induced only transient T-cell responses to replication-competent whole virus in adults. In contrast, stronger influenza virus-specific secretory IgA (sIgA) responses were induced by LAIV than by IIV.Citation41 A previous investigation conducted by the same groupCitation40 reported that LAIVs showed a greater ability to induce several T-cell responses (CD4+, CD8+, and γδ T cells) in young children, indicating that they play an important role in providing heterosubtypic immunity.
Measuring sIgA and identifying a subpopulation of resident memory T-cellsCitation43 could constitute valuable alternative tools for assessing the effectiveness of LAIV (). In addition, a recently published meta-analysis performed by the group of WenCitation44 allowed the identification of differentially expressed genes responsible for distinct immune responses following LAIV and TIV vaccinations. Specifically, whereas LAIV mainly promoted the upregulation of genes associated with the innate immune system, TIV up-regulated genes correlated with both the innate and the humoral immune responses. The importance of these data lies in the fact that they provide more information about the activating pathways underlying the different immune responses to LAIV and TIV immunization – knowledge which may enable us to enhance the efficacy of vaccinations in children, adults and the elderly.Citation44 Wang and colleaguesCitation45 first investigated the correlates of protection by calculating Spearman’s rank correlation coefficient (r) for antibody levels for SRH, HI and MN against H3N2 influenza in children and adolescents; in these two age-groups, few data on the transferability of the two thresholds established for HI and SRH are available.Citation31 They reported significant correlations among HI, MN and SRH. Specifically, correlation of 0.50 (P < .01), 0.53 (P < .01) and 0.82 (P < .01) were observed between HI and MN, between HI and SRH, and between MN and SRH. MN was the most sensitive of the three serological assays investigated for the evaluation of antibody response against influenza H3N2.Citation45 This result could be directly linked to the principle underlying the three tests. While SRH and HI assays are based on complement fixation and HA binding, respectively, MN recognizes specifically functional antibodies involved in virus neutralization. Hence, MN can detect a higher proportion of protective antibodies than SRH and HI.Citation25 Specifically, HI has been seen to have lower sensitivity than SRH and MN, as demonstrated by the fact that 34% and 16% of the subjects, respectively, showed HI and SRH titers below the detection limit, in comparison with 7% on the MN assay. Previous investigations had also reported a higher sensitivity of MN than HI.Citation11,Citation19,Citation20 Moreover, MN is reckoned to be more sensitive than HI in detecting protective antibodies towards some pandemic influenza strains.Citation46,Citation47 With regard to the MN assay, a recent study conducted by TsangCitation48 reported a correlation between antibody titers ≥ 40 and the establishment of 49% protection against H3N2 influenza virus within households.
Another limitation that should be considered is that the currently available correlates of protection for influenza vaccines regard healthy subjects and not high risk groups, such as older adults, young children and subjects affected by certain medical conditions;Citation46 in these groups, HI displays lower effectiveness in predicting protection. Indeed, the efficacy of an influenza vaccine is not always correlated with the extent of the humoral immune response.Citation49 Evaluation of the efficacy of novel influenza vaccines is heavily based on serological assays; however, both seroconversion and seroprotection rates, as well as antibody titers, when used as the only predictors of vaccine efficacy, present limitations, as has increasingly been recognized.Citation50-Citation54 Immune senescence is responsible for the age-related decline of immune responses, since the elderly present alterations in the immune function and inflammatory response; this results in more serious outcomes of viral and bacterial infections as well as lower vaccine responses.Citation55 Specifically, elderly subjects who are affected by febrile influenza illness may not be able to mount an antibody response (with reductions in antibody titers and lower antibody avidity) although they test PCR+ for influenza virus.Citation56 For this reason, an optimal correlation between antibody titer and strain-specific vaccine efficacy cannot be obtained by using the traditional measures of immune response to influenza vaccines. Hence, in the elderly, the cell-mediated immune response can help to establish clinical protection against the increased risk for complications of influenza infections.Citation57,Citation58 In addition, in the elderly cytotoxic T-lymphocyte (CTL) response and granzyme B synthesis have shown a stronger correlation with protection than that provided by antibodies.Citation59,Citation60
Another limitation of standard serology methods is that new vaccines may not contain HA in their formulations, but other viral proteins, which means that their protective actions cannot be determined; some example are DNA- or RNA-based vaccines with sequences encoding nucleoprotein (NP) and M proteins.Citation61
In young adults, correlates of protection for T-lymphocytes have been identified,Citation62,Citation63 but these have not been transferred to older adults. Moreover, attempts to transfer the thresholds indicating clinical protection against influenza infection to older adults, with a view to developing novel influenza vaccines, could be unsuccessful, since these thresholds may need to be associated with antibody responses. Supporting the need for new correlates of protection, a recent study by NeidichCitation64 on influenza-vaccinated adults suffering from obesity revealed for the first time that, although obese subjects displayed similar seroconversion and seroprotection rates to healthy-weight subjects, they were twice as likely to develop influenza or influenza-like illness (ILI). Not only did an HI titer ≥ 40 not represent a serological correlate of protection in obese adults, but also MN titers could not be applied to this group at high risk of influenza and ILI, in accordance with studies performed on obese mice.Citation65
The use of flow cytometry
Cell-mediated immunity (CMI) plays an important role in host immune response in protecting against virus-related illnesses, including influenza, and in the establishment of long-term immunological memory.Citation66
Today, clinical trials aimed at evaluating vaccine immunogenicity and, in particular, at increasing our knowledge of the mechanisms underlying the immune response are making greater use of techniques involving the simultaneous and accurate measurement of subpopulations of stimulated peripheral blood mononuclear cells (PBMCs) and several extracellular and intracellular cytokines, chemokines and cytotoxic activity, by means of flow cytometry () or Enzyme-linked ImmunoSPOT (ELISPOT) assays.Citation67
Identifying significant changes in the phenotype, differentiation and activity of T-lymphocytes induced by vaccine administration could provide reliable correlates of protection.Citation68
Although our knowledge of memory T-cell responses has increased, we still know little about the duration of these responses and their involvement in various pathologies. Protection against influenza involves both B and T lymphocytes. Specifically, CD8 T-cell memory and antigen-selective B cells require CD4 T-cells. Although conserved influenza peptides/antigens have been seen to induce the formation of both CD8 and CD4 T-lymphocytes, their generation does not reach a sufficiently elevated level to maintain immunological protection for years.Citation69 Long-term heterotypic protection against several influenza viruses have been induced by memory T-lymphocytes,Citation70-Citation72 as demonstrated by the fact that seasonal influenza viruses induced CD4 TCitation70,Citation73 and cytotoxic T-lymphocytesCitation71 that are able to recognize the pandemic H1N1 2009 (pdmH1N1) virus.
