ABSTRACT
Background: Two new formulations of an investigational 15-valent pneumococcal conjugate vaccine (PCV15-A and PCV15-B) were developed using 2 different protein-polysaccharide conjugation processes and evaluated in separate phase I/II studies (NCT02037984 [V114-004] and NCT02531373 [V114-005]) to assess optimal concentrations of pneumococcal polysaccharide (PnPs) and Aluminum Phosphate Adjuvant.
Methods: Various lots of PCV15-A and PCV15-B containing different concentrations of PnPs and/or adjuvant were compared to PCV13 in young adults and infants. Adults received single dose and infants received 4 doses at 2, 4, 6, and 12–15 months of age. Adverse events (AEs) were collected after each dose. Serotype-specific immunoglobulin G (IgG) concentrations and opsonophagocytic activity (OPA) were measured prior and 30 days postvaccination in adults, at 1 month postdose 3 (PD3), pre-dose4, and postdose 4 (PD4) in infants.
Results: Safety profiles were comparable across vaccination groups. At PD3, serotype-specific IgG GMCs were generally lower for either PCV15 formulation than PCV13 for most shared serotypes. PCV15 consistently elicited higher antibody responses to the 2 serotypes unique to the vaccine (22F and 33F) and serotype 3 for which PCV13 was shown to be ineffective. Except for serotypes 6A and 6B, no dose-response effect was observed with increasing concentrations of PnPs and/or adjuvant.
Conclusion: PCV15 is safe and induces IgG and OPA responses to all 15 serotypes in the vaccine. No significant differences in antibody responses were observed with increases in PnPs and/or Aluminum Phosphate Adjuvant.
Introduction
Streptococcus pneumoniae (pneumococcus) is a major cause of morbidity and mortality in children (<5 years of age) and older adults (≥65 years of age) worldwide.Citation1 A comprehensive study estimated that in the year 2000, there were 14.5 million cases of pneumococcal disease worldwide and ~800,000 children < 5 years of age died of the disease.Citation2 Humans are the sole reservoir of the bacterium, and it is carried in the nasopharynx. Pneumococcal carriage is asymptomatic, but it is a necessary precursor to the pathogenesis of pneumococcal disease (PD). The majority of children carry one or more pneumococcal serotypes by their first birthday although carriage is time limited. PD is classified as either invasive pneumococcal disease (IPD), defined as the presence of S. pneumoniae in a normally sterile body site or noninvasive pneumococcal disease (non-IPD). The primary invasive clinical syndromes caused by S. pneumoniae include meningitis, bacteremic pneumonia, bacteremia without focus and septic arthritis; the primary noninvasive clinical syndromes include acute otitis media (AOM), non bacteremic pneumonia, and sinusitis.Citation2,Citation3 Furthermore, the complications of these diseases can be significant with some studies reporting up to 8% mortality and 25% neurologic sequelae with pneumococcal meningitis.Citation4
Multivalent pneumococcal polysaccharide vaccines were first developed and licensed several decades ago for the prevention of pneumococcal disease. Such vaccines have proved invaluable in preventing pneumococcal disease in adults, particularly the elderly and those at high-risk. However, infants and young children respond poorly to unconjugated pneumococcal polysaccharides because bacterial polysaccharides are T-cell-independent immunogens and elicit poor immune responses in infants. Pneumococcal conjugate vaccine (PCV) consists of the chemical conjugation of bacterial polysaccharide immunogen to a carrier protein, converting the immune response to T-cell-dependent in infants and improvement of vaccine-induced immunity against pneumococcal disease. Widespread use of PCVs has significantly decreased incidence of IPD in children targeted by vaccination and unvaccinated individuals of various ages in many countries worldwide.Citation5-Citation10 Despite these reductions, there remains a substantial burden of PD in children and adults, mostly related to disease caused by serotypes not included in the licensed PCVs. Similar to the trend seen in IPD, many studies have observed that the incidences of pneumococcal pneumonia and acute otitis media (AOM) in children have also significantly decreased after immunization with PCVs; however, the estimate of the true impact of PCVs on serotype-specific disease varies somewhat by study and is difficult to precisely determine due to methodological challenges in case ascertainment for pneumonia or AOM.Citation11-Citation14 However, there are limitations in serotype coverage with PCV7 (Pneumococcal 7-valent Conjugate Vaccine [Diphtheria CRM197 Protein]; Prevnar; Wyeth Pharmaceuticals Inc. Philadelphia, PA) and PCV13 (Pneumococcal 13-valent Conjugate Vaccine [Diphtheria CRM197 Protein]; PREVNAR 13; Wyeth Pharmaceuticals Inc. Philadelphia, PA) in certain regions of the world and some evidence of certain emerging serotypes causing pneumococcal disease in both children and adults.Citation5-Citation10 Coincidentally, an increase in disease caused by serotypes not included in PCV13 was also observed. In United States (U.S.), IPD caused by serotypes 22F and 33F were <1% in children while representing respectively 4.5% and 0.9% of cases in adults ≥65 years in 1998.Citation5 By 2013, overall incidence of IPD has significantly declined while absolute number of cases caused by some non-vaccine types had increased, leading to an increase in their relative proportions. IPD caused by serotype 22F among children <5 years and adults ≥18 years were 11% and 13%, respectively, while serotype 33F caused 10% and 5% of residual IPD cases in children <5 years and adults ≥18 years, respectively.Citation6
A candidate 15-valent PCV (PCV15: 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F) has been developed, including 2 serotypes (22F and 33F) that are among leading causes of IPD following implementation of PCVs. Citation5-Citation7,Citation10 Although PCV15 utilizes diphtheria toxoid CRM197 as carrier protein similar to PCV7/PCV13, these carrier proteins are manufactured using different techniques and each protein likely possesses unique characteristics that are associated with specific and unique immunological properties. A dose-ranging study of the first formulation of the investigational PCV15 was evaluated in preclinical animal studies using rabbits and infant rhesus monkeys but not in humans. Study results of the first generation PCV15 in infants, toddlers, and adults showed that PCV15 displayed safety profile comparable to PCV13, but vaccine-induced immune responses were generally lower to PCV13 for most shared serotypes.Citation15 Subsequently, several modifications were implemented, and 2 new formulations (Formulation A and Formulation B) were developed; dose ranging of PnPs and adjuvant in each PCV15 formulation were evaluated in 2 different Phase 1/2 clinical trials () involving small number of healthy young adults and infants in order to determine the optimal concentrations of PnPs and aluminum adjuvant in PCV15, and ultimately the best PCV15 formulation to evaluate in Phase 2 adult and pediatric clinical development.
