ABSTRACT
Pertussis is resurgent worldwide. Currently available acellular pertussis vaccines contain chemically detoxified pertussis toxin (PTc); a highly immunogenic genetically detoxified pertussis toxin (PTg) vaccine has been off the market for over a decade. We compared CD4+ T cell and B cell responses induced by genetically detoxified pertussis toxin (PTg) and chemically detoxified pertussis toxin (PTc) using naive human neonatal cells. Responses to novel adjuvants were also assessed. PTg induced significant antigen-specific CD4+ T cell activation and IL17 secretion than PTc. TLR agonist combinations improved PTg induced T cell-CD69 expression and IL17 secretion.
Introduction
Despite high vaccine coverage, pertussis is resurgent in the US and other developed countries that replaced whole cell pertussis (wP) vaccine with acellular pertussis (aP) vaccines.Citation1 Many potential reasons for resurgence of pertussis had been proposed.Citation2 A number of laboratories, including ours, have shown that DTaP (aP vaccine combined with diphtheria and tetanus toxoids) vaccination elicits robust humoral responses in children, adolescents and adults.Citation3–Citation5 However it is becoming clear that aP vaccines do not induce durable protection due to lower peak antibody titers and inadequate induction of immune memory. Studies in baboons by Warfel et al.Citation6 suggest that wP vaccine protects against pertussis by eliciting antibody as well as robust Th1 and Th17 responses.
Current alum based aP vaccines predominantly induce a Th2 response.Citation7 Optimal immunogenicity at the time of priming with aP vaccines might benefit from more potent Th1 and Th17 polarizing adjuvants.Citation1,Citation2,Citation7 All currently marketed aP vaccines contain a form of chemically detoxified pertussis toxin (PTc) that induce 10–20 fold lower antibody levels compared to genetically detoxified recombinant PT (PTg; Arg9_Lys and Glu129_Gly).Citation8 Vaccination with PTg elicits a native antigen-specific response.Citation9 Studies sponsored by NIAID over two decades ago showed the PTg was superior among all PT preparations evaluated in generating a primary immune response in infants and booster responses in young children.Citation10,Citation11 PTg was shown to be safe and was included in a DTaP vaccine that was marketedCitation3,Citation12 and widely used in Italy for several years before the manufacturer discontinued production. Neonates are especially prone to infectious diseases and methods to safely vaccinate neonates against pertussis have long been sought.
In this study, using umbilical cord blood mononuclear cells as a source of naïve cells and to test for neonatal immune responses, we compared PTg and PTc for their ability to induce T cell activation, cytokine secretion and antigen-specific CD4 + T cells. We also tested whether TLR agonist combinations [TLR3 agonist, Poly I:C; TLR2 agonist, PAM3CSK4; TLR4 agonists, E. coli lipopolysaccharide (LPS) or monophosphoryl lipid A (MPLA); TLR7/8 agonist, Resiquimod (R848)] known to induce Th1 polarizing responses improved naïve neonatal T or B cell responses to PTg or PTc.
Results
PTg but not PTc induces naïve t cell activation
We measured cord blood T cell activation when cells were treated with PTg or PTc in the presence or absence of TLR agonists. PTg alone induced significant T cell – CD69 expression while PTc did not. When TLR agonists were added to PTg, the number of CD4 T cells expressing CD69 increased significantly from 46% to 60–80% depending on the combination ()). Addition of TLR agonists to PTc failed to induce CD69 expression ()).
Figure 1. PTg but not PTc induces cord blood T cell response. Cord blood mononuclear cells (n = 7) were stimulated with PT antigens or TLR agonists (TLR3 agonist, Poly I:C; TLR4 agonist, MPLA and TLR7/8 agonist, R848)as indicated. Surface expression of CD4 T cell early activation marker CD69 (a), intracellular IL17 (b) and IL2 (c) levels and, surface co-expression of OX40 and CD25 (d) were determined by flow cytometry. Results are expressed as mean ± standard deviation. * p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 denotes significance compared to unstimulated control.
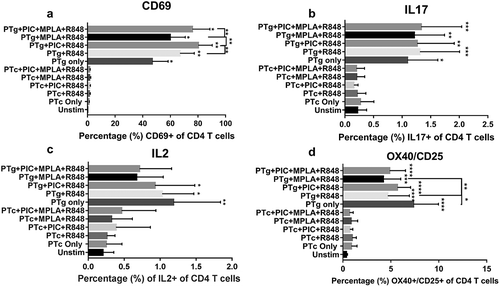
We measured CD4 T cell CD69 expression in response to TLR agonist combinations of Poly I:C, LPS and R848 and found CD69 was significantly increased by a TLR3 + 4 + 7/8 combination (data not shown). TLR agonists alone did not induce significant intracellular T cell – IL2 or IFN-γ secretion (data not shown).