Intracellular cytokines released by an entire population can be identified through the use of intracellular cytokine staining (ICS). However, the extracellular release of cytokines by the Golgi is prevented by the use of an inhibitor of protein transport, such as monensin or Brefeldin A in the last 4 or 16 hours of cell culture.Citation74,Citation75 PBMCs or diluted whole blood can then be stimulated overnight by using different stimuli, such as Staphylococcus enterotoxin B (SEB), recombinant ESAT-6 protein or anti-CD28 and anti-CD49d co-stimulatory antibodies. The end of the incubation is followed by fixing, permeabilization and staining with fluorescent-labeled anti-cytokine antibodies. The PBMCs are then analyzed by means of a flow cytometer.Citation76,Citation77 The profile of secreted cytokines enables T-lymphocyte sub-populations to be distinguished.
ICS can be conducted both on isolated PBMCs, either fresh or cryopreserved in freezing medium (before at −80ºC and then in liquid nitrogen)Citation78 and on whole blood; in the former case, however, a lower inter-laboratory coefficient of variation has been observed.Citation79
Evaluation of t and B cell responses
Natural influenza virus infection stimulates CD4+ and CD8+ T cells, which act in synergy to provide protection in the case of vaccine mismatch or pandemic outbreak. By contrast, only the currently available LAIVs, and not IIVs, have been seen to efficiently elicit T-cell responses, especially in children.Citation40,Citation80,Citation81 The establishment of T-cell-mediated immunity induced by next-generation vaccines could overcome limitations linked to both specific subtype protection and antigenic mismatch. The different T-cell sub-populations can be identified through the use of specific antibody combinations that recognize cytokines secreted by a specific T-cell subset, such as Interferon (IFN)-γ by CD4+ T helper (Th)1 T-cellsCitation82 and cytotoxic T (Tc)1 CD8+ T cells,Citation83 Interleukin (IL)-17A by Th17 CD4+ T-cells,Citation84 or IL-4, IL-5, IL-9, IL-10 and IL-13 synthetized by CD4+ Th2 cells.Citation84
Although the cell-mediated immune response upon influenza vaccination is increasingly being investigated, and the observation that granzyme B production correlates with protection and increased CTL response to influenza vaccination in the elderly (vide supra), no correlates of protection regarding either the phenotype or the magnitude of the T-cell response following vaccination have yet been established. Evaluation of the cytotoxic potential of CD8+ T-lymphocytes is a further method of evaluating immune response; this involves measuring the degranulation and granule contents of specific T-cell subsets. Degranulation is typically measured in terms of CD107a expression on the cell surface. In normal conditions, CD107a is expressed in internal granular membranes, whereas during degranulation its transient expression can be identified on the cell surface.Citation85 T-cell responses can be evaluated by analyzing several cytokines, cell surface markers and other functional markers, such as perforin, CD107a, and CD154, with up to 10-color resolutionCitation86 and CD40 ligand expression with regard to CD4+T cell response.
The profile of ab-producing B lymphocytes has been investigated in infected or immunized subjects by monitoring the surface markers CD19, CD20, CD27, CD38, and CD138. Acute plasmablasts constitute the cell population which usually appears in the blood after infection during the phase of immune response. These cells are CD19lowCD20−CD27highCD38highCD138+/−cell populations, which differ from steady-state plasmablasts.Citation87 Their number has been seen to peak on day 6 or 7 in the case of booster responses, and somewhat later (∼day 10) in the case of new responses.Citation11,Citation88-Citation91
In order to identify novel correlates of protection, Nakaya and colleagues investigated early features of the innate and adaptive immune responses that could predict the HI titer 4 weeks after vaccination in 56 healthy young adults immunized with TIV or LAIV during the annual influenza seasons in 2007, 2008 and 2009.Citation92 The study highlighted the presence of a large number of genes showing a different expression; most of these participated in the response involving type I IFN and had a high expression in antibody secreting cells (ASCs), the latter probably due to rapid plasmablast proliferation 7 days after vaccination,Citation89 in the PBMCs of LAIV and TIV vaccinees, respectively.Citation92
Gijzen et al.Citation49 utilized granzyme B as a marker of T cell-mediated cytotoxicity and the production of Th1 and Th2 cytokines, such as IFN-γ, TNF-α, IL-2, IL-10, IL-4, IL-13, GM-CSF, to determine the cellular immune response with a view to establishing correlates of protection. Their study demonstrated that both granzyme B and cytokine assays could be used to evaluate cellular immunity and thus be examined as correlates of protection.