Table 1. Vaccine Lots Evaluated in Study #1 (PCV15-Formulation A) and Study #2 (PCV15-Formulation B) (Infant Per-Protocol Population).
Results
Study #1 (PCV15 formulation A)
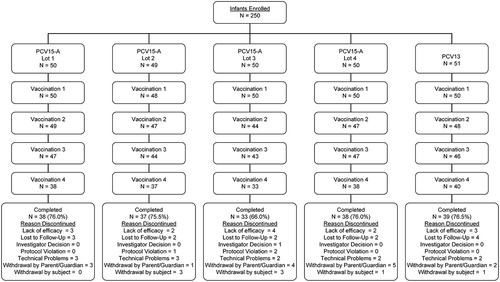
The proportions of infants who received dose 1, dose 2, dose 3, and dose 4 were comparable across the different vaccination groups. Furthermore, the reasons and proportions of subjects who discontinued from the study were also comparable across the groups (). Overall, frequencies of solicited injection site and systemic AEs were generally comparable across all PCV15 Formulation A (PCV15-A) lots and PCV13. No dose-response effects were observed when comparing frequencies of these AEs between PCV15-A lots with low, medium, or high content of PnPs but similar concentration of adjuvant (lot 2 vs lot 3 vs lot 4) or lots with medium and high amounts of adjuvant but similar content in PnPs (lot 1 vs lot 3). Most frequently reported AEs were those solicited in the trial. Following any vaccination and in all vaccination groups, rates of injection site AEs were highest for pain while rates of other solicited local AEs (erythema, swelling, and induration) were comparable across vaccination groups. Although recipients of lot 2 of PCV15-A had numerically lower rate of injection site pain than other vaccination groups, they reported numerically higher rates of other injection site AEs than recipients of other PCV15 Formulation B (PCV15-B) lots or PCV13. We could not identify any scientific reason to explain these observed differences in injection site AEs between recipients of PCV15-A lot 2 and other vaccination groups, especially as lot 2 contained the lowest concentration of PnPs than any other vaccination group and similar amount of adjuvant as lot 3 and lot 4 of PCV15-A. The observation of such numerical differences between recipients of PCV15-A lot 2 and other vaccination groups is likely related to the small number of subjects in the study (). Irritability, somnolence, and decreased appetite were the most frequently reported systemic AEs in decreasing order and rates were comparable across all PCV15-A lots and PCV13. Rates of infants reporting body temperature ≥100.4°F (38.0°C) were also comparable across vaccination groups, and no subject reported body temperature ≥103.1°F (39.5°C). The numbers of reported serious adverse events (SAEs) varied between vaccination groups but were generally comparable across vaccination groups, and none of these SAEs were deemed to be related to the study vaccine (). Although PCV15-A containing the lowest concentration of PnPs for serotypes 6A and 6B (lot 2) tended to induce higher anti-pneumococcal antibodies directed to these serotypes than other lots with higher PnPs content, PCV15-A lot 1 containing PnPs at 2µg per dose (for all serotypes except 6B at 4µg per dose) and adjuvant at 125µg per dose displayed satisfactory immune responses (for most serotypes) at levels comparable to those observed to other PCV15 lots containing lower or higher concentrations of PnPs and/or adjuvant (). However, serotype-specific IgG GMCs measured in recipients of various lots of PCV15-A were generally lower than those observed in recipients of PCV13 for most shared serotypes between PCV15 and PCV13, regardless of the concentrations of PnPs and/or adjuvant contained in PCV15 (). Recipients of different lots of PCV15-A had more robust antibody responses (IgG and OPA) than recipients of PCV13 for the 2 serotypes unique to V114 (22F and 33F) and serotype 3 at 1 month PD3 (, Supplemental Table 1). Kinetics of vaccine-induced immune responses followed the same trends for all vaccination groups at pre-dose 4 and 1 month PD4, with a significant decrease prior to receipt of toddler dose in comparison to peak PD3 levels followed by an increase post-toddler dose (Supplemental Table 2). Analysis of the serotype-specific IgG response rates (proportion of subjects achieving the WHO-accepted IgG threshold value of 0.35µg/mL) showed similar trends to those observed with IgG GMCs, with no dose-response in relation to PnPs and/or adjuvant concentrations across PCV15-A lots, and generally lower response rates in recipients of any PCV15-A lot in comparison to recipients of PCV13 (). Despite the generally lower serotype-specific IgG GMCs and response rates in recipients of PCV15-A than PCV13, proportions of infants who met the rescue criteria at 1 month postdose 3 (lack of efficacy) and needed to be given PCV13 prior to their first birthday was comparable across various lots of PCV15-A, and among recipients of PCV13 (). In adults, safety and immunogenicity profiles of various lots of PCV15-A containing medium to high PnPs and/or adjuvant were comparable (Supplement Table 3).