PTg but not PTc induces IL17 responses from naive CD4 t cells
PTg induced significant IL17 and IL2 secretion from naive CD4 T cells (). Addition of TLR agonists to PTg did not significantly increase IL17 or IL2 levels. PTc did not induce significant IL17 or IL2 levels and addition of TLR agonists had no effect (). When IFN-γ or IL4 or IL13 levels were measured following PTg or PTc exposure with or without TLR agonists, there were no significant differences compared to un-stimulated cells (data not shown).
PTg but not PTc induces antigen specific naive CD4 t cell responses
Detection of antigen-specific CD4 T cell response is a challenge using naïve neonatal cells because of the rarity and heterogeneity of antigen specific cells. This will lead to gross underestimation of the size of antigen specific response. Therefore, we utilized a novel cytokine independent activation induced marker (AIM) assayCitation13,Citation14 wherein CD4 T cells double positive for OX40/CD25 were described to most reliably identify antigen specific peripheral blood cells in humans and non-human primates. We examined the quantitative difference between PTg and PTc in inducing antigen specific CD4 T cell responses using the AIM assay. We measured the percentage of OX40+/CD25+ CD4 T cells after stimulation with PTg or PTc in the presence or absence of TLR agonist combinations. CD4 T cells stimulated with PTg induced significantly high OX40/CD25 expression indicative of antigen specificity compared to un-stimulated cells. PTc did not stimulate OX40/CD25 expression on CD4 T cells ()). Adding TLR agonists to PTg did not increase OX40/CD25 expression compared to PTg alone. Similarly, adding TLR agonists to PTc did not increase OX40/CD25 expression compared to PTc alone.
TLR agonist combinations induce naive b cell activation
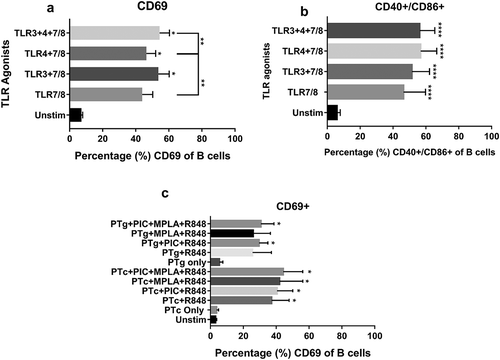
We measured whether selected TLR agonist combinations had a direct B cell stimulatory function. CBMCs were stimulated with combinations of Poly I:C, LPS and R848 and B cell activation was measured. TLR3 and/or TLR4 agonists with R848 induced significantly high B cell – CD69 expression as well as CD40/CD86 co-stimulatory markers compared to un-stimulated controls (). We measured B cell activation when cells were treated with PTg or PTc in the presence or absence of TLR agonists. Neither PTg nor PTc induced B cell CD69 expression and PT antigens did not improve TLR agonist induced B cell-CD69 expression when combined ()).
Figure 2. TLR agonist combinations but not PT induces cord blood B cell activation. Cord blood mononuclear cells (n = 4) were stimulated with PT antigens or TLR agonists (TLR3 agonist, Poly I:C; TLR4 agonist, MPLA and TLR7/8 agonist, R848) as indicated. Flow cytometric analysis of B cell – CD69 (a) as well as costimulatory marker CD40/CD86 expression (b) were measured after TLR agonist stimulation. B cell – CD69 expression in the presence of PT antigens (c) were also tested. Results are expressed as mean ± standard deviation. * p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 denotes significance compared to unstimulated control.
Discussion
We found PTg induces significant T cell activation and addition of TLR agonists further improved neonatal T cell activation. A similar effect was seen with naïve neonatal T cell-IL17 and IL2 secretion. Moreover, PTg induced significant antigen-specific OX40/CD25 expression. Interestingly, when combined with TLR agonists, PTg-induced OX40/CD25 expression was lower than induced by PTg alone. Some T regulatory cells can upregulate CD25 upon antigen stimulation and we speculate that with the addition of TLR agonists to PTg the T regulatory cell fraction was lost from total antigen specific cell pool. PTc did not activate naïve neonatal T cells, stimulate IL17 or IL2 secretion from T cells nor induce antigen-specific OX40/CD25 expression. Overall, our data suggest that PTg possesses superior T cell activating and Th17 polarizing capacity compared to PTc in naïve neonatal cells. This may be due to the inherent adjuvant/mitogenic activity retained in PTg but lost in PTc due to chemical detoxification. We also tested whether Poly I:C, MPLA and R848 TLR agonist combinations improved neonatal T cell responses to PT antigens and found they enhanced the effect of PTg on the number of neonatal CD4 T cells expressing CD69 whereas addition of TLR agonists to PTc had no effect.