The group of JürchottCitation93 evaluated the baseline protective immune response to the A(H1N1)pdm2009 influenza strain following seasonal vaccination of 17 young (<31 years old) and 20 older (≥50 years) subjects who were seronegative against this strain by analyzing 36 sub-populations of lymphocytes. They also correlated this response with the serological immune response to the A(H1N1)pdm2009 strain after seasonal influenza vaccination. The seasonal vaccine for the season 2011–2012 (and 2013–2014 season) contained A(H1N1)pdm09/California/7/2009, together with A(H3N2)/Perth/16/2009 and B/Brisbane/60/2008 (or A(H3N2)/Texas/50/2012 and B/Massachusetts/2/2012) as vaccine strains.
The A(H3N2) and the B strains circulated before 2009 in humans and accumulated slight modifications by means of antigenic drift over the time,Citation94 whereas the California strain was a new virus of the subtype A(H1N1). The study revealed that the serological response to A/California/7/2009 depended on age and number of strains for which the donors were sero-negative at the baseline. More specifically, a trend toward a higher risk of no response and no seroprotection was observed in elderly donors. In addition, the analysis of several cell counts of immune sub-populations allowed these authors to identify the axis of CD4+ T cells, CD4+ naïve T-cells and CD4+ recent thymic emigrant T-cells as good candidates for response predictors. Specifically, they reported that the baseline CD4+ T-cell count, and especially that of naive CD4+ T-cells, constituted the best correlates for the evaluation of a successful immune response to A(H1N1)pdm09, but not to the A(H3N2) Perth or the influenza B Brisbane strains. Indeed, no marked deviations in CD4+ T-cells and their subsets were noted in Brisbane and Perth seronegative donors regarding the response to these strains, while the cell counts of CD8+ T-cells and CD19+ B cells in Brisbane seronegative donors, and of monocytes and dendritic cells in Perth seronegative donors, differed considerably between the protected and non-protected groups.Citation67 The significant differences between non-responders and responders concerning the immune cell sub-populations could be due to the fact that the H3N2 and the influenza B strains – or similar strains – were circulating in humans before 2009.
In agreement with Jürchott’s results, Nayak’s group also reported that CD4+ T-cell expansion was predictive of neutralizing antibody responses to a monovalent 2009 A(H1N1)pdm09 vaccine.Citation95 By contrast TebasCitation96 found that the A(H1N1)pdm09 vaccine was poorly immunogenic in well-controlled HIV-infected patients as a consequence of their low CD4+ T-cell counts. The further subdivision of naïve CD4+ T-lymphocytes into CD31+ recent thymic emigrants (RTE) and CD31− non-RTE fractions was not correlated with improved prediction, though both sub-populations were predictive of protection. Conversely, no association between baseline influenza selective CD4+ CD40L+ T-cells and protection against the A(H1N1)/California/7/2009 strain was observed.
Recently, Tsang and colleagues revealed that the analyses of human immune changes highlights the presence of baseline predictors of post-vaccination immune responses.Citation97
A recent investigation conducted by MbawuikeCitation98 evaluated cell-mediated immune responses upon re-vaccination of 177 subjects by using an inactivated influenza A/H5N1 (A/H5N1/Vietnam/1203/2004 and A/H5N1/Indonesia/05/05) vaccine; they also considered the effects exerted by the vaccine dose (15- or 90-mcg), adjuvant and the age of the subjects immunized.
Concerning LAIV vaccines, neither the quantization of mucosal or serum antibodies, nor that of chemokines or cytokines provide information regarding protection, and no association between protection and the administration of either IIV or LAIV vaccines was observed when the commonly used methods for the evaluation of immunity were implemented.Citation99
Conclusions
The formulation of an influenza vaccine that provides broad protection even in high risk groups, and the optimization of the vaccines currently available, require more thorough knowledge of the immune response of the host following influenza vaccination. A further need is to establish new correlates of protection for influenza vaccines, particularly for the evaluation of next-generation vaccines, since correlates of protection can vary according to the vaccine type, vaccine formulation, and the age and medical status of vaccinees. For the last 70 years, serological assays have been the only tests used to assess influenza vaccine efficacy, and the HI assay is a well-standardized and widely used test. However, the standardization of a T-cell assay may constitute a valuable approach, not least with a view to the development of more immunogenic, effective and cross-reactive novel vaccines. Traditionally, influenza vaccines have been aimed at eliciting antibodies involved in virus neutralization. However, those which recognize the HA-head region, even though potently neutralizing, can usually target only related viruses that do not present marked antigenic diversity. By contrast, although antibodies that target the conserved stem region have less neutralizing activity in vitro, they are endowed with cross-reactivity.Citation100,Citation101 Even though HI plays a paramount role as a correlate of protection for conventional influenza vaccines, in addition to CMI, assays based on other vaccination-induced antibodies, and which recognize epitopes different from HA, could constitute valid alternatives. Indeed, the human immune response is complex, involving both humoral and cellular responses, and various correlates of protection may conceivably exist.