Table 2. Subjects Reporting Local and Systemic Adverse Events Within 14 Days Postvaccination – Study #1 (PCV15-Formulation A) (Infant Per-Protocol Population).
Table 3. Subjects Reporting Local and Systemic Adverse Events Within 14 Days Postvaccination – Study #2 (PCV15-Formulation B) (Infant Per-Protocol Population).
Figure 2. Postdose 3 IgG GMCs : Subject Disposition in Study #1 (PCV15-Formulation A) (Infant Per-Protocol Population).
● Lot 1 = 1x:1x composition (2 μg/dose polysaccharide: 125 μg/dose adjuvant; except 6B)● Lot 2 = 0.5x:2x composition (1 μg/dose polysaccharide: 250 μg/dose adjuvant; except 6B)● Lot 3 = 1x:2x composition (2 μg/dose polysaccharide: 250 μg/dose adjuvant; except 6B)● Lot 4 = 2x:2x composition (4 μg/dose polysaccharide: 250 μg/dose adjuvant; except 6B)● PCV13 = Prevnar 13● IgG=immunoglobulin G; GMC=geometric mean concentration; PCV pneumococcal polysaccharide vaccine
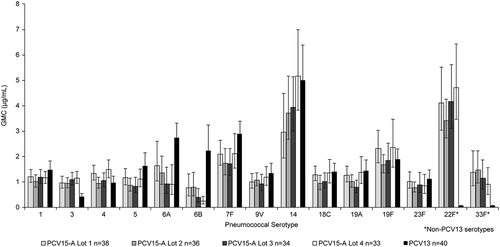
Figure 3. Postdose 3 IgG Response Rates in Study #1 (PCV15-Formulation A) (Infant Per-Protocol Population).
● Lot 1 = 1x:1x composition (2 μg/dose polysaccharide: 125 μg/dose adjuvant; except 6B)● Lot 2 = 0.5x:2x composition (1 μg/dose polysaccharide: 250 μg/dose adjuvant; except 6B)● Lot 3 = 1x:2x composition (2 μg/dose polysaccharide: 250 μg/dose adjuvant; except 6B)● Lot 4 = 2x:2x composition (4 μg/dose polysaccharide: 250 μg/dose adjuvant; except 6B)● PCV13 = Prevnar 13● IgG=immunoglobulin G; GMC=geometric mean concentration; PCV pneumococcal polysaccharide vaccine
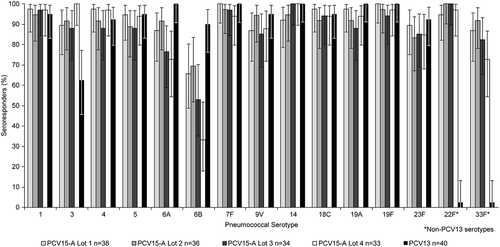
Study #2 (PCV15 formulation B)
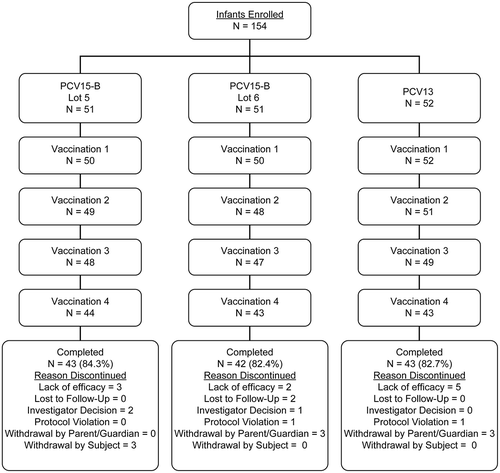
The proportions of infants who received dose 1, dose 2, dose 3, and dose 4 were comparable across the different vaccination groups. Furthermore, the reasons and proportions of subjects who discontinued from the study were also comparable across the groups (). Both PCV15-B/medium (lot 5) and PCV15-B/high (lot 6) displayed acceptable safety profiles and were immunogenic to all 15 serotypes in PCV15 in both adults and infants. Among infants, the safety profiles of the 2 lots of PCV15-B and PCV13 were generally comparable. Overall rate of systemic AEs was similar across all vaccination groups. The most frequently reported injection site AEs included injection site pain, erythema, swelling, induration while irritability, somnolence, and decreased appetite represented the most frequently systemic AEs. The incidences of injection-site AEs were higher in recipients of either lot of PCV15-B than PCV13 (). Proportions of infants reporting body temperature ≥100.4ºF was comparable between recipients of either lot of PCV15-B and recipients of PCV13 (). The reported AEs were mostly of mild to moderate intensity (Data not shown). There were no vaccine-related SAEs reported by any study participants over the duration of the study and no participants discontinued from the study due to an AE or SAE ().