TLR agonist combinations induced significant B cell activation and co-stimulation in naïve neonatal cells whereas neither PTg nor PTc did and addition of PT antigens to TLR agonists did not result in improved B cell activation compared to TLR agonists alone. Selected TLR agonists, based on previously published study from our group,Citation15 stimulate TLRs (TLR3, 4 and 7/8) expressed by antigen presenting cells to induce Th1 polarizing cytokines. However, among the TLRs stimulated, human B cells express TLRs 4&7 at much higher levels than T cells.Citation16 Therefore, we speculate that the selected TLR agonists induced much higher B cell activation than T cell activation in the absence of PT antigen. Although PTg alone induced significant T cell activation ()), the duration of cell culture (18–24 hours) with PT antigens alone might not have been sufficient to generate a B cell activation response ()).
Our study was limited by the amount of neonatal cord blood precluding studies by sorting neonatal T cells and B cells. Future studies will test PTg together with diphtheria and tetanus toxoids to understand the impact of replacing PTc with PTg in pediatric immune responses to DTaP vaccine.
In conclusion, vaccination with a PTg-containing aP vaccine should be reconsidered as a strategy to enhance immune protection against pertussis. Neonatal vaccination may be effective with PTg. Additionally, novel adjuvants that could promote aP vaccine efficacy by inducing a Th1/Th17 response should be further studied.
Materials and methods
Antigens, adjuvants and antibodies
PTg (PT-9K/129G) was obtained from Novartis Vaccines, Siena, Italy (since March 2015, GSK vaccines). PTc was obtained from Sanofi Pasteur. Endotoxin content of PTg was determined by limulus amebocyte lysate (LAL) test (Sigma-Aldrich, St. Louis, MO) and was found to be <0.1 EU/mL. TLR agonists were purchased from Invivogen (San Diego, CA). Antibodies were purchased from Biolegend, (San Diego, CA) except anti-CD86 (BD Biosciences, San Diego, CA)
Blood processing and in vitro cell culture conditions
The study was approved by the Institutional Ethics Review Board at Rochester General Hospital. Cord blood mononuclear cells (CBMCs) from normal full-term healthy deliveries were isolated from buffy coat, washed in phosphate-buffered saline (PBS) and frozen in liquid nitrogen until use. Prior to stimulation, cells were quick-thawed in a 37°C water bath, counted, washed and plated (2 × 106 cells/ml) at 200 µl/well in 96 well round bottom plates and rested for 1 hour at 37°C in 5% CO2 incubator. Then Poly I:C (10 µg/ml), PAM3CSK4 (1 µg/ml), E. coli LPS (50 ng/ml) or MPLA (1 µg/ml), R848 (1 µg/ml) were added to respective wells with or without PT antigens (1 µg/ml) and incubated for 24 hours at 37°C in 5% CO2 incubator. An identical set of samples was used for intracellular cytokine assays with 10 µg/ml brefeldin-A (BFA; Cell signaling, Danvers, MA) added to cultures 2 hours in to the stimulation and incubated at 37°C in 5% CO2 incubator for additional 6 hours.
Activation induced marker assay (AIM assay)
Cryopreserved CBMCs after thawing and washing were counted and plated at 2 × 106 cells/ml, 200 µl/well in 96 well flat bottom plates then stimulated with PT antigens and TLR agonists for 18 hours without BFA. Post stimulation, cells were washed, surface stained and acquired on a BD LSR II flow cytometer.
Surface and intracellular staining and flow cytometry analysis
Post stimulation, cells were washed before incubation with live-dead aqua dead cell stain for 30 minutes, washed again, then surface stained along with Fc receptor blocking solution (human TruStain FcX, Biolegend,) for 20 minutes in the dark at 4°C. For intracellular staining, cells were fixed and permeabilized using BD Cytofix/Cytoperm solution and stained with cytokine antibody cocktail for 30 minutes in the dark at 4°C. After wash steps, cells were acquired on a BD LSR II flow cytometer and data analyzed using FlowJo software.