Novel assays able to measure the Fc-mediated functions of anti-influenza antibodies have been developed, since it has been demonstrated that, in addition to neutralization, Abs can mediate further functions by using their Fc region. Specifically, they are important for anti-influenza immunity in vivo, playing a role in complement-dependent cytotoxicity (CDC),Citation102-Citation106 antibody-dependent phagocytosis (ADP),Citation107,Citation108 and antibody-dependent cellular cytotoxicity (ADCC).Citation109,Citation110 These antibodies represent a connection between the innate and adaptive immune responses. It has been proved that antibody Fc-receptor interaction is not only able to enhance the efficacy of widely neutralizing antibodies,Citation111 but is also necessary for broadly neutralizing anti-influenza Abs to guarantee protection in vivoCitation112 and that these antibodies are correlated with protection against experimental influenza challenge for several candidate universal vaccines.Citation113 Indeed, in the absence of elevated HI titers towards circulating strains in the elderly, older adults usually present ADCC antibodies.Citation114 The majority of the currently used cell-based assays of the Fcγ function of antibodies are based on Natural Killer (NK) cells, and quantify activation marker expression, cytokine and lytic protein release or the killing ability of NK cells through flow cytometry or ELISpot techniques.Citation115-Citation117 These methods include NK viral inhibition assays, rapid-fluorimetric ADCC assay (RFADCC), granzyme delivery assays, lactate dehydrogenase release assay, and NK cell activation assays which assess IFN-γ and/or CD107a. However, these techniques also present limitations, owing to the long execution, complexity and difficulty of reproduction and standardization across laboratories; in addition, the results of the assays may be biased by the possible presence of polymorphisms in the Fc-receptor of effector cells collected from human donors.Citation118
This review focuses on the main aspects of T- and B-cell responses following influenza vaccination, as evaluated by means of flow cytometry. Hence, efforts should be made to identify other immunological parameters, such as T – cell-mediated immune response, as correlates of protection, especially in view of the fact that the scenario of influenza vaccine is evolving rapidly and novel influenza vaccines will probably be developed in the foreseeable future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [accessed 2018 Dec 01].http://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- Wong KK, Jain S, Blanton L, Dhara R, Brammer L, Fry AM, Finelli L. Influenza-associated pediatric deaths in the United States, 2004-2012. Pediatrics. 2013;132(5):796–804. doi:10.1542/peds.2013-1493.
- Heltzer ML, Coffin SE, Maurer K, Bagashev A, Zhang Z, Orange JS, Sullivan KE. Immune dysregulation in severe influenza. J Leukoc Biol. 2009;85(6):1036–43. doi:10.1189/jlb.1108710.
- [accessed 2018 Dec 02] https://www.cdc.gov/flu/consumer/symptoms.htm
- Bailey KL, Smith LM, Heires AJ, Katafiasz DM, Romberger DJ, LeVan TD. Aging leads to dysfunctional innate immune responses to TLR2 and TLR4 agonists. Aging Clin Exp Res. Nov 7 2018. doi: 10.1007/s40520-018-1064-0
- Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15:21. doi:10.1186/s12948-017-0077-0.
- Moro-Garcıa MA, Alonso-Arias R, Lopez-Larrea C. Molecular mechanisms involved in the aging of the T-cell immune response. Current Genomics. 2012;13(8):589–602. doi:10.2174/138920212803759749.
- Zeman AM, Holmes TH, Stamatis S, Tu W, He XS, Bouvier N, Kemble G, Greenberg HB, Lewis DB, Arvin AM, et al. Humoral and cellular immune responses in children given annual immunization with trivalent inactivated influenza vaccine. Pediatr Infect Dis J. 2007;26:107–15. doi:10.1097/01.inf.0000253251.03785.9b.
- Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43–82. doi:10.1007/978-3-540-92165-3_3.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–65. doi:10.1128/CVI.00131-10.
- Trombetta CM, Perini D, Mather S, Temperton N, Montomoli E. Overview of serological techniques for influenza vaccine evaluation: past, present and future. Vaccines. 2014;2:707‐734. doi:10.3390/vaccines2040707.
- Henle W, Stokes J Jr, Shaw DR. Passive immunization of mice against human influenza virus by the intranasal route. J Immunol. 1941;40:201–12.
- Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103.
- Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Engl J Med. 2001;345:1331–35. doi:10.1056/NEJMra012493.
- Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–15564. doi:10.1056/NEJMoa0708630.
- Ramos EL, Mitcham JL, Koller TD, Bonavia A, Usner DW, Balaratnam G, Fredlund P, Swiderek KM. Efficacy and safety of treatment with an anti-m2e monoclonal antibody in experimental human influenza. J Infect Dis. 2015;211:1038–44. doi:10.1093/infdis/jiu539.
- Montomoli E, Torelli A, Manini I, Gianchecchi E. Immunogenicity and safety of the new inactivated quadrivalent influenza vaccine vaxigrip tetra: preliminary results in children ≥6 months and older adults. Vaccines (Basel). 2018:6. doi:10.3390/vaccines6010014.