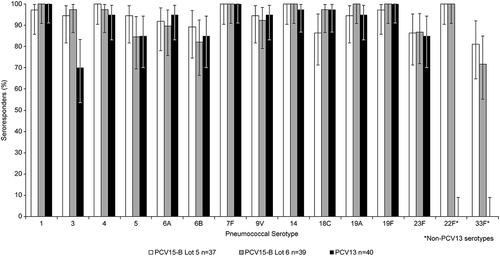
In infants, serotype-specific IgG GMCs measured at 1 month PD3 among recipients of PCV15-B/medium (lot 5) and PCV15-B/high (lot 6) were generally comparable with those measured in recipients of PCV13 for all 13 common serotypes and higher for the 2 serotypes unique to PCV15 (). Serotype-specific IgG response rates showed similar trends to those observed with IgG GMCs, with no dose-response in relation to PnPs and/or adjuvant concentrations across PCV15-B lots, but response rates were generally comparable to those measured in recipients of PCV13 (). These trends in IgG GMCs and response rates at 1 month PD3 were also observed at 1 month PD4 (Supplemental Table 4). No clear benefit in the levels of vaccine-induced antibodies was observed with increases in PnPs and adjuvant in PCV15-B for most vaccine serotypes ( and ). The proportions of infants who met the rescue criteria at 1 month postdose 3 (lack of efficacy) and needed to be given PCV13 prior to their first birthday was comparable between recipients of either PCV15-B lot and PCV13, indicating the overall acceptable clinical performance (based on safety and immunogenicity profiles) of PCV15-B/medium and PCV15-B/high in comparison to the licensed PCV13 (). Proportions of infants achieving OPA titer ≥1:8 and serotype-specific OPA GMTs measured at 1 month PD3 were generally comparable between the 3 vaccination groups (Supplemental Table 5).
Figure 5. Postdose 3 IgG GMCs in Study #2 (PCV15-Formulation B) (Infant Per-Protocol Population).
● Lot 5 = 1x:1x composition (2 μg/dose polysaccharide: 125 μg/dose adjuvant; except 6B)● Lot 6 = 2x:2x composition (4 μg/dose polysaccharide: 250 μg/dose adjuvant; except 6B)● PCV13 = Prevnar 13● IgG=immunoglobulin G; GMC=geometric mean concentration; PCV pneumococcal polysaccharide vaccine
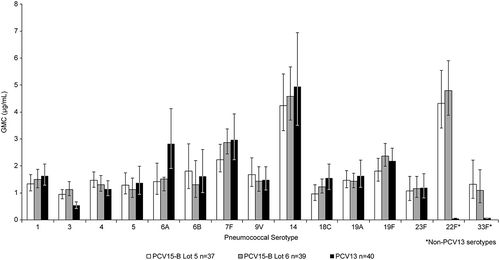
Figure 6. Postdose 3 IgG Response Rates in Study #2 (PCV15-Formulation B) (Infant Per-Protocol Population).
● Lot 5 = 1x:1x composition (2 μg/dose polysaccharide: 125 μg/dose adjuvant; except 6B)● Lot 6 = 2x:2x composition (4 μg/dose polysaccharide: 250 μg/dose adjuvant; except 6B)● PCV13 = Prevnar 13● IgG=immunoglobulin G; GMC=geometric mean concentration; PCV pneumococcal polysaccharide vaccine
In adults, safety profiles of both lots of PCV15-B were comparable. In addition, serotype-specific IgG GMCs were comparable between recipients of PCV15-B/medium (lot 5) and PCV15-high, although PCV15-B/high (lot 6) elicited higher IgG GMCs than PCV15-B/medium (lot 5) for some serotypes included in PCV15 (Supplement Table 6).