Statistical analysis
Statistical analysis were conducted using Graphpad Prism 7.03 using a one-way ANOVA with Tukey’s or Dunnet’s post-hoc correction for multiple comparisons. Multiplicity adjusted p-value < 0.05 was considered significant.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Andrea Simmons for technical help.
Additional information
Funding
References
- Plotkin SA. The pertussis problem. Clin Infect Dis. 2014;58:830–33. doi:10.1093/cid/cit934.
- Eberhardt CS, Siegrist CA. What is wrong with pertussis vaccine immunity? Inducing and recalling vaccine-specific immunity. Cold Spring Harb Perspect Biol. 2017;9:a029629. doi:10.1101/cshperspect.a029629.
- Wood N, McIntyre P, Marshall H, Roberton D. Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age. Pediatr Infect Dis J. 2010;29:209–15. doi:10.1097/INF.0b013e3181bc98d5.
- Pichichero ME, Rennels MB, Edwards KM, Blatter MM, Marshall GS, Bologa M, Wang E, Mills E. Combined tetanus, diphtheria, and 5-component pertussis vaccine for use in adolescents and adults. JAMA. 2005;293:3003–11. doi:10.1001/jama.293.24.3003.
- Pichichero ME, Bernstein H, Blatter MM, Schuerman L, Cheuvart B, Holmes SJ. Immunogenicity and safety of a combination diphtheria, tetanus toxoid, acellular pertussis, hepatitis B, and inactivated poliovirus vaccine coadministered with a 7-valent pneumococcal conjugate vaccine and a Haemophilus influenzae type b conjugate vaccine. J Pediatr. 2007;151:43–9, 49.e1-2. doi:10.1016/j.jpeds.2007.02.013.
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci USA. 2014;111:787–92. doi:10.1073/pnas.1314688110.
- Misiak A, Leuzzi R, Allen AC, Galletti B, Baudner BC, D’Oro U, O’Hagan DT, Pizza M, Seubert A, Mills KHG. Addition of a TLR7 agonist to an acellular pertussis vaccine enhances Th1 and Th17 responses and protective immunity in a mouse model. Vaccine. 2017;35:5256–63. doi:10.1016/j.vaccine.2017.08.009.
- Rappuoli R. The vaccine containing recombinant pertussis toxin induces early and long-lasting protection. Biologicals. 1999;27:99–102. doi:10.1006/biol.1999.0189.
- Seubert A, D’Oro U, Scarselli M, Pizza M. Genetically detoxified pertussis toxin (PT-9K/129G): implications for immunization and vaccines. Expert Rev Vaccines. 2014;13:1191–204. doi:10.1586/14760584.2014.942641.
- Pichichero ME, Deloria MA, Rennels MB, Anderson EL, Edwards KM, Decker MD, Englund JA, Steinhoff MC, Deforest A, Meade BD. A safety and immunogenicity comparison of 12 acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fourth dose in 15- to 20-month-old children. Pediatrics. 1997;100:772–88.
- Pichichero ME, Edwards KM, Anderson EL, Rennels MB, Englund JA, Yerg DE, Blackwelder WC, Jansen DL, Meade BD. Safety and immunogenicity of six acellular pertussis vaccines and one whole-cell pertussis vaccine given as a fifth dose in four- to six-year-old children. Pediatrics. 2000;105:e11. doi:10.1542/peds.105.1.e11.
- Belloni C, De Silvestri A, Tinelli C, Avanzini MA, Marconi M, Strano F, Rondini G, Chirico G. Immunogenicity of a three-component acellular pertussis vaccine administered at birth. Pediatrics. 2003;111:1042–45.
- Dan JM, Lindestam Arlehamn CS, Weiskopf D, Da Silva Antunes R, Havenar-Daughton C, Reiss SM, Brigger M, Bothwell M, Sette A, Crotty S. A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J Immunol. 2016;197:983–93. doi:10.4049/jimmunol.1600318.
- Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Peña A, Kasturi SP, Dan JM, Bothwell M, Sanders RW, et al. Cytokine-Independent detection of antigen-specific germinal center T follicular helper cells in immunized nonhuman primates using a live cell activation-induced marker technique. J Immunol. 2016;197:994–1002. doi:10.4049/jimmunol.1600320.
- Surendran N, Simmons A, Pichichero ME. TLR agonist combinations that stimulate Th type I polarizing responses from human neonates. Innate Immun. 2018;24:240–51. doi:10.1177/1753425918771178.
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–37.