- Pavlova S, D’Alessio F, Houard S, Remarque EJ, Stockhofe N, Engelhardt OG. Workshop report: immunoassay standardisation for “universal” influenza vaccines. Influenza Other Respir Viruses. 2017;11:194–201. doi:10.1111/irv.12445.
- Russell SM, McCahon D, Beare AS. A single radial haemolysis technique for the measurement of influenza antibody. J Gen Virol. 1975;27:1–10.6. doi:10.1099/0022-1317-27-1-1.
- Schild GC, Pereira MS, Chakraverty P. Single-radial-hemolysis: a new method for the assay of antibody to influenza haemagglutinin. Applications for diagnosis and seroepidemiologic surveillance of influenza. Bull World Health Organ. 1975;52:43–50.
- Salk JE. A simplified procedure for titrating hemagglutinating capacity of influenza virus and the corresponding antibody. J Immunol. 1944;49:87–98.
- Trombetta CM, Perini D, Vitale L, Cox RJ, Stanzani V, Piccirella S, Montomoli E. Validation of single radial haemolysis assay: A reliable method to measure antibodies against influenza viruses. J Immunol Methods. 2015;422:95–101. doi:10.1016/j.jim.2015.04.009.
- Trombetta CM, Montomoli E. Influenza immunology evaluation and correlates of protection: a focus on vaccines. Expert Rev Vaccines. 2016;15:967–76. doi:10.1586/14760584.2016.1164046.
- Cox RJ. Correlates of protection to influenza virus, where do we go from here? Hum Vaccin Immunother. 2013;9:405–08.
- Trombetta CM, Remarque EJ, Mortier D, Montomoli E. Comparison of hemagglutination inhibition, single radial hemolysis, virus neutralization assays, and ELISA to detect antibody levels against seasonal influenza viruses. Influenza Other Respir Viruses. 2018;12(6):675–86. doi:10.1111/irv.12591.
- Wilson G, Ye Z, Xie H, Vahl S, Dawson E, Rowlen K, Xing Z. Automated interpretation of influenza hemagglutination inhibition (HI) assays: is plate tilting necessary? PLoS ONE. 2017;12:e0179939. doi:10.1371/journal.pone.0179939.
- Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). 1972;70:767–77. doi:10.1017/S0022172400022610.
- Benoit A, Beran J, Devaster JM, Esen M, Launay O, Leroux-Roels G, McElhaney JE, Oostvogels L, van Essen GA, Gaglani M, et al. Hemagglutination inhibition antibody titers as a correlate of protection against seasonal A/H3N2 influenza disease. Open Forum Infect Dis. 2015;2(2):ofv067. doi:10.1093/ofid/ofv067.
- de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol. (Basel). 2003;115:63–73.
- Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol. 2010;10:18. doi:10.1186/1471-2288-10-18.
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30:1081–85. doi:10.1097/INF.0b013e3182367662.
- Dunning AJ, DiazGranados CA, Voloshen T, Hu B, Landolfi VA, Talbot HK. Correlates of protection against influenza in the elderly: results from an influenza vaccine efficacy trial. Clin Vaccine Immunol. 2016;23(3):228–35. doi:10.1128/CVI.00604-1519.
- Mögling R, Richard MJ, Vliet SV, Beek RV, Schrauwen EJA, Spronken MI, Rimmelzwaan GF, Fouchier RAM. Neuraminidase-mediated haemagglutination of recent human influenza A(H3N2) viruses is determined by arginine 150 flanking the neuraminidase catalytic site. J Gen Virol. 2017;98:1274–81. doi:10.1099/jgv.0.000809.
- Li CK, Rappuoli R, Xu X-N. Correlates of protection against influenza infection in humans–on the path to a universal vaccine? Curr Opin Immunol. 2013;25:470–76. doi:10.1016/j.coi.2013.07.005.
- Grund S, Adams O, Wahlisch S, Schweiger B. Comparison of hemagglutination inhibition assay, an ELISA-based micro-neutralization assay and colorimetric microneutralization assay to detect antibody responses to vaccination against influenza A H1N1 2009 virus. J Virol Methods. 2011;171:369–73. doi:10.1016/j.jviromet.2010.11.024.
- Stephenson I, Heath A, Major D, Newman RW, Hoschler K, Junzi W, Katz JM, Weir JP, Zambon MC, Wood JM. Reproducibility of serologic assays for influenza virus A (H5N1). Emerg Infect Dis. 2009;15:1250–59. doi:10.3201/eid1508.081754.
- Gauger PC, Vincent AL. Serum virus neutralization assay for detection and quantitation of serum-neutralizing antibodies to influenza A virus in swine. Methods Mol Biol. 2014;1161:313–24. doi:10.1007/978-1-4939-0758-8_26.
- Mohn KG, Smith I, Sjursen H, Cox RJ. Immune responses after live attenuated influenza vaccination. Hum Vaccin Immunother. 2018;14:571–78. doi:10.1080/21645515.2017.1377376.
- Gorse GJ, Belshe RB. Enhancement of anti-influenza A virus cytotoxicity following influenza A virus vaccination in older, chronically ill adults. J Clin Microbiol. 1990;28:2539–50.
- Hoft DF, Babusis E, Worku S, Spencer CT, Lottenbach K, Truscott SM, Abate G, Sakala IG, Edwards KM, Creech CB, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis. 2011;204(6):845–53. doi:10.1093/infdis/jir436.