Discussion
Few studies have evaluated the optimal concentration of PnPs and/or adjuvant needed in pneumococcal conjugate vaccines. Dose finding pediatric studies of first generation PCVs showed that optimal concentration of PnPs varies between serotypes and was carrier dependent.Citation16 A dose-ranging study of 4-valent pneumococcal conjugate vaccine (PCV4) containing serotypes 6B, 14, 19F, and 23F conjugated to diphtheria toxoid, at PnPs concentrations of either 1µg, 3µg, and 10µg per dose for all 4 serotypes, was evaluated in infants who were vaccinated at 2, 4, 6 months of age followed by a toddler dose at 14 months of age of either PCV4 (at 3µg per dose) or PPV23; study results showed that highest dose of study vaccine was associated with strongest antibody responses at 1 month postdose 3 and that toddler dose induced highest antibody levels in children who received the lowest dose during primary series for serotypes 14 and 19F but no differences in post-booster antibody levels were observed for serotypes 6B and 23F. Furthermore, serotype-specific antibodies measured at 24 months and 36 months post-booster dose were comparable across various vaccination groups.Citation17 In contrast, a dose-ranging study of another PCV4 containing PnPs at the same concentrations (1µg, 3µg, or 10µg per dose) and conjugated to tetanus toxoid as carrier protein did not find a dose-response in serotype-specific anti-pneumococcal antibodies measured at 1 month after the infant series. As observed for PCV4 conjugated with diphtheria toxoid, responses following to the toddler dose were highest in the group vaccinated with the lowest dose of conjugate vaccine for three vaccine serotypes.Citation18
As PCVs with increasing valency were developed, it was important to determine whether the amount PnPs for each serotype and adjuvant needed to be adjusted. For example, a higher virus potency was necessary for the varicella component of the quadrivalent measles-mumps-rubella-varicella live attenuated virus vaccine in order to achieve comparable levels of antibody responses to that measured in recipients of monovalent live attenuated varicella virus vaccine as the measles component interfered with the VZV component, resulting in lower VZV GMT titers.Citation19-Citation21 Similarly, antigen concentrations for HPV types 6, 16 and 18 needed to be increased in the 9-valent HPV vaccine in comparison to the concentrations found in 4-valent HPV vaccine.Citation22 Dose ranging studies for PCV7 and PCV9 were only conducted in older adults in order to determine whether the pediatric dose level of these vaccines were sufficiently immunogenic in older adults or if a higher dose was required; dose-dependent response was observed when comparing 1x, 2x, and 4x doses of PCV7 but highest dose was associated with more adverse events.Citation23,Citation24
Although our initial formulation of PCV15 induced both IgG and OPA antibodies to all 15 serotypes included in the vaccine in both adults and infants, levels of serotype-specific antibodies measured following the third dose infant series (1 month PD3) were generally lower to those measured in infants vaccinated with PCV13 for 12 out of 13 shared serotypes between the 2 vaccines.Citation15 Several hypotheses were suggested for the observed findings including the need to optimize the concentration of PnPs for each vaccine serotype, the amount of adjuvant, and/or parameters in the conjugation process for glycoconjugates. It was very likely that several factors together were responsible for the less than optimal immune responses observed in the study, especially because antibody responses were comparable to PCV13 in toddlers,Citation25 young adults 18–45 years of age,Citation26 older adults ≥50 years of age,Citation27 but not in infantsCitation15 with known immature immune system.
We therefore conducted 2 clinical studies to test several hypotheses related to the optimal concentrations of PnPs, aluminum phosphate adjuvant, and several conjugation parameters that have been suggested to be important in the processing and immune recognition of glycoconjugates by antigen presenting cells of the immune system. Study #1 tested several hypotheses related to PnPs and adjuvant concentration while study #2 tested parameters that could influence immune recognition of glycoconjugates as well as limited dose range for PnPs and adjuvant. All 4 lots of PCV15-A evaluated in study #1 and #2 lots PCV15-B evaluated in study #2 had acceptable safety profiles and induced serotype-specific IgG and OPA to all 15 serotypes included in the vaccine in both adults and infants. However, PCV15-A did not show significant improvement in vaccine performance in comparison to PCV13 for most vaccine serotypes than the initial formulation evaluated in a previous study of PCV15.Citation15 In contrast, PCV15-B showed significant improvement in vaccine performance for most serotypes in PCV15 in comparison to PCV15-A and the initial formulation evaluated in the study by Greenberg et al.Citation15 Improved immune responses observed for PCV15-B were observed for both IgG GMCs and proportion of subjects achieving threshold value of ≥0.35 µg/mL at both PD3 and PD4. Study results from both studies also confirmed results from previous studies showing that both PCV15-A and PCV15-B induce higher antibodies than PCV13 to serotype 3 for which the effectiveness of the licensed PCV13 appears to be inconsistent, sometimes due the small size of the study cohortCitation28-Citation31; however, the clinical significance of the observed difference in antibody levels between recipients of PCV15 and PCV13 is unknown without real world evidence of PCV15 effectiveness against disease caused by serotype 3. Moreover, both PCV15-A and PCV15-B induce higher antibody responses than PCV13 to the 2 serotypes unique to PCV15 (serotypes 22F and 33F).
The current studies have several limitations due primarily to the small sample sizes and the lack of an accepted immune correlates to infer protection against pneumococcal disease. The studies were not designed to demonstrate statistical differences in serotype-specific antibodies between different lots of each PCV15 formulation nor differences between each lot of PCV15 to PCV13 but only if any trends could be observed. Although WHO-accepted antibody threshold concentration of ≥0.35 µg/mL was used to compare IgG responses between vaccination groups, lack of accepted serotype-specific immune correlates of protection against pneumococcal disease in infants did not allow for meaningful assessment of clinical significance for observed differences between vaccination groups.
In summary, all lots of both PCV15 formulations display comparable safety and immunogenicity profiles to PCV13 when administered as a single dose to young adults and 4-dose regimen in infants. Increased concentration of aluminum phosphate adjuvant was not associated with an increased in PCV15-induced antibodies. Levels of serotype-specific antibodies in recipients of PCV15-A/medium (PnPs at 2µg for all serotypes except 6B at 4µg) tended to be higher than those observed in recipients of PCV15-A/low (PnPs at 1µg for all serotypes except 6B at 2µg) for some serotypes, but were generally comparable to those in recipients of PCV15-A/high (PnPs at 4µg for all serotypes except 6B at 8 µg). More importantly, modifications in the conjugation process for glycoconjugates implemented in PCV15-B appear to improve the overall performance of the investigational PCV15 in comparison to PCV13 for both the proportion of vaccine responders and IgG GMCs in recipients of either PCV15 medium or high. Study results also confirmed results from previous studies showing that PCV15 induces higher antibodies than PCV13 for the 2 serotypes unique to PCV15 (22F and 33F). Taken together, PCV15-B/medium achieved the optimal balance in PnPs/adjuvant that was associated with acceptable safety and immunogenicity profiles in comparison to PCV13; it was therefore selected for further evaluation in Phase 2 adult and pediatric clinical development.
Methods
Vaccines
Formulation A of PCV15 (PCV15-A) was evaluated in Study #1 and Formulation B of PCV15 (PCV15-B) was evaluated in Study #2. Both formulations contain capsular polysaccharides from 15 serotypes, all conjugated with CRM197 protein and formulated with Aluminum Phosphate Adjuvant. Conjugates for all 15 serotypes were manufactured using the same conjugation process in Formulation A. In contrast, only 8 conjugates in Formulation B were manufactured using the same conjugation process as in Formulation A, while the remaining 7 conjugates were manufactured using a modified conjugation process. In both studies, several lots of the same PCV15 formulation containing various concentrations of PnPs and adjuvant were first evaluated in adults and then infants. In study #1, four different lots of PCV15-A containing pneumococcal polysaccharide (PnPs) ranging from 1µg/dose to 4µg/dose (except for serotype 6B at 2µg/dose to 8µg/dose) formulated with aluminum phosphate adjuvant at 125µg/dose or 250µg/dose were evaluated in the trial. In study #2, a limited dose ranging was evaluated. Two different lots of PCV15-B containing PnPs ranging from 2µg/dose to 4µg/dose (except for serotype 6B at 4µg/dose to 8µg/dose) and aluminum phosphate adjuvant (125µg/dose to 250µg/dose) were evaluated in the trial.
The vaccine lots evaluated in the 2 studies are described in .
Participants and study design
Two phase 1/2 randomized, double-blind, multicenter studies were conducted at 27 (study #1) and 24 (study #2) clinical centers in United States. These studies evaluated safety and immunogenicity profiles of two different formulations of PCV15 (PCV15-A and PCV15-B) in young adults 18–49 years of age and infants 6–12 weeks of age. Adults were given a single dose of study vaccine and safety of study vaccine was to be demonstrated in adults before enrollment of infants who were then given a 4-dose regimen at 2, 4, 6, and 12–15 months of age. Routine pediatric vaccines were allowed and given following US-ACIP recommended immunization schedules. Protocol was approved by ethical review committees of each study site and conducted in conformance with applicable country or local requirements. Written informed consent was obtained from each subject’s parents/guardians.
Subjects were followed for adverse events (AEs) for 14 days following each vaccination using validated vaccination report card (VRC). In both adult and infant cohorts, solicited injection-site (local) AEs included redness, swelling, hard lump, and pain/tenderness. Solicited systemic AEs in the adult cohort included headache, fatigue, muscle pain, and joint pain while irritability, drowsiness, hives/welts, and appetite lost were solicited in the infant cohort. Serious AEs (SAEs) were collected during entire study period and/or study completion. Body temperatures (rectal/axillary) were recorded for 5 days and 7 days following each vaccination in adults and infants, respectively. In infants, body temperature was also taken Days 8–14 postvaccination if fever was suspected.
In adults, blood samples were collected immediately prior and 30 days postvaccination. In the infant cohort, blood was collected approximately 30 days postdose 3 (PD3), pre-dose 4 (Pre-D4), and 30 days PD4. Sera were used to measure IgG using pneumococcal electrochemiluminescence (Pn-ECL) assay,Citation32 and opsonophagocytic (OPA) killing activity using multiplex OPA (MOPA-4) assayCitation33 to all 15 vaccine serotypes. Serotype-specific IgG was measured for all subjects, but OPA was measured in approximately 50% of infants across both studies that had sufficient volume of blood to perform both Pn ECL and OPA assays at PD3 and on a subset of those infants at pre-D4 and PD4. Proportion of vaccine responders (infants achieving WHO-accepted serotype-specific IgG of ≥0.35µg/mL), serotype-specific IgG GMCs, and OPA GMTs were compared between groups at all three time-points. Pn-ECL assays were performed by PPD® Vaccines and Biologics (Wayne, PA) and PPD® Laboratories Bioanalytical Lab (Richmond, VA). MOPA-4 assays were performed by the University of Alabama (Birmingham, AL).
In order to ensure that infants achieved adequate protection against vaccine-type (VT) pneumococcal disease before reaching 12 months of age, any study subject with serotype-specific IgG GMC <0.35µg/mL for serotype 19A individually or 4 or more serotypes in common between PCV15 and PCV13 at 7 months of age (corresponding to 1 month PD3) was given one dose of licensed PCV13 as soon as serological results were available, typically by 10–11 months of age.