- Hoft DF, Lottenbach KR, Blazevic A, Turan A, Blevins TP, Pacatte TP, Yu Y, Mitchell MC, Hoft SG, Belshe RB. Comparisons of the humoral and cellular immune responses induced by live attenuated influenza vaccine and inactivated influenza vaccine in adults. Clin Vaccine Immunol. 2017;24(1). doi:10.1128/CVI.00414-16.
- Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM. CAIV-T comparative efficacy study group. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–96. doi:10.1056/NEJMoa065368.
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–97. doi:10.1016/j.immuni.2014.12.007.
- Wen F, Guo J, Huang S. A meta-analysis identified genes responsible for distinct immune responses to trivalent inactivated and live attenuated influenza vaccines. J Cell Physiol. Sep 10 2018. doi: 10.1002/jcp.2732
- Wang B, Russell ML, Brewer A, Newton J, Singh P, Ward BJ, Loeb M. Single radial haemolysis compared to haemagglutinin inhibition and microneutralization as a correlate of protection against influenza A H3N2 in children and adolescents. Influenza Other Respir Viruses. 2017;11:283–88. doi:10.1111/irv.12450.
- Reber A, Katz J. Immunological assessment of influenza vaccines and immune correlates of protection. Expert Rev Vaccines. 2013;12:519–36. doi:10.1586/erv.13.35.
- [accessed 2018 Dec 02] www.who.int/immunization/research/meetings_workshops/Jackie_Katz_Chicago_August_2016.pdf
- Tsang TK, Cauchemez S, Perera RAPM, Freeman G, Fang VJ, Ip DK, Leung GM, Malik Peiris JS, Cowling BJ. Association between antibody titers and protection against influenza virus infection within households. J Infect Dis. 2014;210:684–92. doi:10.1093/infdis/jiu186.
- Gijzen K, Liu WM, Visontai I, Oftung F, van der Werf S, Korsvold GE, Pronk I, Aaberge IS, Tütto A, Jankovics I, et al. Standardization and validation of assays determining cellular immune responses against influenza. Vaccine. 2010;28:3416–22. doi:10.1016/j.vaccine.2010.02.076.
- Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272:1661–65.
- Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. Role of humoral and cell-mediated immunity in protection from infuenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37:427–39.
- Efros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25(4):599–604. doi:10.1016/j.vaccine.2006.08.032.
- McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21(4):418–24. doi:10.1016/j.coi.2009.05.023.
- McElhaney JE. Prevention of infectious diseases in older adults through immunization: the challenge of the senescent immune response. Expert Rev Vaccines. 2009;8(5):593–606. doi:10.1586/erv.09.12.
- Fuentes E, Fuentes M, Alarcón M, Palomo I. Immune system dysfunction in the elderly. An Acad Bras Cienc. 2017;89(1):285–99. doi:10.1590/0001-3765201720160487.
- Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. 2010;28:6145–51. doi:10.1016/j.vaccine.2010.07.036.
- McElhaney JE, Kuchel GA, Zhou X, Swain SL, Haynes L. T-cell immunity to influenza in older adults: a pathophysiological framework for development of more effective vaccines. Front Immunol. 2016;7:41. doi:10.3389/fimmu.2016.00041.
- Andrew MK, Bowles SK, Pawelec G, Haynes L, Kuchel GA, McNeil SA, McElhaney JE. Influenza vaccination in older adults: recent innovations and practical applications. Drugs Aging. Nov 9 2018. doi: 10.1007/s40266-018-0597-4
- McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–39.
- McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, Barry MB, Kleppinger A, Wang Y, Bleackley RC, et al. Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27(18):2418–25. doi:10.1016/j.vaccine.2009.01.136.
- Berlanda Scorza F, Tsvetnitsky V, Donnellym JJ. Universal influenza vaccines: shifting to better vaccines. Vaccine. 2016;34:2926–33. doi:10.1016/j.vaccine.2016.03.085.
- Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protec-tion against influenza challenge in humans. Nat Med. 2012;18:274–80. doi:10.1038/nm.2612.
- Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19:1305–12. doi:10.1038/nm.3350.
- Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, Chakladar S, Hudgens MG, Weir SS, Beck MA. Increased risk of influenza among vaccinated adults who are obese. Int J Obes (Lond). 2017;41:1324–30. doi:10.1038/ijo.2017.131.
- Karlsson EA, Hertz T, Johnson C, Mehle A, Krammer F, Schultz-Cherry S. Obesity outweighs protection conferred by adjuvanted influenza vaccination. MBio. 2016;7:e01144–16. doi:10.1128/mBio.01144-16.
- Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi:10.3201/eid1201.051237.
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–25. doi:10.1038/ni.1688.
- Slifka MK, Amanna I. How advances in immunology provide insight into improving vaccine efficacy. Vaccine. 2014;32:2948–57. doi:10.1016/j.vaccine.2014.03.078.
- Coughlan L, Lambe T. Measuring cellular immunity to influenza: methods of detection, applications and challenges. Vaccines (Basel). 2015;3:293–319. doi:10.3390/vaccines3020293.
- Richards KA, Topham D, Chaves FA, Sant AJ. Cutting edge: CD4 T cells generated from encounter with seasonal influenza viruses and vaccines have broad protein specificity and can directly recognize naturally generated epitopes derived from the live pandemic H1N1 virus. J Immunol. 2010;185:4998–5002. doi:10.4049/jimmunol.1001395.
- Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G, Chan PL, Lam KT, Guan J, Zhang L, et al. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. J Virol. 2010;84:6527–35. doi:10.1128/JVI.00519-10.
- McKinstry KK, Strutt TM, Swain SL. Hallmarks of CD4 T cell immunity against influenza. J Intern Med. 2011;269:507–18. doi:10.1111/j.1365-2796.2011.02367.x.
- Schmidt T, Dirks J, Enders M, Gärtner BC, Uhlmann-Schiffler H, Sester U, Sester M. CD4+ T-cell immunity after pandemic influenza vaccination cross-reacts with seasonal antigens and functionally differs from active influenza infection. Eur J Immunol. 2012;42:1755–66. doi:10.1002/eji.201242393.
- Suni MA, Picker LJ, Maino VC. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J. Immunol Methods. 1998;212:89–98. doi:10.1016/S0022-1759(98)00004-0.
- Nomura LE, Walker JM, Maecker HT. Optimization of whole blood antigen-specific cytokine assays for CD4+ T cells. Cytometry. 2000;40:60–68. doi:10.1002/(ISSN)1097-0320.
- Waldrop SL, Pitcher CJ, Peterson DM, Maino VC, Picker LJ. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J Clin Invest. 1997;99:1739–50. doi:10.1172/JCI119338.
- De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, Evans TG, Koup R, Miller CJ, Roederer M. Vaccination in humans generates broad T cell cytokine responses. J Immunol. 2004;173:5372–80. doi:10.4049/jimmunol.173.9.5372.
- Smits K, Pottier G, Smet J, Dirix V, Vermeulen F, De Schutter I, Carollo M, Locht C, Ausiello CM, Mascart F. Different T cell memory in preadolescents after whole-cell or acellular pertussis vaccination. Vaccine. 2013;32:111–18. doi:10.1016/j.vaccine.2013.10.056.
- Maecker HT, Rinfret A, D’Souza P, Darden J, Roig E, Landry C, Hayes P, Birungi J, Anzala O, Garcia M, et al. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005;6:13. doi:10.1186/1471-2172-6-13.
- He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80(23):11756–66. doi:10.1128/JVI.01460-06.
- Basha S, Hazenfeld S, Brady RC, Subbramanian RA. Comparison of antibody and T-cell responses elicited by licensed inactivated- and live-attenuated influenza vaccines against H3N2 hemagglutinin. Hum Immunol. 2011;72(6):463–69. doi:10.1016/j.humimm.2011.03.001.
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi:10.1186/1471-2172-6-13.
- Mittrucker HW, Visekruna A, Huber M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch Immunol Ther Exp (Warsz.). 2014;62:449–58. doi:10.1007/s00005-014-0293-y.
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi:10.1016/j.immuni.2009.05.001.
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78.
- Maecker HT. Multiparameter flow cytometry monitoring of T cell responses. Methods Mol Biol. 2009;485:375–91. doi:10.1007/978-1-59745-170-3_25.
- Mei HE, Yoshida T, Sime W, Hiepe F, Thiele K, Manz RA, Radbruch A, Dörner T. Blood-bornehuman plasma cells in steady state are derived from mucosal immuneresponses. Blood. 2009;113:2461–69. doi:10.1182/blood-2008-04-153544.
- Cox RJ, Brokstad KA, Zuckerman MA, Wood JM, Haaheim LR, Oxford JS. Anearly humoral immune response in peripheral blood following parenteralinactivated influenza vaccination. Vaccine. 1994;12:993–99.
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapidcloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi:10.1038/nature06890.
- Halliley JL, Kyu S, Kobie JJ, Walsh EE, Falsey AR, Randall TD, Treanor J, Feng C, Sanz I, Lee FE. Peak frequen-cies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–87. doi:10.1016/j.vaccine.2010.02.088.
- He XS, Sasaki S, Narvaez CF, Zhang C, Liu H, Woo JC, Kemble GW, Dekker CL, Davis MM, Greenberg HB. Plasmablast-derivedpolyclonal antibody response after influenza vaccination. J Immunol Methods. 2011;365:67–75. doi:10.1016/j.jim.2010.12.008.
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol. 2011;12:786–95. doi:10.1038/ni.2067.
- Jürchott K, Schulz AR, Bozzetti C, Pohlmann D, Stervbo U, Warth S, Mälzer JN, Waldner J, Schweiger B, Olek S, et al. Highly predictive model for a protective immune response to the A(H1N1)pdm2009 influenza strain after seasonal vaccination. PLoS One. 2016;11:e0150812. doi:10.1371/journal.pone.0150812.
- Kreijtz JHCM, Fouchier RAM, Rimmelzwaan GF. Immune responses to influenza virus infection. Virus Res. 2011;162:19–30. doi:10.1016/j.virusres.2011.09.022.
- Nayak JL, Fitzgerald TF, Richards K, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis. 2013;207:297–305. doi:10.1093/infdis/jis684.
- Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, Thomas A, Kenney T, Kappes R, Wagner W, Maffei K, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. Aids. 2010;24:2187–92. doi:10.1097/QAD.0b013e32833c6d5c.
- Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, Germain RN, Wang E, Olnes MJ, Narayanan M, Golding H, et al. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell. 2014;157:499–513. doi:10.1016/j.cell.2014.03.031.