Statistical methods
Both studies were descriptive in nature aimed at describing the safety of the 2 new formulations of the investigational vaccine in reasonably sized Phase 1/2 clinical trials. No formal hypotheses were tested, and no statistical criteria for success were defined. In each trial, primary immunogenicity objectives were to compare serotype-specific IgG GMCs between the various lots of the new formulation. Responses measured at PD3 and PD4 in infants vaccinated with PCV13 were used as benchmark for expected responses with licensed PCV.
Serotype-specific IgG GMCs measured at 1 month PD3 in infants at 1 month PD in adults along with two-sided 95% CIs were computed for the 15 serotypes in PCV15 and 13 serotypes in PCV13. Point estimates of GMCs were exponentiated estimates of the mean loge concentrations. The confidence intervals for IgG GMCs were the exponentiated confidence intervals for the mean loge concentrations, based on 1-sample t-distributions. Additionally, IgG GMC ratios (PCV15/PCV13) along with two-sided 95% CIs were computed in infants for each PCV15 lot relative to PCV13 for the 13 common serotypes. The CIs for the ratios were calculated using a 2-sample t-test approach based on the natural logarithm of the antibody concentration.
Proportion of infants achieving the serotype-specific threshold value of ≥ 0.35µg/mL (response rates) for the 15 serotypes measured at 1 month PD3 along with the 95% CI were calculated for each PCV15 lot and the 13 serotypes in PCV15. The one-sample two-sided CIs were computed using the exact CI method for a single binomial proportion.Citation34 Additionally, the difference (PCV15 minus PCV13) in the proportion of infants achieving an IgG concentration ≥0.35µg/mL along with two-sided 95% CIs were computed for each PCV15 lot relative to PCV13 for the 13 serotypes in common. The CIs for the differences in response rates were computed using the Miettinen and Nurminen method.Citation35
Author contributions
RR and DH: enrollment of subjects and/or data collection, review of the manuscript.
SG, JL, RDM, JH, LM: analysis and interpretation of data, and preparation of manuscript.
KN, RDM, JH, CA, MW, HP, PB, and LM: study concept and design, analysis and interpretation of data, and preparation of manuscript.
CA, MW, and HP: vaccine design and supply.
Disclosure of potential conflicts of interest
SG, JL, KN, RDM, JH, CA, MW, HP, PB, and LM are employees of the study sponsor.
Supplemental Material
Download MS Word (95.4 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Cherian T. WHO expert consultation on serotype composition of pneumococcal conjugate vaccines for use in resource-poor developing countries, 26-27 October 2006, Geneva. Vaccine. 2007;25:6557–64. doi:10.1016/j.vaccine.2007.06.044.
- O’Brien K, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi:10.1016/S0140-6736(09)61204-6.
- Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20 Supple 5:45–51. doi:10.1111/1469-0691.12461.
- Arditi M, Mason EO Jr, Bradley JS, Tan TQ, Barson WJ, Schutze GE, Wald ER, Givner LB, Kim KS, Yogev R, et al. Three-year multicenter surveillance of pneumococcal meningitis in children: Clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics. 1998;102(5):1087–1097.
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi:10.1086/648593.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Lepoutre A, Varon E, Georges S, Dorleans F, Janoir C, Gutmann L, Lévy-Bruhl D. Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001-2012. Vaccine. 2015;33(2):359–66. doi:10.1016/j.vaccine.2014.11.011.
- Weiss S, Falkenhorst G, van der Linden M, Imohl M, von Kries R. Impact of 10- and 13-valent pneumococcal conjugate vaccines on incidence of invasive pneumococcal disease in children aged under 16 years in Germany, 2009 to 2012. Euro Surveill. 2015;20(10):21057. doi:10.2807/1560-7917.ES2015.20.10.21057.
- Martinelli D, Pedalino B, Cappelli MG, Caputi G, Sallustio A, Fortunato F, Tafuri S, Cozza V, Germinario C, Chironna M, et al. Towards the 13-valent pneumococcal conjugate universal vaccination: effectiveness in the transition era between PCV7 and PCV13 in Italy, 2010-2013. Hum Vaccin Immunother. 2014;10(1):33–39. doi:10.4161/hv.26650.
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–43. doi:10.1016/S1473-3099(15)70044-7.
- Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997-2004. Pediatrics. 2008;121(2):253–60. doi:10.1542/peds.2007-0619.
- Greenberg D, Hoffman S, Leibovitz E, Dagan R. Acute otitis media in children: association with day care centers–antibacterial resistance, treatment, and prevention. Paediatr Drugs. 2008;10(2):75–83. doi:10.2165/00148581-200810020-00002.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63(5):611–18. doi:10.1093/cid/ciw347.
- International Vaccine Access Center. Pneumococcal conjugate vaccine (PCV) product assessment [Internet]. Baltimore (MD): Johns Hopkins Bloomberg School of Public Health; 2013. http://www.jhsph.edu/research/centers-and-institutes/ivac/resources/pcv-product-assessment-april-25-2017.pdf.
- Greenberg D, Hoover PA, Vesikari T, Peltier C, Hurley DC, McFetridge RD, Dallas M, Hartzel J, Marchese RD, Coller BG, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine. 2018;36(45):6883–91. doi:10.1016/j.vaccine.2018.02.113.
- Poolman JT, Peeters CC, van Den Dobbelsteen GP. The history of pneumococcal conjugate vaccine development: dose selection. Expert Rev Vaccines. 2013;12(12):1379–94. doi:10.1586/14760584.2013.852475.
- Ahman H, Käyhty H, Lehtonenm H, Leroy O, Eskola J. Streptococcus pneunoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Ped Infect Dis J. 1998;17(3):211–16. doi:10.1097/00006454-199803000-00008.
- Ahman H, Käyhty H, Vuorela A, Leroy O, Eskola J. Dose dependency of antibody response in infants and children to pneumococcal polysaccharides conjugated to tetanus toxoid. Vaccine. 1999;17:2726–32.
- Berger R, Just M. Interference between strains in live virus vaccine II: combined vaccination with varicella and measles-mumps-rubella vaccine. J Biol Stand. 1988;16(4):275–79. doi:10.1016/0092-1157(88)90015-7.
- Shinefield H, Black S, Digilio L, Reisinger K, Blatter M, Gress JO, Brown ML, Eves KA, Klopfer SO, Schödel F, et al. Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J. 2005;24(8):665–69. doi:10.1097/01.inf.0000172902.25009.a1.
- Shinefield H, Williams WR, Marchant C, Reisinger K, Stewart T, Meissner C, Meissner HC, Guerrero J, Klopfer SO, Xu J, et al. Dose-response study of a quadrivalent measles, mumps, rubella, and varicella vaccine in healthy children. Pediatr Infect Dis J. 2005;24(8):670–75. doi:10.1097/01.inf.0000172901.29621.e9.
- Luxembourg A, Brown D, Bouchard C, Giuliano AR, Iversen OE, Joura EA, Penny ME, Restrepo JA, Romaguera J, Maansson R, et al. Phase II studies to select the formulation of a multivalent HPV L1 virus-like particle (VLP) vaccine. Hum Vaccin Immunother. 2015;11(6):1313–22. doi:10.1080/21645515.2015.1012010.
- Jackson LA, Neuzil KM, Nahm MH, Whitney CG, Yu O, Nelson JC, Starkovich PT, Dunstan M, Carste B, Shay DK, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2007;25(20):4029–37. doi:10.1016/j.vaccine.2007.02.062.
- Lode H, Scmoele-Thoma B, Gruber W, Ahlers N, Fernsten P, Baker S, Razmpour A, Siber G, Hackell J, Lockhart S, et al. Dose-ranging study of a single injection of pneumococcal conjugate vaccine (1x, 2x, or 4x) in healthy subjects aged 70 years or older. Vaccine. 2011;29(31):4940–46. doi:10.1016/j.vaccine.2011.04.132.
- Sobanjo-Ter Meulen A, Vesikari T, Malacaman EA, Shapiro SA, Dallas MJ, Hoover PA, McFetridge R, Stek JE, Marchese RD, Hartzel J, et al. Safety, tolerability and immunogenicity of 15-valent pneumococcal conjugate vaccine in toddlers previously vaccinated with 7-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2015;34(2):186–94. doi:10.1097/INF.0000000000000516.
- McFetridge R, Meulen AS, Folkerth SD, Hoekstra JA, Dallas M, Hoover PA, Marchese RD, Zacholski DM, Watson WJ, Stek JE, et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2015;33(24):2793–99. doi:10.1016/j.vaccine.2015.04.025.
- Ermlich SJ, Andrews CP, Folkerth S, Rupp R, Greenberg D, McFetridge RD, Hartzel J, Marchese RD, Stek JE, Abeygunawardana C, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naïve adults ≥50 years of age. Vaccine. 2018;36(45):6875–82. doi:10.1016/j.vaccine.2018.03.012.
- Ladhani SN, Collins S, Djennad A, Shepaprd CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18:441–51. doi:10.1016/S1473-3099(18)30052-5.
- Domínguez Á, Ciruela P, Hernández S, García-García JJ, Soldevila N, Izquierdo C, Moraga-Llop F, Díaz AF, de Sevilla M, González-Peris S, et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing inasive pneumococcal disease in children aged 7-59 months. A matched case-control study. PLoS ONE. 2017;12(8):e0183191. doi:10.1371/journal.pone.0183191.
- Naucler P, Galanis I, Morfeldt E, Darenberg J, Ortqvist A, Henriques-Normark B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis. 2017;65:1780–90.e1. doi:10.1093/cid/cix685.
- Sings HL, De Wals P, Gessner BD, Isturiz R, Laferriere C, McLaughlin JM, Pelton S, Schmitt H-J, Suaya JA, Jodar L. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive disease caused by serotype 3 in children: a systematic review and meta-analysis of observational studies. Clin Infect Dis. 2018 Oct 23. [Epub ahead of print]. doi:10.1093/cid/ciy920.
- Marchese RD, Puchalski D, Miller P, Antonello J, Hammond O, Green T, Rubinstein LJ, Caulfield MJ, Sikkema D. Optimization and validation of a multiplex, electrochemiluminescence-based detection assay for the quantitation of immunoglobulin G serotype-specific antipneumococcal antibodies in human serum. Clin Vaccine Immunol. 2009;16(3):387–96. doi:10.1128/CVI.00415-08.
- Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13(9):1004–09. doi:10.1128/CVI.00112-06.
- Collett D. Statistical inference for binary data. In: Collett D, editor. Modelling binary data. New York (NY): Chapman & Hall; 1991. p. 17–42.
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26.