- Mbawuike IN, Atmar RL, Patel SM, Corry DB, Winokur PL, Brady RC, Chen WH, Edwards KM, Creech CB, Walter EB Jr, et al. Cell mediated immune responses following revaccination with an influenza A/H5N1 vaccine. Vaccine. 2016;34:547–54. doi:10.1016/j.vaccine.2015.11.055.
- Wright PF, Hoen AG, Ilyushina NA, Brown EP, Ackerman ME, Wieland-Alter W, Connor RI, Jegaskanda S, Rosenberg-Hasson Y, Haynes BC, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis. 2016;3:ofw108. doi:10.1093/ofid/ofw108.
- He W, Mullarkey CE, Duty JA, Moran TM, Palese P, Miller MS. Broadly neutralizing anti-influenza virus antibodies: enhancement of neutralizing potency in polyclonal mixtures and IgA backbones. J Virol. 2015;89:3610–18. doi:10.1128/JVI.03099-14.
- He W, Mullarkey CE, Miller MS. Measuring the neutralization potency of influenza A virus hemagglutinin stalk/stem-binding antibodies in polyclonal preparations by microneutralization assay. Methods. 2015;90:95–100. doi:10.1016/j.ymeth.2015.04.037.
- Verbonitz MW, Ennis FA, Hicks JT, Albrecht P. Hemagglutinin-specific complement-dependent cytolytic antibody response to influenza infection. J Exp Med. 1978;147:265–70. doi:10.1084/jem.147.1.265.
- Quinnan GV, Ennis FA, Tuazon CU, Wells MA, Butchko GM, Armstrong R, McLaren C, Manischewitz JF, Kiley S. Cytotoxic lymphocytes and antibody-dependent complement-mediated cytotoxicity induced by administration of influenza vaccine. Infect Immun. 1980;30:362–69.
- O’Brien KB, Morrison TE, Dundore DY, Heise MT, Schultz-Cherry S, Vartanian J-P. A protective role for complement C3 protein during pandemic 2009 H1N1 and H5N1 influenza A virus infection. PLoS One. 2011;6:e17377. doi:10.1371/journal.pone.0017377.
- Ohta R, Torii Y, Imai M, Kimura H, Okada N, Ito Y. Serum concentrations of complement anaphylatoxins and proinflammatory mediators in patients with 2009 H1N1 influenza. Microbiol Immunol. 2011;55:191–98. doi:10.1111/j.1348-0421.2011.00309.x.
- Terajima M, Co MD, Cruz J, Ennis FA. High antibody-dependent cellular cytotoxicity antibody titers to H5N1 and H7N9 avian influenza a viruses in healthy US adults and older children. J Infect Dis. 2015;212:1052–60. doi:10.1093/infdis/jiv181.
- Ana-Sosa-Batiz F, Vanderven H, Jegaskanda S, Johnston A, Rockman S, Laurie K, Barr I, Reading P, Lichtfuss M, Kent SJ. Influenza-specific antibody-dependent phagocytosis. PLoS One. 2016;11:e0154461. doi:10.1371/journal.pone.0154461.
- Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, Novakowski KE, Bowdish DM, Miller MS, Palese P. broadly neutralizing hemagglutinin stalk-specific antibodies induce potent phagocytosis of immune complexes by neutrophils in an Fc-dependent manner. mBio. 2016;7:e01624–16. doi:10.1128/mBio.01624-16.
- DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med. 2014;20:143–51. doi:10.1038/nm.3443.
- Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol. 2014;193:469–75. doi:10.4049/jimmunol.1400432.
- Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015;349:1301–06. doi:10.1126/science.aac7263.
- DiLillo DJ, Palese P, Wilson PC, Ravetch JV. Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest. 2016;126:605–10. doi:10.1172/JCI84428.
- Jegaskanda S, Vanderven HA, Wheatley AK, Kent SJ. Fc or not Fc; that is the question: antibody Fc-receptor interactions are key to universal influenzavaccine design. Hum Vaccin Immunother. 2017;13(6):1–9. doi:10.1080/21645515.2017.1290018.
- Vanderven HA, Jegaskanda S, Wines BD, Hogarth PM, Carmuglia S, Rockman S, Chung AW, Kent SJ. Antibody-dependent cellular cytotoxicity responses to seasonal influenza vaccination in older adults. J Infect Dis. 2017;217(1):12–23. doi:10.1093/infdis/jix554.
- Zaritskaya L, Shurin MR, Sayers TJ, Malyguine AM. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev Vaccines. 2010;9(6):601–16. doi:10.1586/erv.10.49.
- Valiathan R, Lewis JE, Melillo AB, Leonard S, Ali KH, Asthana D. Evaluation of a flow cytometry-based assay for natural killer cell activity in clinical settings. Scand J Immunol. 2012;75(4):455–62. doi:10.1111/j.1365-3083.2011.02667.x.
- Memarnejadian A, Meilleur CE, Mazzuca DM, Welch ID, Haeryfar SM. Quantification of alloantibody-mediated cytotoxicity in vivo. Transplantation. 2016;100(5):1041–51. doi:10.1097/TP.0000000000001154.
- Wines BD, Billings H, Mclean MR, Kent SJ, Hogarth PM. Antibody functional assays as measures of Fc receptor-mediated immunity to HIV - new technologies and their impact on the HIV vaccine field. Curr HIV Res. 2017;15(3):202–15. doi:10.2174/1570162X15666170320112247.